The Impact of Exogenous Sodium Selenite Treatment on the Nutritional Value and Active Constituents of Pueraria lobata
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment Methods
2.2. Biomass Measurement
2.3. Determination of the Photosynthetic Pigment Content
2.4. Determination of Antioxidant Index
2.5. Determination of Nutrient Content
2.6. Determination of the Total Se and Se-Species Content
2.7. Determination of Flavonoid Content
2.8. Transcriptome Sequencing and Data Analysis
2.9. Statistical Analysis
3. Results
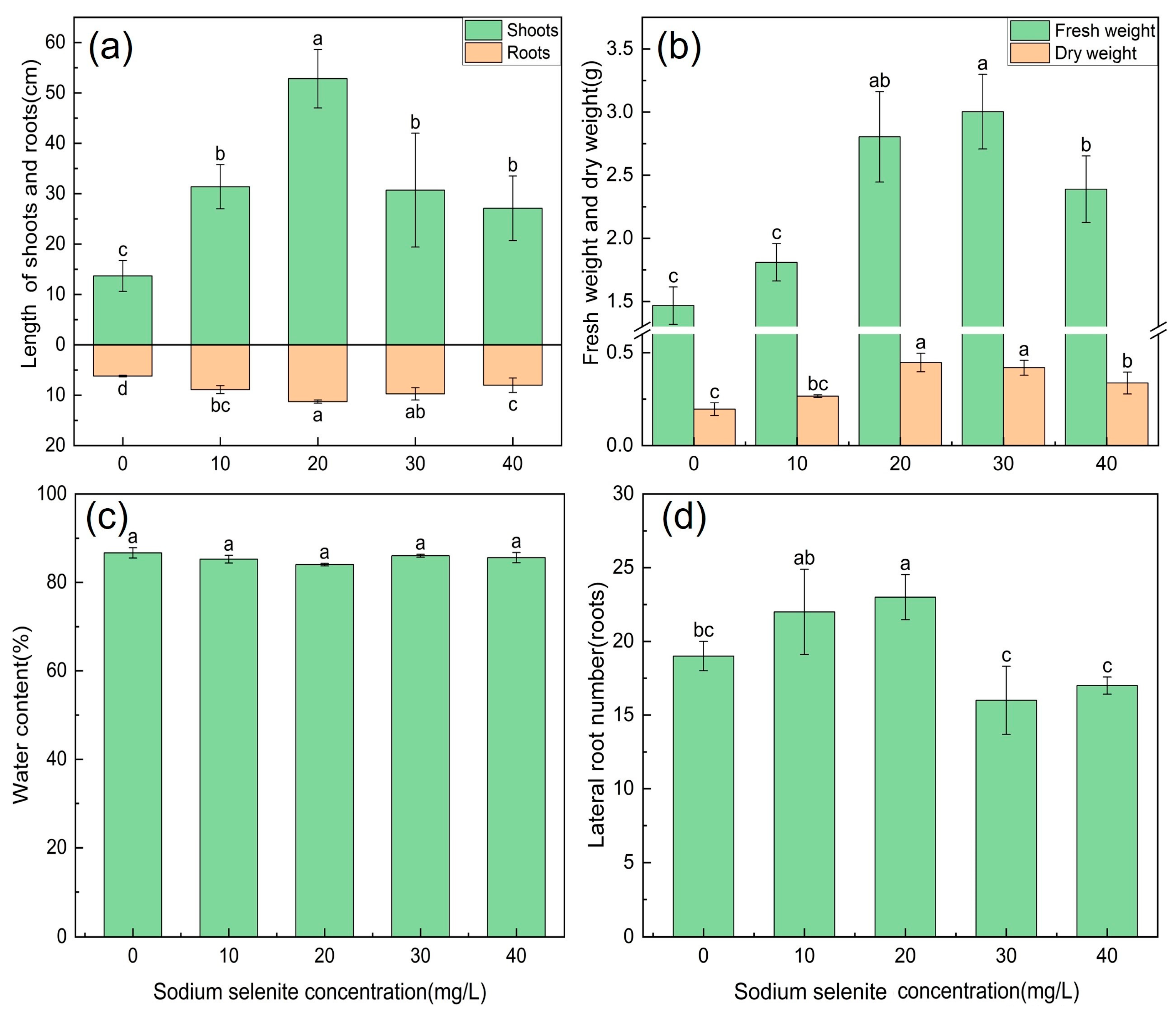
3.1. Effect of Na2SeO3 Treatment on the Biomass of Kudzu
3.2. Impact of Na2SeO3 Treatment on the Photosynthetic Pigment Content in Kudzu
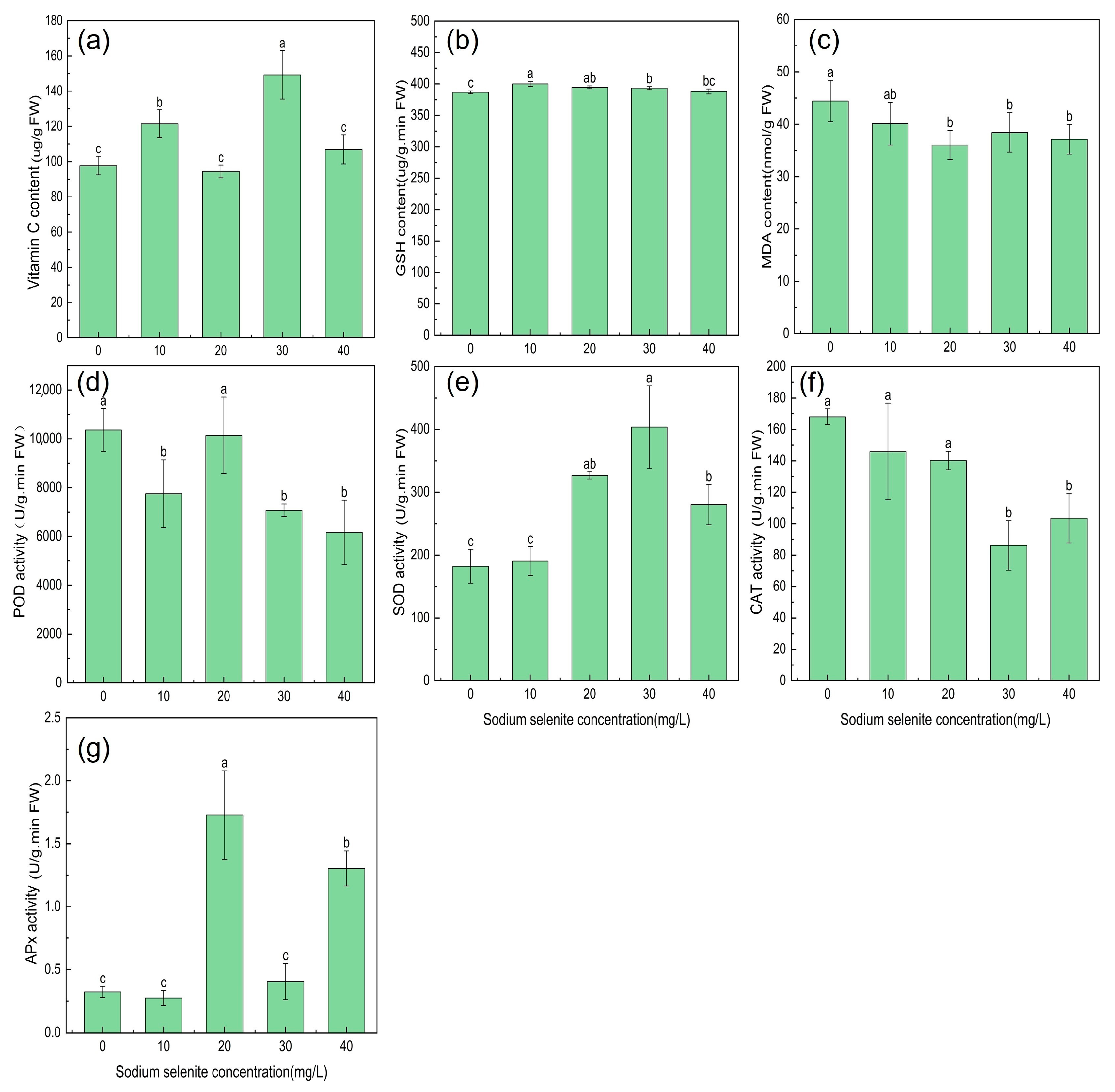
3.3. Effect of Na2SeO3 Treatment on Antioxidant Indexes of Kudzu
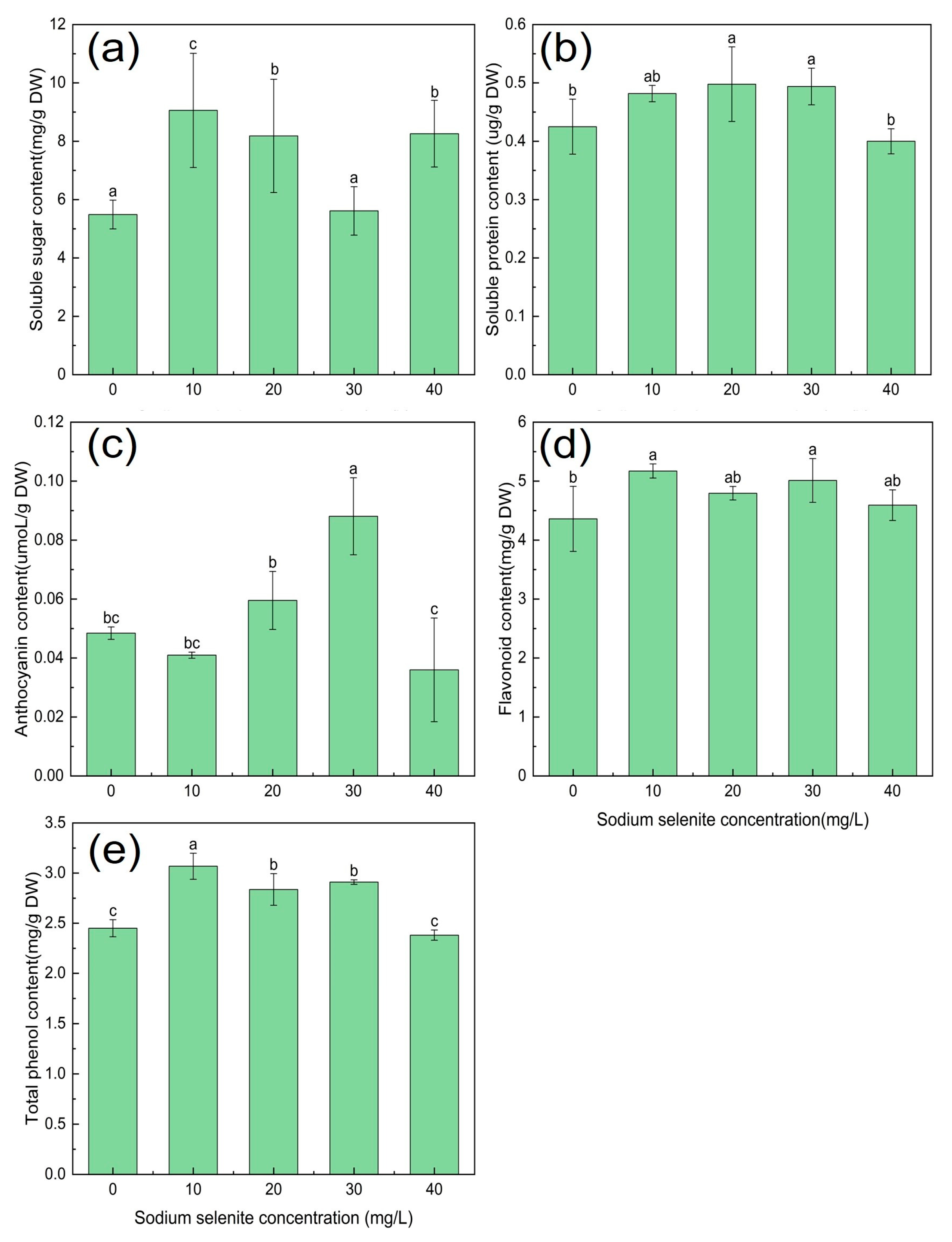
3.4. Effect of Na2SeO3 Treatment on the Nutritional Quality of Kudzu
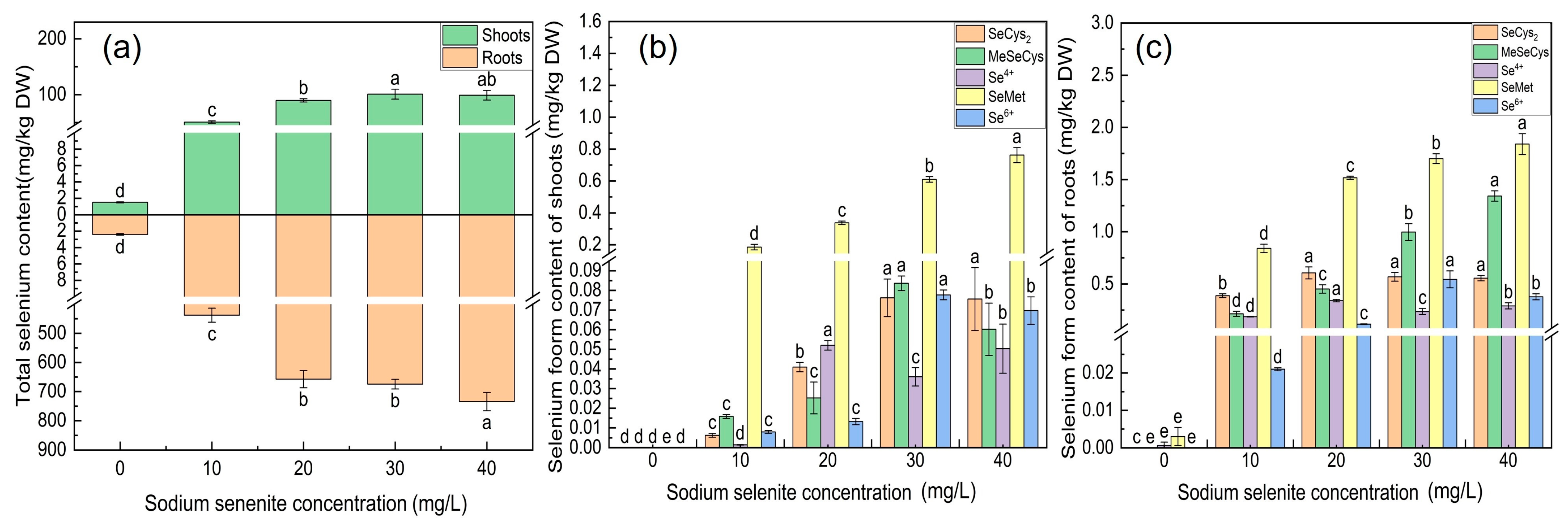
3.5. The Effect of Na2SeO3 Treatment on the Content of Total Se and Se-Species
3.6. The Effect of Na2SeO3 Treatment on the Content of Flavonoids
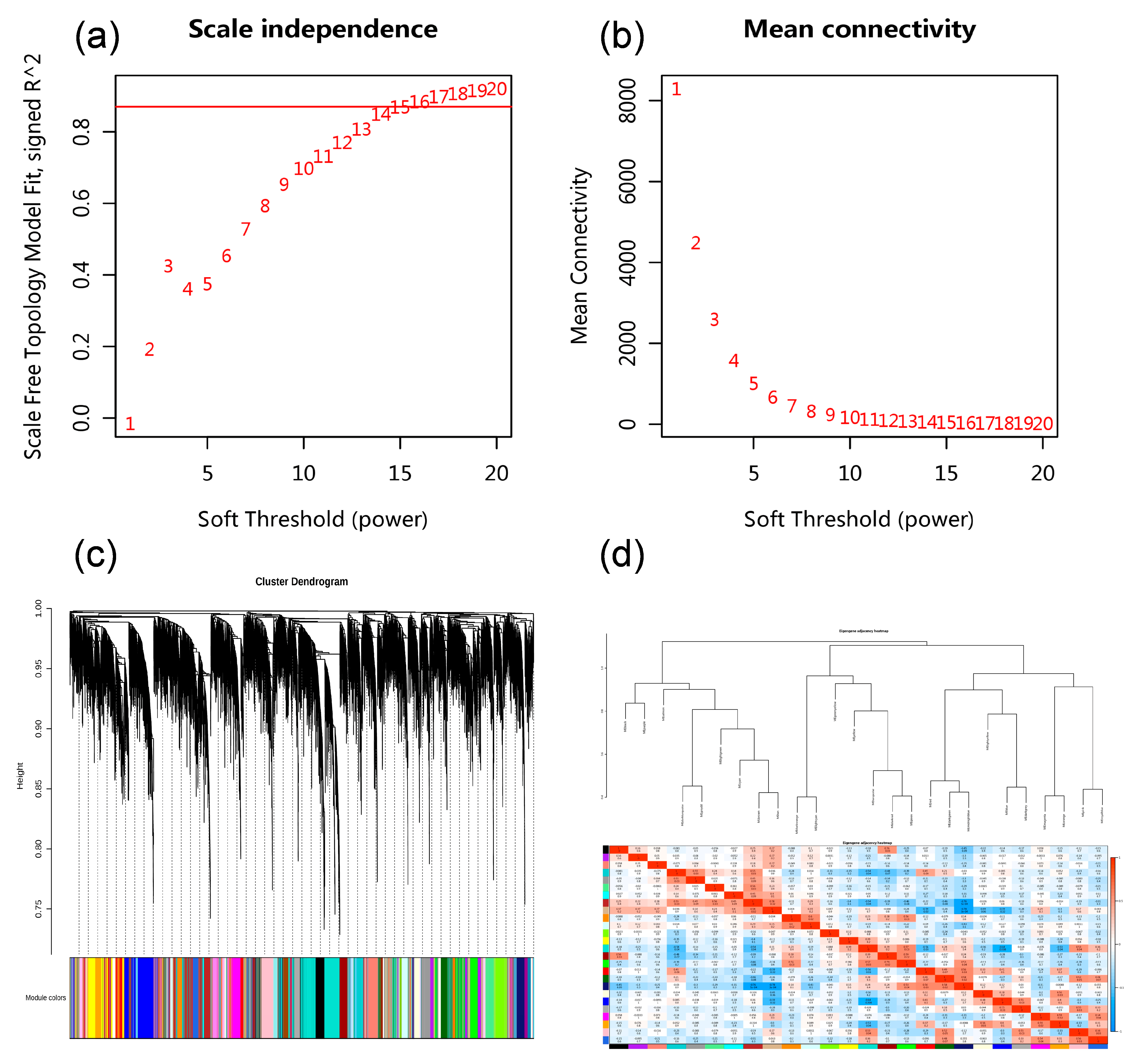
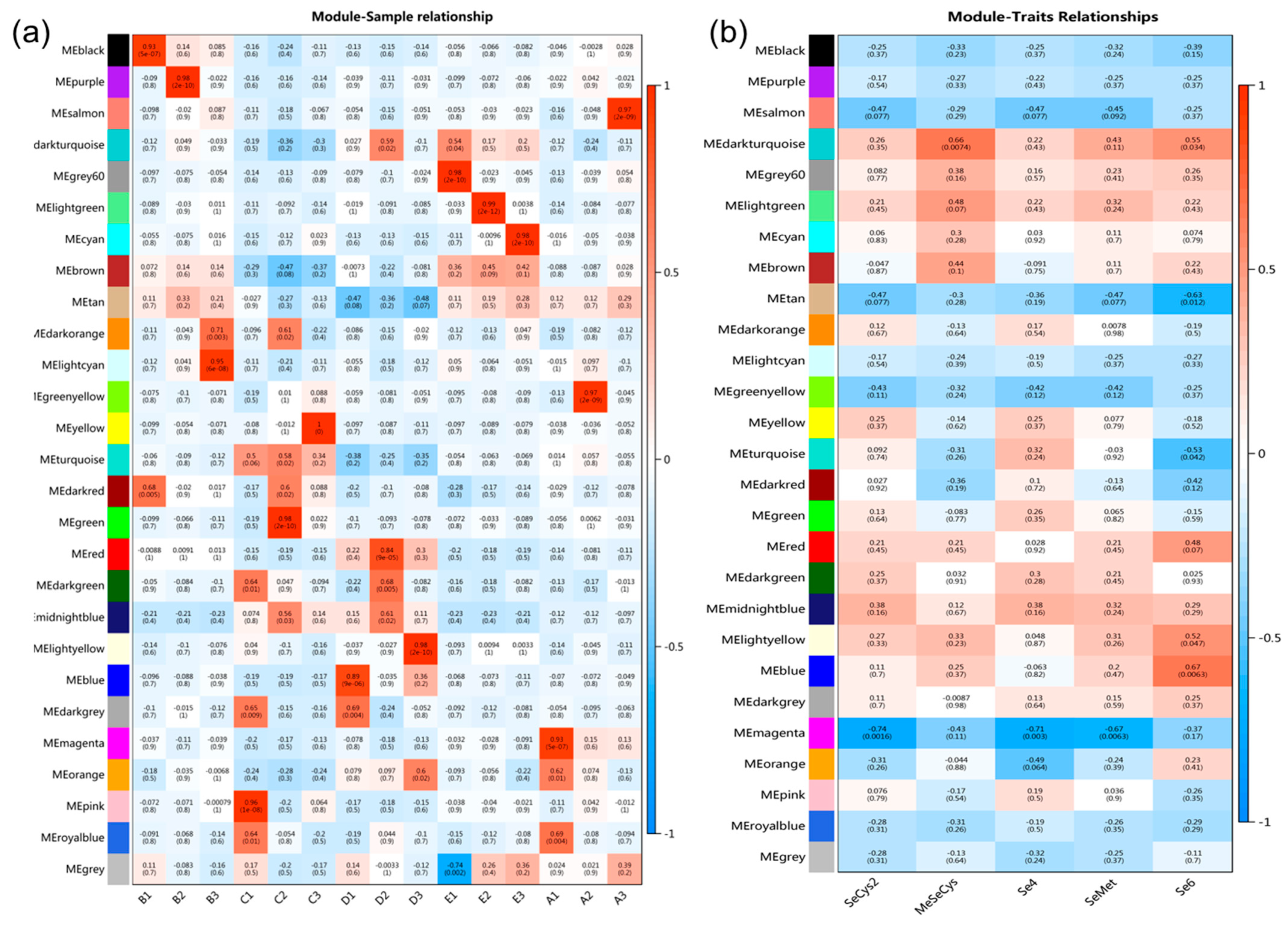
3.7. The Effect of Na2SeO3 Treatment on the Transcriptome Data of Kudzu
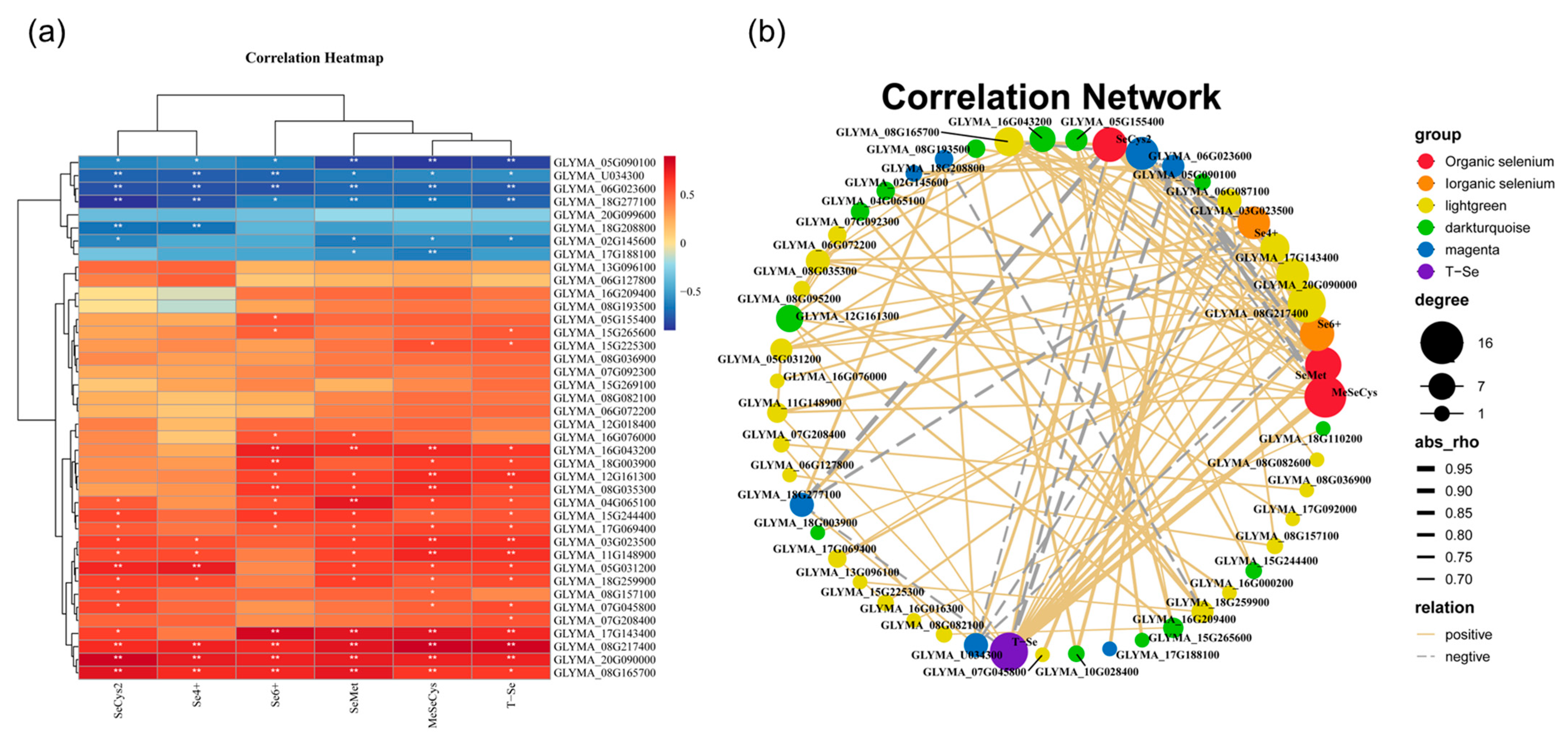
3.8. Screening of Hot Genes Related to Se-Species Metabolism
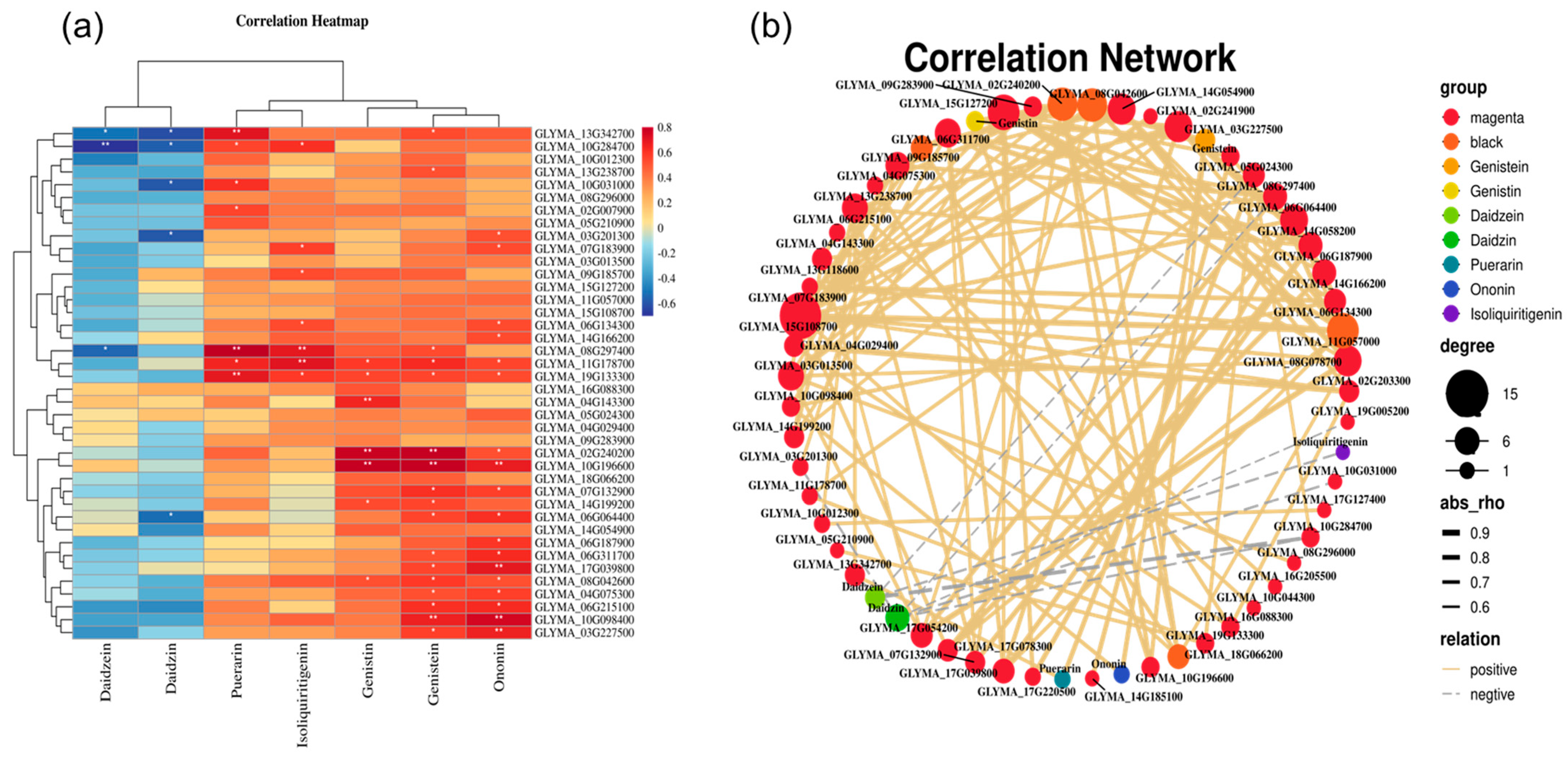
3.9. Correlation Analysis between Flavonoid Content and DEGs
4. Discussion
4.1. The Effect of Se on the Growth of Kudzu
4.2. The Effect of Se on the Nutritional Quality of Kudzu
4.3. Absorption and Transformation of Se in Kudzu
4.4. The Effect of Se on the Content of Flavonoids in Kudzu
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; van Zanten, A.R.H. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. (Edinb. Scotl.) 2019, 38, 982–995. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Beecher, G.R.; Burk, R.F.; Chan, A.C.; Erdman, J.J.; Jacob, R.A.; Jialal, I.; Kolonel, L.N.; Marshall, J.R.; Taylor Mayne, P.R. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Inst. Med. 2000, 19, 95–185. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.E.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition Society. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. The fascinating facets of plant selenium accumulation–biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium biofortification: Strategies, progress and challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium biofortification and phytoremediation phytotechnologies: A Review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 2017, 217, 194–205. [Google Scholar] [CrossRef]
- Bai, B.; Zhang, S.; Suo, X.; Chen, W.; Shen, Y. Selenite uptake by Medicago sativa L. roots. Grassl. Sci. 2022, 68, 328–335. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium biofortification: Roles, mechanisms, responses and prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.; Zhang, J.; An, Q.; Zhou, C.; Li, D.; Pan, C. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, E.; Cao, Q.; Yang, F.; Li, G. Physiological function and absorption enrichment of selenium in staple crops. J. Northeast Agric. Sci. 2020, 45, 115–118. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Lin, L.; Liao, M.; Lv, X. Effects of different selenium concentrations on photosynthetic physiology of grape seedlings. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 042062. [Google Scholar] [CrossRef]
- Zhong, Y.; Cheng, J.J. Effects of selenite on unicellular green microalga Chlorella pyrenoidosa: Bioaccumulation of selenium, enhancement of photosynthetic pigments, and amino acid production. J. Agric. Food Chem. 2017, 65, 10875–10883. [Google Scholar] [CrossRef]
- Li, L.; Wu, S.; Wang, S.; Shi, X.; Cheng, S.; Cheng, H. Molecular mechanism of exogenous selenium affecting the nutritional quality, species and content of organic selenium in mustard. Agronomy 2023, 13, 1425. [Google Scholar] [CrossRef]
- Cunha, M.L.O.; de Oliveira, L.C.A.; Silva, V.M.; Montanha, G.S.; Dos Reis, A.R. Selenium increases photosynthetic capacity, daidzein biosynthesis, nodulation and yield of peanuts plants (Arachis hypogaea L.). Plant Physiol. Biochem. 2022, 190, 231–239. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Y.; Zhu, N.; Jin, H. Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Saf. 2022, 240, 113681. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wu, Q.; Lu, P.; Kang, Q.; Zhao, M.; Wang, A.; Dong, Q.; Sun, M.; Yang, Z.; et al. Selenium application enhances the accumulation of flavones and anthocyanins inbread wheat (Triticum aestivum L.) grains. J. Agric. Food Chem. 2022, 70, 13431–13444. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Gou, J.; Zhang, Y. Molecular cloning and functional characterization of a novel isoflavone 3′-O-methyltransferase from Pueraria lobata. Front. Plant Sci. 2016, 7, 793. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, X.; Wu, S.; Wang, S.; Rao, S.; Li, L.; Cheng, S.; Li, L. Chromosome-Level genome assembly and multi-omics dataset provide insights into isoflavone and puerarin biosynthesis in Pueraria lobata (Wild.) ohwi. Biomolecules 2022, 12, 1731. [Google Scholar] [CrossRef]
- Liu, D.; Ma, L.; Zhou, Z.; Liang, Q.; Xie, Q.; Ou, K.; Liu, Y.; Su, Y. Starch and mineral element accumulation during root tuber expansion period of Pueraria thomsonii Benth. Food Chem. 2021, 343, 128445. [Google Scholar] [CrossRef]
- Li, Y.; He, M.; Li, J.; Yao, Y.; Zhu, L.; Wu, B. Regulatory protein genes and microRNAs in response to selenium stimuli in Pueraria lobata (Willd.) Ohwi. Metallomics 2021, 13, mfaa004. [Google Scholar] [CrossRef]
- Shi, X.B.; Liu, F.Z.; Wang, X.D.; Zeng, G.J.; Shi, H.G.; Wei, C.C.; Wang, H.B. Production of selenium-rich soybean and mung bean sprouts. Food Sci. Technol. 2017, 42, 72–77. [Google Scholar] [CrossRef]
- Deng, X.; Li, R.; Deng, S. Determination of the total content of arsenic, antimony, selenium and mercury in Chinese herbal food by chemical vapor generation-four-channel non-dispersive atomic fluorescence spectrometry. J. Fluoresc. 2020, 30, 949–954. [Google Scholar] [CrossRef]
- Herath, I.; Kumarathilaka, P.; Bundschuh, J.; Marchuk, A.; Rinklebe, J. A fast analytical protocol for simultaneous speciation of arsenic by Ultra-High Performance Liquid Chromatography (UHPLC) hyphenated to Inductively Coupled Plasma Mass Spectrometry (ICP-MS) as a modern advancement in liquid chromatography approaches. Talanta 2020, 208, 120457. [Google Scholar] [CrossRef]
- Shumate, A.; Wong, B.; Pertea, G.; Pertea, M. Improved transcriptome assembly using a hybrid of long and short reads with StringTie. PLoS Comput. Biol. 2022, 18, e1009730. [Google Scholar] [CrossRef]
- Cheng, H.; Chang, S.; Shi, X.; Chen, Y.; Cong, X.; Cheng, S.; Li, L. Molecular mechanisms of the effects of sodium selenite on the growth, nutritional quality, and species of organic selenium in dandelions. Horticulturae 2024, 10, 209. [Google Scholar] [CrossRef]
- Cheng, H.; Li, L.; Dong, J.; Wang, S.; Wu, S.; Rao, S.; Li, L.; Cheng, S.; Li, L. Transcriptome and physiological determination reveal the effects of selenite on the growth and selenium metabolism in mung bean sprouts. Food Res. Int. 2023, 169, 112880. [Google Scholar] [CrossRef]
- He, R.; Gao, M.; Shi, R.; Song, S.; Zhang, Y.; Su, W.; Liu, H. The combination of selenium and LED light quality affects growth and nutritional properties of broccoli sprouts. Molecules 2020, 25, 4788. [Google Scholar] [CrossRef]
- Abdoun, K.A.; Altahir, O.A.; Alsagan, A.A.; Alsaiady, M.Y.; Alshaikhi, A.M.; Alshamiry, F.A.; Al-Haidary, A.A. Effect of irrigation frequency and selenium fertilization on the vegetative growth and biomass yield of Moringa oleifera and Moringa peregrina. Sci. Rep. 2022, 12, 22379. [Google Scholar] [CrossRef]
- Araujo, M.A.; Melo, A.A.R.; Silva, V.M.; Reis, A.R.D. Selenium enhances ROS scavenging systems and sugar metabolism increasing growth of sugarcane plants. Plant Physiol. Biochem. 2023, 201, 107798. [Google Scholar] [CrossRef]
- Mateus, M.P.B.; Tavanti, R.F.R.; Tavanti, T.R.; Santos, E.F.; Jalal, A.; Reis, A.R.D. Selenium biofortification enhances ROS scavenge system increasing yield of coffee plants. Ecotoxicol. Environ. Saf. 2021, 209, 111772. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S. Influence of selenite and selenate on growth, leaf physiology and antioxidant defense system in wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 5700–5710. [Google Scholar] [CrossRef]
- Ren, G.; Ran, X.; Zeng, R.; Chen, J.; Wang, Y.; Mao, C.; Wang, X.; Feng, Y.; Yang, G. Effects of sodium selenite spray on apple production, quality, and sucrose metabolism-related enzyme activity. Food Chem. 2021, 339, 127883. [Google Scholar] [CrossRef]
- Dai, Z.; Imtiaz, M.; Rizwan, M.; Yuan, Y.; Huang, H.; Tu, S. Dynamics of selenium uptake, speciation, and antioxidant response in rice at different panicle initiation stages. Sci. Total Environ. 2019, 691, 827–834. [Google Scholar] [CrossRef]
- Ozakman, G.; Yayman, S.G.; Sezer Zhmurov, C.; Serdaroglu Kasikci, E.; Catal, T. The influence of selenium on expression levels of the rbcL gene in Chlorella vulgaris. 3 Biotech 2018, 8, 189. [Google Scholar] [CrossRef]
- Jing, D.; Du, Z.; Ma, H.; Ma, B.; Liu, F.; Song, Y.; Xu, Y.; Li, L. Selenium enrichment, fruit quality and yield of winter jujube as affected by addition of sodium selenite. Sci. Hortic. 2017, 225, 1–5. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.J.; Kang, H.M.; Lee, Y.T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Gui, J.; Rao, S.; Gou, Y.; Xu, F.; Cheng, S. Comparative study of the effects of selenium yeast and sodium selenite on selenium content and nutrient quality in broccoli florets (Brassica oleracea L. var. italica). J. Sci. Food Agric. 2022, 102, 1707–1718. [Google Scholar] [CrossRef]
- Farooq, M.U.; Tang, Z.; Zeng, R.; Liang, Y.; Zhang, Y.; Zheng, T.; Ei, H.H.; Ye, X.; Jia, X.; Zhu, J. Accumulation, mobilization, and transformation of selenium in rice grain provided with foliar sodium selenite. J. Sci. Food Agric. 2019, 99, 2892–2900. [Google Scholar] [CrossRef]
- Gong, R.; Ai, C.; Zhang, B.; Cheng, X. Effect of selenite on organic selenium speciation and selenium bioaccessibility in rice grains of two Se-enriched rice cultivars. Food Chem. 2018, 264, 443–448. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Zhou, C.; Li, J.; Zhang, Y. Molecular characterization of the C-glucosylation for puerarin biosynthesis in Pueraria lobata. Plant J. 2017, 90, 535–546. [Google Scholar] [CrossRef]
- Deng, K.; Li, L.; Li, L.; Xu, F.; Yuan, H.; Zha, S.; Xiao, X.; Yu, J.; Cheng, S.; Cheng, H. Molecular mechanism of selenium affecting the synthesis of flavonoids in G. biloba leaves. Plant Mol. Biol. Report. 2022, 40, 232–246. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, N.; Xiong, Y.; Liu, Y.; Tong, L.; Wang, F.; Fan, B.; Maesen, P.; Blecker, C. Influence of selenium biofortification of soybeans on speciation and transformation during seed germination and sprouts quality. Foods 2022, 11, 1200. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Wang, Y.; Ying, Z.; Bian, Z.; Zhu, W.; Liu, W.; Yang, L.; Jiang, D. How exogenous selenium affects anthocyanin accumulation and biosynthesis-related gene expression in purple lettuce. Pol. J. Environ. Stud. 2017, 26, 717–723. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, W.; Wei, L.; Chen, P.; Yuan, F.; Wang, Z.; Ying, X. Molecular evolution and expression divergence of three key Met biosynthetic genes in plants: CGS, HMT and MMT. PeerJ 2018, 6, e6023. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, G.; Fan, Z.; Xiao, Z.; Lu, Y.; Xiao, Q.; Hou, Z.; Tang, Q.; Zhou, Y. Whole genome identification, molecular docking and expression analysis of enzymes involved in the selenomethionine cycle in Cardamine hupingshanensis. BMC Plant Biol. 2024, 24, 199. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, L.; Chao, W.; Xiang, J.; Yang, X.; Ye, J.; Liao, X.; Zhou, X.; Rao, S.; Cheng, S.; et al. Comparative physiological and transcriptome analysis reveals the potential mechanism of selenium accumulation and tolerance to selenate toxicity of Broussonetia papyrifera. Tree Physiol. 2022, 42, 2578–2595. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Hu, H.; Hu, J.; Xiang, M.; Yang, Q. Comparative proteomics analysis of the responses to selenium in selenium-enriched alfalfa (Medicago sativa L.) leaves. Plant Physiol. Biochem. 2021, 165, 265–273. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiang, X.; Xiao, C.; Zhang, N.; Cheng, H.; Rao, S.; Cheng, S.; Li, L. Illumina RNA and SMRT sequencing reveals the mechanism of uptake and transformation of selenium nanoparticles in soybean seedlings. Plants 2023, 12, 789. [Google Scholar] [CrossRef]
- Wang, X.; Song, B.; Wu, Z.; Zhao, X.; Song, X.; Adil, M.F.; Riaz, M.; Lal, M.K.; Huang, W. Insights into physiological and molecular mechanisms underlying efficient utilization of boron in different boron efficient Beta vulgaris L. varieties. Plant Physiol. Biochem. 2023, 197, 107619. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Indoliya, Y.; Chauhan, A.S.; Mishra, S.; Agrawal, L.; Srivastava, S.; Dwivedi, S.; Singh, P.C.; Mallick, S.; et al. Transcriptome and proteome analyses reveal selenium mediated amelioration of arsenic toxicity in rice (Oryza sativa L.). J. Hazard. Mater. 2020, 390, 122122. [Google Scholar] [CrossRef]
- Lin, X.; Dong, L.; Tang, Y.; Li, H.; Cheng, Q.; Li, H.; Zhang, T.; Ma, L.; Xiang, H.; Chen, L.; et al. Novel and multifaceted regulations of photoperiodic flowering by phytochrome A in soybean. Proc. Natl. Acad. Sci. USA 2022, 119, e2208708119. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, Y.; Zhang, W.; Song, T.; Hou, X.; Kong, L.; Cui, G. Overexpression of an NF-YC2 gene confers alkali tolerance to transgenic alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 960160. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, W.; Li, C.; Wang, J.; Shang, X.; Xiao, L.; Cao, S.; Zhang, Y.; Xu, S.; Yan, H. Genome-wide identification and expression pattern analysis of R2R3-MYB transcription factor gene family involved in puerarin biosynthesis and response to hormone in Pueraria lobata var. thomsonii. BMC Plant Biol. 2023, 23, 107. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Hua, Y.; Wang, K.; Guo, Y.; Lu, Y.; Zhu, S.; Zhang, P.; Hu, G. Transcriptome and metabolome analysis of selenium treated alfalfa reveals influence on phenylpropanoid biosynthesis to enhance growth. Plants 2023, 12, 2038. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, M.; Cui, S.; Guan, J. Weighted gene coexpression correlation network analysis reveals a potential molecular regulatory mechanism of anthocyanin accumulation under different storage temperatures in ‘Friar’ plum. BMC Plant Biol. 2021, 21, 576. [Google Scholar] [CrossRef]
- Wu, X.; Tao, M.; Meng, Y.; Zhu, X.; Qian, L.; Shah, A.; Wang, W.; Cao, S. The role of WRKY47 gene in regulating selenium tolerance in Arabidopsis thaliana. Plant Biotechnol. Rep. 2020, 14, 121–129. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Chen, L.; Lin, J.; Wang, Z.; Pan, J.; Yang, F.; Ni, X.; Wang, Y.; Wang, Y.; et al. Multifaceted roles of WRKY transcription factors in abiotic stress and flavonoid biosynthesis. Front. Plant Sci. 2023, 14, 1303667. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Zeng, Q.; Wang, S.; Luo, Y.; Huang, Y.; Xin, Y.; He, N. Abnormal expression of bHLH3 disrupts a flavonoid homeostasis network, causing differences in pigment composition among mulberry fruits. Hortic. Res. 2020, 7, 83. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Chen, J.; Liu, Z.; Zhang, Y.; Zhang, Z.; Li, R. Two 4-coumarate: Coenzyme A ligase genes involved in acteoside and flavonoids biosynthesis in Rehmannia glutinosa. Ind. Crops Prod. 2022, 185, 115117. [Google Scholar] [CrossRef]
- Ishihama, N.; Choi, S.-w.; Noutoshi, Y.; Saska, I.; Asai, S.; Takizawa, K.; He, S.Y.; Osada, H.; Shirasu, K. Oxicam-type non-steroidal anti-inflammatory drugs inhibit NPR1-mediated salicylic acid pathway. Nat. Commun. 2021, 12, 7303. [Google Scholar] [CrossRef]
- Khalid, M.; Saeedur, R.; Ali, M.; Hassani, D.; Rauf, A.; Jan, F.; Hui, N. Salicylic acid mediated protection of Brassica campestris sp. chinensis from saline stress via SA receptor NPR1 dependent transcriptional regulation and biosynthesis of related biochemicals. Environ. Technol. Innov. 2021, 24, 101950. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Wang, L.; Gong, H.; Wang, L.; Chen, Y.; Cheng, S.; Li, L. The Impact of Exogenous Sodium Selenite Treatment on the Nutritional Value and Active Constituents of Pueraria lobata. Horticulturae 2024, 10, 1081. https://doi.org/10.3390/horticulturae10101081
Cheng H, Wang L, Gong H, Wang L, Chen Y, Cheng S, Li L. The Impact of Exogenous Sodium Selenite Treatment on the Nutritional Value and Active Constituents of Pueraria lobata. Horticulturae. 2024; 10(10):1081. https://doi.org/10.3390/horticulturae10101081
Chicago/Turabian StyleCheng, Hua, Lu Wang, Huiyi Gong, Li Wang, Yuanfei Chen, Shuiyuan Cheng, and Linling Li. 2024. "The Impact of Exogenous Sodium Selenite Treatment on the Nutritional Value and Active Constituents of Pueraria lobata" Horticulturae 10, no. 10: 1081. https://doi.org/10.3390/horticulturae10101081
APA StyleCheng, H., Wang, L., Gong, H., Wang, L., Chen, Y., Cheng, S., & Li, L. (2024). The Impact of Exogenous Sodium Selenite Treatment on the Nutritional Value and Active Constituents of Pueraria lobata. Horticulturae, 10(10), 1081. https://doi.org/10.3390/horticulturae10101081



