Integrated Metabolome and Transcriptome Analyses Reveal the Mechanisms Regulating Flavonoid Biosynthesis in Blueberry Leaves under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Salt Stress Treatments
2.2. Metabolomics Analysis by UHPLC-MS/MS
2.3. Transcriptomic Analysis by RNA Sequencing
2.4. RNA Sequencing Data Validation
2.5. Integrative Analysis of Transcriptomic and Metabolomic Data
3. Results
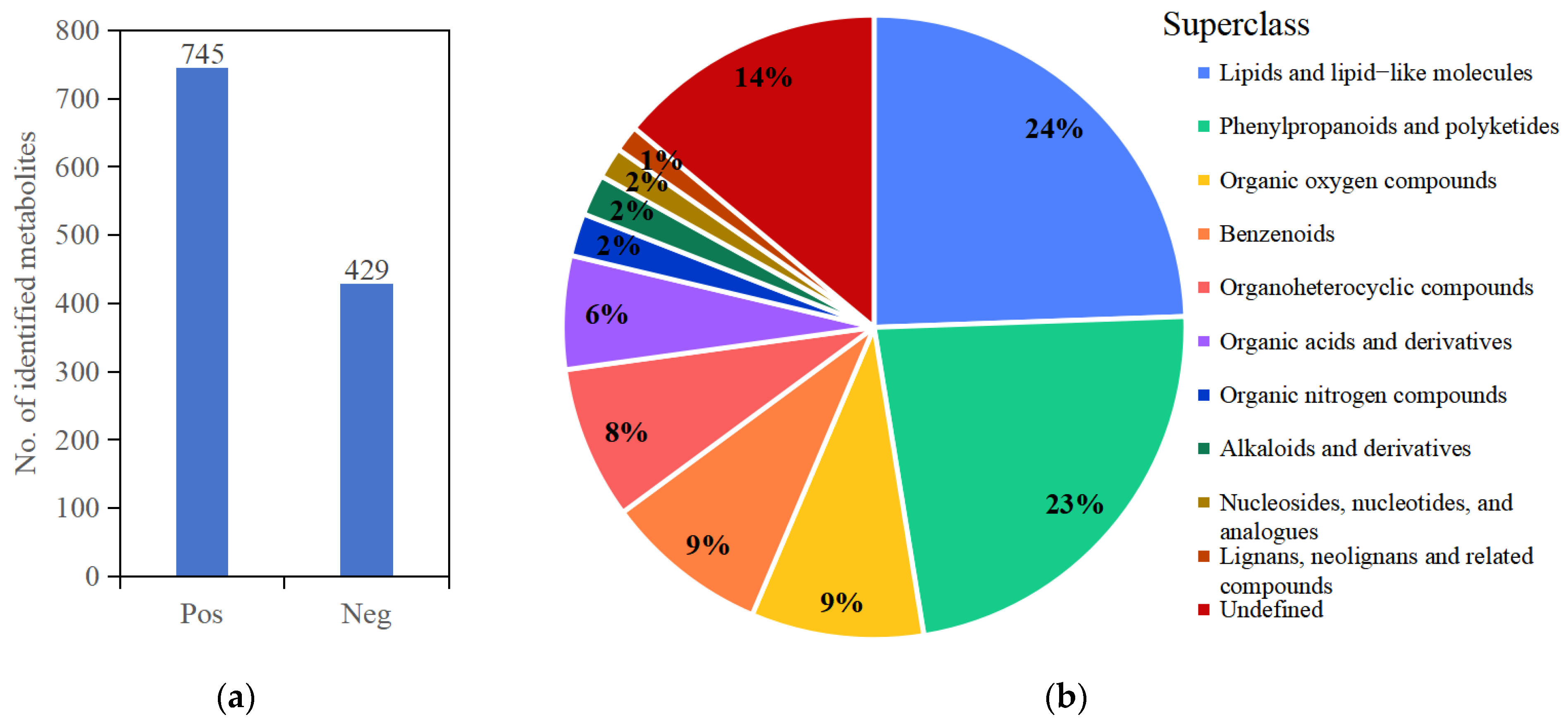
3.1. Metabolome Analysis for Blueberry Leaves under Salt Stress
3.2. Analysis of DAMs for Blueberry Leaves under Salt Stress
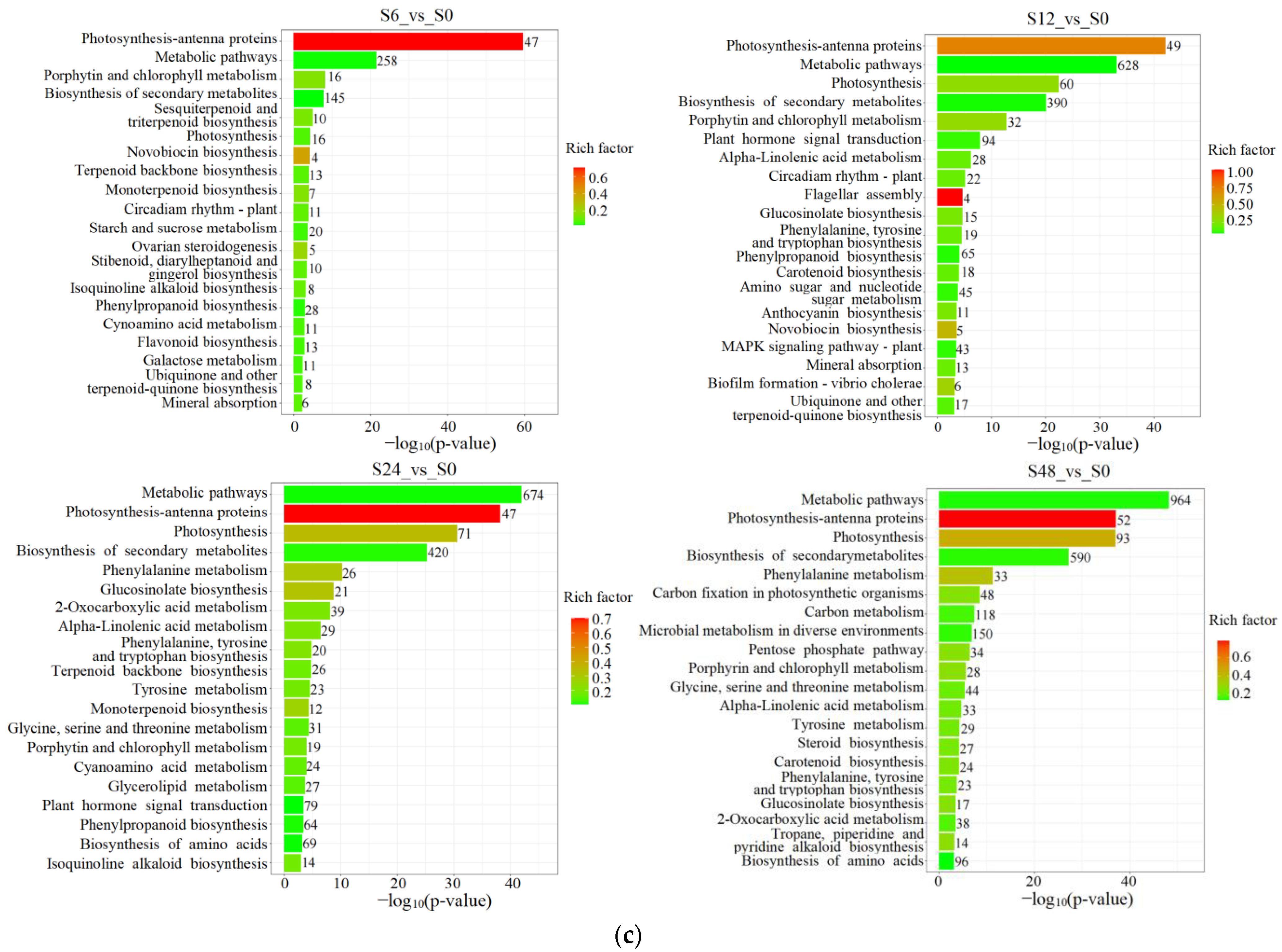
3.3. Enrichment Analysis of the KEGG Pathways of the DAMs
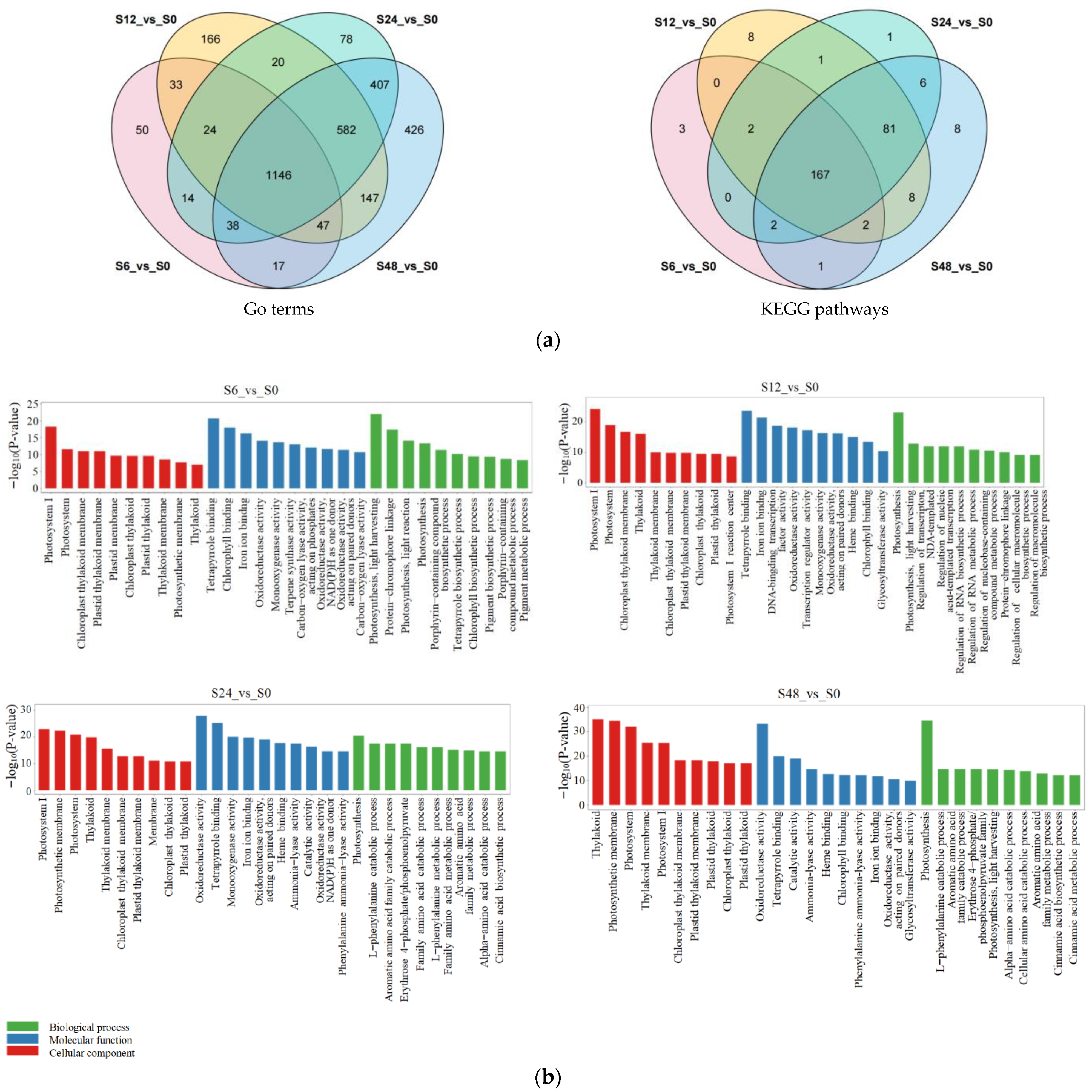
3.4. Transcriptome Analysis and DEGs for Blueberry Leaves under Salt Stress
3.5. Functional Annotation and Enrichment Analysis of DEGs
3.6. Identification of Differentially Expressed Transcription Factors (TFs) under Salt Stress
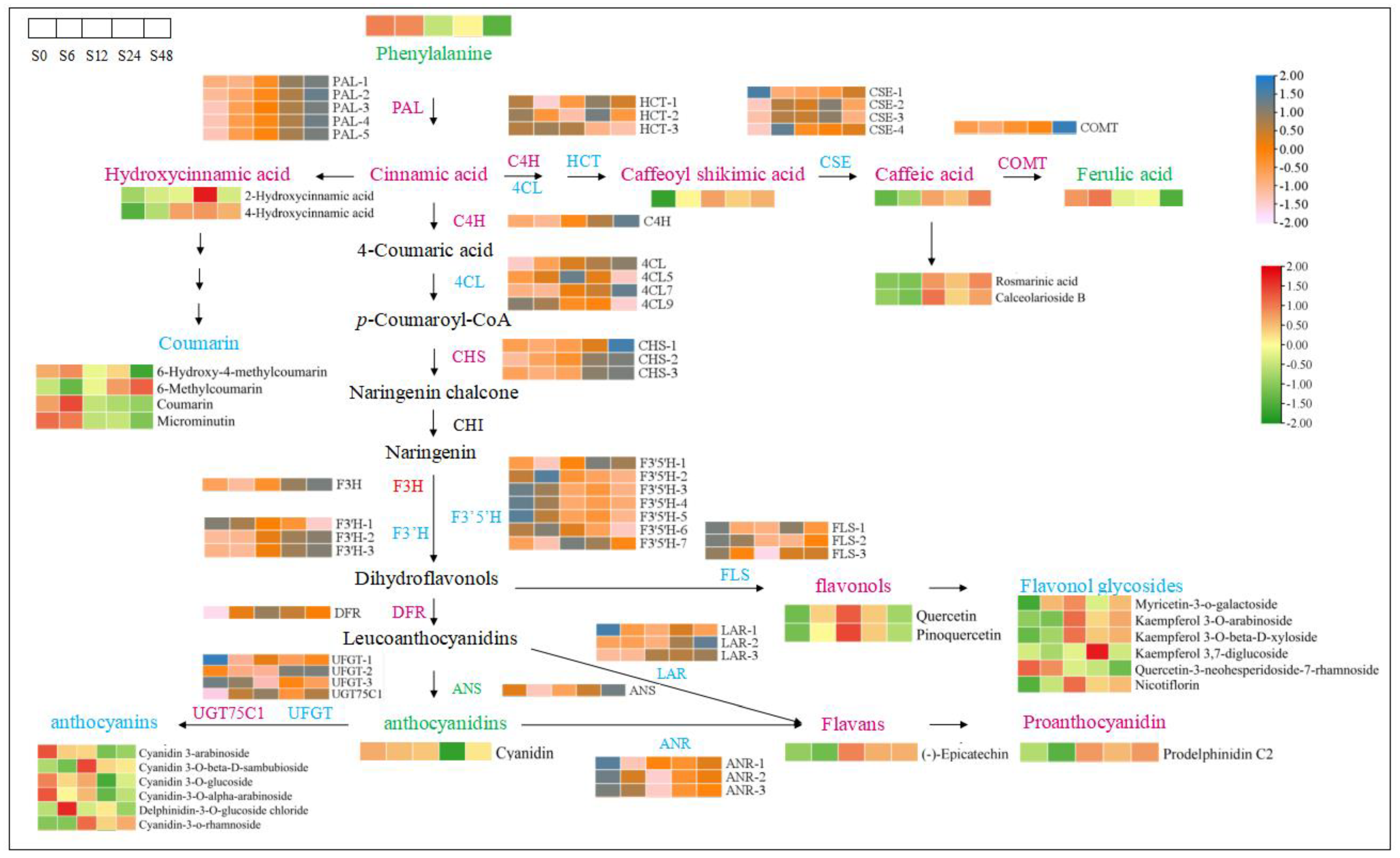
3.7. DAMs and DEGs in Phenylpropanoid and Flavonoid Biosynthetic KEGG Pathways under Salt Stress
3.8. Combined Metabolome and Transcriptome Analysis of the Flavonoid Biosynthetic Pathway under Salt Stress
4. Discussion
4.1. Salt Stress Regulates the Accumulation of Phenylpropanoid and Flavonoid Metabolites
4.2. Salt Stress Regulates the Expression of Genes from the Phenylpropanoid and Flavonoid Pathways
4.3. The Differentially Expressed Transcription Factors (TFs) in Response to Salt Stress
4.4. Salt Stress Promotes Flavonoid Biosynthesis by Activating the Expression of Flavonoid Biosynthesis Pathway Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. R. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X.; Guo, H.; Bai, S.; Khan, A.; Wang, X.; Gao, Y.; Li, J. Tomato salt tolerance mechanisms and their potential applications for fighting salinity: A review. Front. Plant Sci. 2022, 13, 949541. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Fang, S.; Liu, Y.; Shang, X. Integrative analysis of metabolome and transcriptome reveals molecular regulatory mechanism of flavonoid biosynthesis in Cyclocarya paliurus under salt stress. Ind. Crops Prod. 2021, 170, 113823. [Google Scholar] [CrossRef]
- Lin, S.; Zeng, S.; Biao, A.; Yang, X.; Yang, T.; Zheng, G.; Mao, G.; Wang, Y. Integrative analysis of transcriptome and metabolome reveals salt stress orchestrating the accumulation of specialized metabolites in Lycium barbarum L. Fruit. Int. J. Mol. Sci. 2021, 22, 4414. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.; Mohsin, S.M.; Fujita, A.M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef]
- Yiu, J.; Tseng, M.; Liu, C.; Kuo, C. Modulation of NaCl stress in Capsicum annuum L. seedlings by catechin. Sci. Hortic. 2012, 134, 200–209. [Google Scholar] [CrossRef]
- Truong, H.A.; Lee, W.J.; Jeong, C.Y.; Trịnh, C.S.; Lee, S.; Kang, C.S.; Cheong, Y.K.; Hong, S.W.; Lee, H. Enhanced anthocyanin accumulation confers increased growth performance in plants under low nitrate and high salt stress conditions owing to active modulation of nitrate metabolism. J. Plant Physiol. 2018, 231, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, F.; Liu, G.; Li, W.; Li, W.; Zhuang, J.; Zhang, X.; Wang, L.; Lei, B.; Hu, C.; et al. Effects and mechanisms of proanthocyanidins-derived carbon dots on alleviating salt stress in rice by muti-omics analysis. Food Chem. X 2024, 22, 101422. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Määttä, K.; Pirttilä, A.M.; Törrönen, R.; Kärenlampi, S.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.; Qin, B.; Sun, H.; Yuan, X.; Wang, Q.; Xu, J.; Yin, Z.; Du, Y.; Du, J.; et al. Analysis of the transcriptome and metabolome reveals phenylpropanoid mechanism in common bean (Phaseolus vulgaris) responding to salt stress at sprout stage. Food Energy Secur. 2023, 12, e481. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Wang, Y.; Xu, Y.; Li, J.; Zhao, S.; Wang, D.; Ma, Z.; Yan, F.; Liu, Y. Combined transcriptomic and metabolomic analysis reveals the role of phenylpropanoid biosynthesis pathway in the salt tolerance process of Sophora alopecuroides. Int. J. Mol. Sci. 2021, 22, 2399. [Google Scholar] [CrossRef]
- Wang, M.; Ren, T.; Huang, R.; Li, Y.; Zhang, C.; Xu, Z. Overexpression of an Apocynum venetum flavonols synthetase gene confers salinity stress tolerance to transgenic tobacco plants. Plant Physiol. Biochem. 2021, 162, 667–676. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef]
- Mahajan, M.; Yadav, S.K. Overexpression of a tea flavanone 3-hydroxylase gene confers tolerance to salt stress and alternaria solani in transgenic tobacco. Plant Mol. Biol. 2014, 85, 551–573. [Google Scholar] [CrossRef]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods. 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. J. Soil Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Vincent, C.; Isaacs, R. Blueberry IPM: Past successes and future challenges. Annu. Rev. Entomol. 2019, 64, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Muralitharan, M.S.; Chandler, S.; Van, S.R. Effects of NaCl and Na2SO4 on growth and solute composition of highbush blueberry (Vaccinium corymbosum). Funct. Plant Biol. 1992, 19, 155–164. [Google Scholar] [CrossRef]
- Bryla, D.R.; Scagel, C.F.; Lukas, S.B.; Sullivan, D.M. Ion-specific limitations of sodium chloride and calcium chloride on growth, nutrient uptake, and mycorrhizal colonization in northern and southern highbush blueberry. J. Am. Soc. Hort. Sci. 2021, 146, 399–410. [Google Scholar] [CrossRef]
- Molnar, S.; Clapa, D.; Pop, V.C.; Hârța, M.; Andrecan, F.A.; Bunea, C.I. Investigation of salinity tolerance to different cultivars of highbush blueberry (Vaccinium corymbosum L.) grown in vitro. Not. Bot. Horti Agrobot. 2024, 52, 13691. [Google Scholar] [CrossRef]
- Wright, G.C.; Patten, K.D.; Drew, M.C. Salinity and supplemental calcium influence growth of rabbiteye and southern highbush blueberry. J. Am. Soc. Hortic. Sci. 1992, 117, 749–756. [Google Scholar] [CrossRef]
- Gan, T.; Lin, Z.; Bao, L.; Hui, T.; Cui, X.; Huang, Y.; Wang, H.; Su, C.; Jiao, F.; Zhang, M.; et al. Comparative proteomic analysis of tolerant and sensitive varieties reveals that phenylpropanoid biosynthesis contributes to salt tolerance in mulberry. Int. J. Mol. Sci. 2021, 22, 9402. [Google Scholar] [CrossRef]
- Wu, Q.; Bai, X.; Zhao, W.; Xiang, D.; Wan, Y.; Yan, J.; Zou, L.; Zhao, G. De novo assembly and analysis of tartary buckwheat (Fagopyrum tataricum Garetn.) transcriptome discloses key regulators involved in salt-stress response. Genes 2017, 8, 255. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- Zhang, H.; Han, B.; Wang, T.; Chen, S.; Li, H.; Zhang, Y.; Dai, S. Mechanisms of plant salt response: Insights from proteomics. J. Proteome Res. 2012, 11, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Törrönen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’ blueberry (Vaccinium corymbosum × V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant actvities of Vaccinium vitis-idaea L. leaves within cultivars and their phenolic compounds. Molecules 2019, 24, 844. [Google Scholar] [CrossRef]
- Vyas, P.; Kalidindi, S.; Chibrikova, L.; Igamberdiev, A.U.; Weber, J.T. Chemical analysis and effect of blueberry and lingonberry fruits and leaves against glutamate-mediated excitotoxicity. J. Agric. Food Chem. 2013, 61, 7769–7776. [Google Scholar] [CrossRef]
- Ren, G.; Yang, P.; Cui, J.; Gao, Y.; Yin, C.; Bai, Y.; Zhao, D.; Chang, J. Multiomics analyses of two sorghum cultivars reveal the molecular mechanism of salt tolerance. Front. Plant Sci. 2022, 13, 886805. [Google Scholar] [CrossRef]
- Ma, W.; Kim, J.K.; Jia, C.; Yin, F.; Kim, H.J.; Akram, W.; Hu, X.; Li, X. Comparative transcriptome and metabolic profiling analysis of buckwheat (Fagopyrum tataricum (L.) Gaertn.) under salinity stress. Metabolites 2019, 9, 225. [Google Scholar] [CrossRef]
- Abdallah, S.B.; Aung, B.; Amyot, L.; Lalin, I.; Lachal, M.; Karray-Bouraou, N.; Hannoufa, A. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant 2016, 38, 72. [Google Scholar] [CrossRef]
- Ma, S.; Lv, L.; Meng, C.; Zhang, C.; Li, Y. Integrative analysis of the metabolome and transcriptome of Sorghum bicolor reveals dynamic changes in flavonoids accumulation under saline-alkali stress. J. Agric. Food Chem. 2020, 68, 14781–14789. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Pan, X.; Yang, L.; Guo, W. Proteins expression and metabolite profile insight into phenolic biosynthesis during highbush blueberry fruit maturation. Food Chem. 2019, 290, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, Y.; Li, Y.; Wang, X.; Guo, Q.; Zhou, L.; Zhang, Y.; Zhang, C. Key genes for phenylpropanoid metabolite biosynthesis during half-highbush blueberry (Vaccinium angustifolium × Vaccinium corymbosum) fruit development. J. Berry Res. 2022, 12, 297–311. [Google Scholar] [CrossRef]
- Pi, E.; Zhu, C.; Fan, W.; Huang, Y.; Qu, L.; Li, Y.; Zhao, Q.; Ding, F.; Qiu, L.; Wang, H.; et al. Quantitative phosphoproteomic and metabolomic analyses reveal GmMYB173 optimizes flavonoid metabolism in soybean under salt stress. Mol. Cell. Proteom. 2018, 17, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, H.; Kong, W.; Peng, R.; Liu, Q.; Yao, Q. The Antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis. Planta 2016, 244, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.; Rugină, D.; Sconţa, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tăbăran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef]
- Müller, D.; Schantz, M.; Richling, E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. J. Food Sci. 2012, 77, C340–C345. [Google Scholar] [CrossRef]
- Gavrilova, V.; Kajdzanoska, M.; Gjamovski, V.; Stefova, M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Sun, M.; Feng, X.; Gao, J.; Peng, R.; Yao, Q.; Wang, L. VvMYBA6 in the promotion of anthocyanin biosynthesis and salt tolerance in transgenic Arabidopsis. Plant Biotechnol. Rep. 2017, 11, 299–314. [Google Scholar] [CrossRef]
- Kim, J.; Lee, W.J.; Vu, T.T.; Jeong, C.Y.; Hong, S.W.; Lee, H. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep. 2017, 36, 1215–1224. [Google Scholar] [CrossRef]
- Jia, C.; Guo, B.; Wang, B.; Li, X.; Yang, T.; Li, N.; Wang, J.; Yu, Q. Integrated metabolomic and transcriptomic analysis reveals the role of phenylpropanoid biosynthesis pathway in tomato roots during salt stress. Front. Plant Sci. 2022, 13, 1023696. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, L.; Liu, K.; Liang, K.; Yang, S.; Cao, Y.; Zhang, L. Comparative transcriptome profiling reveals the defense pathways and mechanisms in the leaves and roots of blueberry to drought stress. Fruit Res. 2022, 2, 18. [Google Scholar] [CrossRef]
- Zhang, F.; Ji, S.; Wei, B.; Cheng, S.; Wang, Y.; Hao, J.; Wang, S.; Zhou, Q. Transcriptome analysis of postharvest blueberries (Vaccinium corymbosum ‘Duke’) in response to cold stress. BMC Plant Biol. 2020, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qi, Y.; Zhao, C.; Wang, X.; Zhang, Q. Transcriptome profiling of the salt stress response in the leaves and roots of halophytic Eutrema salsugineum. Front. Genet. 2021, 12, 770742. [Google Scholar] [CrossRef]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta. 2018, 248, 1063–1078. [Google Scholar] [CrossRef]
- Lavhale, S.G.; Joshi, R.S.; Kumar, Y.; Giri, A.P. Functional insights into two Ocimum kilimandscharicum 4-coumarate-CoA ligases involved in phenylpropanoid biosynthesis. Int. J. Biol. Macromol. 2021, 181, 202–210. [Google Scholar] [CrossRef]
- Gao, S.; Liu, X.; Ni, R.; Fu, J.; Tan, H.; Cheng, A.; Lou, H. Molecular cloning and functional analysis of 4-coumarate: CoA ligases from Marchantia paleacea and their roles in lignin and flavanone biosynthesis. PLoS ONE 2024, 19, e0296079. [Google Scholar] [CrossRef]
- Ma, J.; Zuo, D.; Zhang, X.; Li, H.; Ye, H.; Zhang, N.; Li, M.; Dang, M.; Geng, F.; Zhou, H.; et al. Genome-wide identification analysis of the 4-Coumarate: CoA ligase (4CL) gene family expression profiles in Juglans regia and its wild relatives J. Mandshurica resistance and salt stress. BMC Plant Biol. 2024, 24, 211. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, T.; Luo, W.; Xu, J.; Liu, J.; Wan, D. Identification of 4CL genes in desert poplars and their changes in expression in response to salt stress. Genes 2015, 6, 901–917. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhao, S.; Xi, X.; Feng, J.; Han, R. Brachypodium BdCHS is a homolog of Arabidopsis AtCHS involved in the synthesis of flavonoids and lateral root development. Protoplasma 2023, 260, 999–1003. [Google Scholar] [CrossRef]
- Huang, H.; Hu, K.; Han, K.; Xiang, Q.; Dai, S. Flower colour modification of chrysanthemum by suppression of F3′H and overexpression of the exogenous Senecio cruentus F3′5′H gene. PLoS ONE 2013, 8, e74395. [Google Scholar] [CrossRef]
- Baba, S.A.; Ashraf, N. Functional characterization of flavonoid 3′-hydroxylase, CsF3′H, from Crocus sativus L: Insights into substrate specificity and role in abiotic stress. Arch. Biochem. Biophys. 2019, 667, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jia, W.; Duan, Q.; Du, W.; Li, X.; Cui, G.; Wang, X.; Wang, J. Heterologous expression of Platycodon grandiflorus PgF3′5′H modifies flower color pigmentation in tobacco. Genes 2023, 14, 1920. [Google Scholar] [CrossRef] [PubMed]
- Isıyel, M.; İlhan, E.; Kasapoğlu, A.G.; Muslu, S.; Öner, B.M.; Aygören, A.S.; Yiğider, E.; Aydın, M.; Yıldırım, E. Identification and characterization of Phaseolus vulgaris CHS genes in response to salt and drought stress. Genet. Resour. Crop Evol. 2024, 9, 189. [Google Scholar] [CrossRef]
- Wang, J.; Lan, Z.; Wang, H.; Xu, C.; Zhou, Z.; Cao, J.; Liu, Y.; Sun, Z.; Mu, D.; Han, J.; et al. Genome-wide identification of chalcone synthase (CHS) family members and their expression patterns at the sprouting stage of common bean (Phaseolus vulgaris) under abiotic stress. Sci. Hortic. 2024, 334, 113309. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Li, C.; Wang, Z.; Lu, C.; Cheng, J.; Wei, S.; Yang, J.; Yang, Q. Integrated transcriptomic and metabolomic analyses elucidate the mechanism of flavonoid biosynthesis in the regulation of mulberry seed germination under salt stress. BMC Plant Biol. 2024, 24, 132. [Google Scholar] [CrossRef]
- Sui, D.; Wang, B.; El-Kassaby, Y.A.; Wang, L. Integration of physiological, transcriptomic, and metabolomic analyses reveal molecular mechanisms of salt stress in Maclura tricuspidata. Plants 2024, 13, 397. [Google Scholar] [CrossRef]
- Saad, K.R.; Kumar, G.; Mudliar, S.N.; Giridhar, P.; Shetty, N.P. Salt stress-induced anthocyanin biosynthesis genes and MATE transporter involved in anthocyanin accumulation in Daucus carota cell culture. ACS Omega 2021, 6, 24502–24514. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Zhang, F.; Zhang, G.; Jiang, X.; Yu, H.; Hou, B. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef]
- Singh, K.B.; Foley, R.C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Bokolia, M.; Kumar, A.; Singh, B. Plant tolerance to salinity stress: Regulating transcription factors and their functional role in the cellular transcriptional network. Gene Rep. 2024, 34, 101873. [Google Scholar] [CrossRef]
- Golldack, D.; Lüking, I.; Yang, O. Plant tolerance to drought and salinity: Stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2021, 30, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, D.; Li, W.; Cao, X.; Ma, F.; Wang, Q.; Zhan, X.; Hu, T. Overexpression of a tomato AP2/ERF transcription factor SlERF.B1 increases sensitivity to salt and drought stresses. Sci. Hortic. 2022, 304, 111332. [Google Scholar] [CrossRef]
- Pan, Y.; Seymour, G.B.; Lu, C.; Hu, Z.; Chen, X.; Chen, G. An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep. 2012, 31, 349–360. [Google Scholar] [CrossRef]
- Schmidt, R.; Mieulet, D.; Hubberten, H.M.; Obata, T.; Hoefgen, R.; Fernie, A.R.; Fisahn, J.; San Segundo, B.; Guiderdoni, E.; Schippers, J.H.; et al. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 2013, 25, 2115–2131. [Google Scholar] [CrossRef]
- Guo, T.; Wang, S.; Fan, B.; Zou, S.; Chen, S.; Liu, W.; Wang, S.; Ai, L.; Han, L. Overexpression of the Zoysia japonica ZjABR1/ERF10 regulates plant growth and salt tolerance in transgenic Oryza sativa. Environ. Exp. Bot. 2023, 206, 105117. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, J.; Zhang, L.; Jin, H.; Fang, S. Genome-wide identification of bHLH transcription factors and their response to salt stress in Cyclocarya paliurus. Front. Plant Sci. 2023, 14, 1117246. [Google Scholar] [CrossRef]
- Ye, D.; Liu, J.; Tian, X.; Wen, X.; Zhang, Y.; Zhang, X.; Sun, G.; Xia, H.; Liang, D. Genome-wide identification of bHLH gene family and screening of candidate gene in response to salt stress in kiwifruit. Environ. Exp. Bot. 2024, 222, 105774. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple functions of MYB transcription factors in abiotic stress responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, X.; Li, S.; Fu, Y.; Xu, J.; Wang, G. The AabHLH35 transcription factor identified from Anthurium andraeanum is involved in cold and drought tolerance. Plants 2019, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Y.; Zhou, Z.; Xiang, X.; Liu, X.; Wang, J.; Hu, Z.; Xiang, S.; Li, W.; Xiao, Q.; et al. Tobacco transcription factor bHLH123 improves salt tolerance by activating NADPH oxidase NtRbohE expression. Plant Physiol. 2021, 186, 1706–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Q.; Yan, C.; Sun, Q.; Wang, J.; Li, C.; Yuan, C.; Mou, Y.; Shan, S. The bHLH transcription factor AhbHLH121 improves salt tolerance in peanut. Int. J. Biol. Macromol. 2024, 256, 128492. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Z.; Su, L.; Gong, L.; Xin, H. Vitis Myb14 confer cold and drought tolerance by activating lipid transfer protein genes expression and reactive oxygen species scavenge. Gene 2024, 890, 147792. [Google Scholar] [CrossRef] [PubMed]
- Denekamp, M.; Smeekens, S.C. Integration of wounding and osmotic stress signals determines the expression of the AtMYB102 transcription factor gene. Plant Physiol. 2003, 132, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Hagymasi, A.T.; Dempsey, J.P.; Srivastava, P.K. Heat-shock proteins. Curr. Protoc. 2022, 2, e592. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; He, J.; Liu, H.; Zhang, H. A class I cytosolic HSP20 of rice enhances heat and salt tolerance in different organisms. Sci. Rep. 2020, 10, 1383. [Google Scholar] [CrossRef]
- Vogel, J.T.; Zarka, D.G.; Van Buskirk, H.A.; Fowler, S.G.; Thomashow, M.F. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2005, 41, 195–211. [Google Scholar] [CrossRef]
- Rizhsky, L.; Davletova, S.; Liang, H.; Mittler, R. The zinc-finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J. Biol. Chem. 2004, 279, 11736–11743. [Google Scholar] [CrossRef]
- Davletova, S.; Schlauch, K.; Coutu, J.; Mittler, R. The zinc-finger Protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Yang, D.; Yamanaka, N.; Watanabe, S.; Harada, K.; Takahashi, R. A single-base deletion in soybean flavonoid 3′-hydroxylase gene is associated with gray pubescence color. Plant Mol. Biol. 2002, 50, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, Q.; Liu, Y.; Liu, H.; Wang, F.; Jia, C. Molecular cloning and functional analysis of a flavanone 3-hydroxylase gene from blueberry. J. Hortic. Sci. Biotechnol. 2017, 92, 57–64. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, N.; Feng, C.; Sun, S.; Tang, M.; Tang, X.; Ju, Z.; Yi, Y. Functional analysis of a dihydroflavonol 4-reductase gene in Ophiorrhiza japonica (OjDFR1) reveals its role in the regulation of anthocyanin. PeerJ 2021, 9, e12323. [Google Scholar] [CrossRef] [PubMed]
- Vainio, J.; Mattila, S.; Abdou, S.M.; Sipari, N.; Teeri, T.H. Petunia dihydroflavonol 4-reductase is only a few amino acids away from producing orange pelargonidin-based anthocyanins. Front. Plant Sci. 2023, 14, 1227219. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Putterill, J.; Dare, A.P.; Plunkett, B.J.; Cooney, J.; Peng, Y.; Souleyre, E.J.F.; Albert, N.W.; Espley, R.V.; Günther, C.S. Two genes, ANS and UFGT2, from Vaccinium spp. are key steps for modulating anthocyanin production. Front. Plant Sci. 2023, 14, 1082246. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Jia, C.; Liu, Y.; Wang, F.; Wang, J. Cloning, characterization and functional analysis of a flavonol synthase from Vaccinium corymbosum. Trees-Struct. Funct. 2016, 30, 1595–1605. [Google Scholar] [CrossRef]
- Vu, T.T.; Jeong, C.Y.; Nguyen, H.N.; Lee, D.; Lee, S.A.; Kim, J.H.; Hong, S.W.; Lee, H. Characterization of Brassica napus Flavonol Synthase Involved in Flavonol Biosynthesis in Brassica napus L. J. Agric. Food Chem. 2015, 63, 7819–7829. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Jiang, X.; Dai, X.; Xu, L.; Li, T.; Xing, D.; Li, Y.; Li, M.; Gao, L.; et al. Evolutionary and functional characterization of leucoanthocyanidin reductases from Camellia sinensis. Planta 2018, 247, 139–154. [Google Scholar] [CrossRef]





| Gene ID | Fold Change | ||||
|---|---|---|---|---|---|
| Gene Name | S6_vs_S0 | S12_vs_S0 | S24_vs_S0 | S48_vs_S0 | |
| VaccDscaff3-augustus-gene-84.42 | ERF114 | 4.924 | 9.832 | 9.964 | 11.758 |
| VaccDscaff13-augustus-gene-7.21 | ABR1 | 5.842 | 7.966 | 9.182 | 7.754 |
| VaccDscaff39-processed-gene-249.7 | ERF4 | 6.942 | 11.268 | 9.92 | 10.268 |
| VaccDscaff38-augustus-gene-106.21 | ERF110 | 6.986 | 9.476 | 10.994 | 10.43 |
| VaccDscaff21-processed-gene-16.2 | ERF011 | 5.714 | 9.802 | 5.024 | 6.278 |
| VaccDscaff30-processed-gene-33.28 | HSP20 | 6.736 | 9.258 | 6.182 | 8.018 |
| VaccDscaff99-augustus-gene-4.57 | bHLH35 | 4.930 | 7.946 | 4.868 | 4.190 |
| VaccDscaff10-augustus-gene-14.13 | bHLH123 | 9.756 | 8.986 | 7.578 | 10.622 |
| VaccDscaff9-augustus-gene-346.25 | bHLH162 | 5.108 | 9.73 | 6.768 | 7.444 |
| VaccDscaff9-augustus-gene-136.29 | MYB102 | 6.452 | 7.546 | 6.276 | 7.056 |
| VaccDscaff3-augustus-gene-219.29 | MYB13 | 6.262 | 10.038 | 8.412 | 6.076 |
| VaccDscaff43-augustus-gene-40.46 | MYB14 | 5.066 | 5.700 | 7.13 | 6.102 |
| VaccDscaff21-processed-gene-155.6 | ZAT12 | 7.062 | 9.660 | 6.632 | 8.080 |
| Flavonol | Flavonol Glycosides | Flavan | Proanthocyanidin | Anthocyanindin | Anthocyanin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Que | Pin | Myr-Gal | Kae-Ara | Kae-Xyl | Que-Neo-Rha | Nic | Epi | Pro C2 | Cya | Cya-Ara | Cya-Sam | Cya-Glu | Cya-a-Ara | Del-Glu-Chl | Cya-Rha | |
| PAL-3 | -- | -- | -- | -- | -- | −0.895 * | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| PAL-4 | -- | -- | -- | -- | -- | −0.902 * | -- | -- | -- | -- | −0.899 * | -- | -- | -- | -- | -- |
| PAL-5 | -- | -- | -- | -- | -- | −0.886 * | -- | -- | -- | -- | −0.894 * | -- | -- | -- | -- | -- |
| C4H | -- | -- | -- | -- | -- | −0.878 * | -- | -- | -- | −0.912 * | −0.967 ** | -- | 0.944 * | -- | -- | -- |
| 4CL1 | -- | -- | -- | -- | -- | −0.989 ** | -- | 0.909 * | 0.907 * | -- | −0.928 * | -- | -- | -- | -- | -- |
| 4CL5 | 0.932 * | 0.930 * | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 4CL7 | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.949 * | −0.903 * | -- | −0.942 * | -- | -- | -- |
| 4CL9 | -- | -- | -- | -- | -- | 0.954 * | -- | -- | −0.893 * | -- | -- | -- | -- | -- | -- | -- |
| CHS-2 | -- | -- | -- | -- | -- | −0.911 * | -- | -- | -- | −0.885 * | −0.898 * | -- | -- | -- | -- | -- |
| F3′5′H-1 | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.898 * | -- | -- | 0.879 * | -- | -- | -- |
| F3′5′H-2 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.890 * | -- |
| F3′5′H-3 | -- | -- | -- | -- | −0.947 * | 0.982 ** | −0.947 * | −0.894 * | -- | -- | -- | -- | -- | -- | -- | −0.892 * |
| F3′5′H-4 | -- | -- | −0.919 * | -- | −0.928 * | 0.965 ** | −0.941 * | -- | -- | -- | 0.893 * | -- | -- | -- | -- | -- |
| F3′5′H-5 | -- | -- | -- | -- | −0.892 * | 0.942 * | −0.921 * | -- | -- | -- | 0.900 * | 0.936 * | -- | -- | -- | -- |
| F3′5′H-7 | -- | -- | -- | -- | -- | -- | -- | 0.884 * | -- | -- | -- | -- | -- | -- | -- | -- |
| FLS-1 | -- | -- | −0.942 * | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| FLS-2 | -- | -- | -- | −0.892 * | −0.944 * | 0.907 * | −0.921 * | −0.946 * | −0.881 * | -- | -- | -- | -- | -- | -- | −0.909 * |
| FLS-3 | −0.923 * | −0.929 * | -- | -- | −0.895 * | 0.906 * | −0.938 * | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| ANR-1 | -- | -- | −0.904 * | -- | -- | -- | -- | -- | -- | -- | 0.900 * | -- | -- | -- | -- | -- |
| ANR-2 | -- | −0.903 * | -- | -- | −0.960 ** | 0.909 * | −0.985 ** | −0.885 * | -- | -- | -- | -- | -- | -- | -- | −0.899 * |
| ANR-3 | -- | -- | -- | −0.934 * | −0.979 ** | -- | −0.983 ** | −0.943 * | -- | -- | -- | −0.915 * | -- | -- | -- | −0.949 * |
| LAR-1 | -- | -- | −0.973 ** | -- | -- | -- | −0.883 * | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| LAR-3 | -- | -- | -- | 0.971 ** | 0.984 * | −0.946 * | 0.946 * | 0.990 ** | 0.969 ** | -- | -- | 0.882 * | -- | -- | -- | 0.978 * |
| DFR | -- | 0.954 * | -- | -- | -- | -- | 0.895 * | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| UFGT-1 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.879 * | -- | -- |
| UFGT-3 | -- | -- | -- | −0.977 * | −0.996 ** | 0.936 * | 0.979 ** | −0.968 ** | −0.945 * | -- | -- | −0.880 * | -- | -- | -- | −0.985 ** |
| UGT75C1 | -- | -- | 0.990 ** | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Song, Y.; Feng, X.; Guo, P.; Zhou, L.; Jia, S.; Guo, Q.; Zhang, C. Integrated Metabolome and Transcriptome Analyses Reveal the Mechanisms Regulating Flavonoid Biosynthesis in Blueberry Leaves under Salt Stress. Horticulturae 2024, 10, 1084. https://doi.org/10.3390/horticulturae10101084
Ma B, Song Y, Feng X, Guo P, Zhou L, Jia S, Guo Q, Zhang C. Integrated Metabolome and Transcriptome Analyses Reveal the Mechanisms Regulating Flavonoid Biosynthesis in Blueberry Leaves under Salt Stress. Horticulturae. 2024; 10(10):1084. https://doi.org/10.3390/horticulturae10101084
Chicago/Turabian StyleMa, Bin, Yan Song, Xinghua Feng, Pu Guo, Lianxia Zhou, Sijin Jia, Qingxun Guo, and Chunyu Zhang. 2024. "Integrated Metabolome and Transcriptome Analyses Reveal the Mechanisms Regulating Flavonoid Biosynthesis in Blueberry Leaves under Salt Stress" Horticulturae 10, no. 10: 1084. https://doi.org/10.3390/horticulturae10101084
APA StyleMa, B., Song, Y., Feng, X., Guo, P., Zhou, L., Jia, S., Guo, Q., & Zhang, C. (2024). Integrated Metabolome and Transcriptome Analyses Reveal the Mechanisms Regulating Flavonoid Biosynthesis in Blueberry Leaves under Salt Stress. Horticulturae, 10(10), 1084. https://doi.org/10.3390/horticulturae10101084






