Abstract
The application of wounding stress can induce the accumulation of phenolic antioxidants in carrots. This study aimed to investigate the possible regulation role of invertase (INV) on the biosynthesis of phenolics in wounded carrots. In this study, carrots were cut into two different wounding intensities of slices and cubes, then stored at 20 °C for 2 days. The results showed that wounding stress caused an obvious increase in phenolic content and antioxidant capacity in carrot tissues, and a positive correlation was observed between the biosynthesis of phenolic compounds and the degradation of sucrose. Simultaneously, wounding activated the sucrose-cleaving enzymes of INVs, including acid INV (AI) and neutral INV (NI), and up-regulated the expressions of most encoding genes of INVs. In addition, treatment with INV activators accelerated the accumulation of phenolic antioxidants, while treatment with INV inhibitors suppressed this process, suggesting that the synthesis of phenolic compounds in wounded carrots is closely related to the availability of sugars. Our findings provide new insights into the regulation role of INV on the wound-induced accumulation of phenolic compounds in carrots, which may be helpful in using wounded plants to produce more phenolic antioxidants.
1. Introduction
Epidemiological and clinical investigations have shown that diets rich in phenolic antioxidants can not only prevent oxidative damage to cells, proteins and DNA, but also have been shown to inhibit carcinogenesis, diminish inflammation and enhance immune function [1,2,3]. Phenolic substances are mainly found in plants, therefore, obtaining sufficient polyphenols from limited plant resources is a goal that has been pursued. Over the past decade, cutting has been demonstrated as an effective and simple way to induce the accumulation of phenolic compounds in many fresh fruits and vegetables such as carrot, pitaya, broccoli, and potato, thereby largely improving their nutritional effects and health benefits [4,5,6,7]. Among this produce, particular attention has been given to carrots because of their pronounced increase in phenolic substances, especially chlorogenic acid. For instance, Surjadinata and Cisneros-Zevallos [8] showed that the increment of total soluble phenolic (TSP) content in shredded carrots after 4 days of storage at 15 °C was 252%, and the chlorogenic acid, the main phenolic in carrots, increased 11.3 fold, as compared to non-wounded carrots.
To date, carrots have emerged as an ideal model material for studying the effect of wounding associated with other postharvest abiotic stresses on the accumulation of phenolic compounds in horticultural crops. Several treatments including UV-light irradiation, exogenous ethylene (ET), hyperoxia, water stress, glyphosate and higher storage temperature storage can play a synergistic role with wounding in improving the level of phenolic antioxidants in carrots [9,10,11,12,13,14]. To better manipulate this biochemical reaction, the mechanisms of wound-induced accumulation of phenolic compounds are constantly revealed. Previously, it has been confirmed that wounding can trigger the increase in phenylalanine ammonia-lyase (PAL) activity, a pivotal enzyme in the phenylpropanoid biosynthesis pathway, thereby causing the enhancement of phenolic compounds [15]. More recently, the effects of signaling molecules in regulating wound-induced accumulation of phenolic compounds were evaluated. As reported by Cisneros-Zevallos, the results obtained in wounded carrots through suppressing the action of signaling molecules indicated a complex cross-talk between reactive oxygen species (ROS), ET and jasmonic acid (JA) that ROS act as the main signal for wounding or other stresses like UV, whereas ET and JA are activated by ROS and can also modulate ROS levels [16,17]. As the intermediate products in the phenylpropane metabolic pathway, the production of phenolic compounds requires the supply of precursor substances. However, currently, much information about the conversion process of phenolic compounds from primary metabolites is still not very clear.
The phenylpropanoid biosynthesis pathway continues from L-phenylalanine (L-phe), the main aromatic amino acid synthesized via the shikimic acid pathway [18]. In previous studies, higher concentrations of L-phe and shikimic acid were observed in wounded carrots, as compared with the intact individuals, which matched with the accumulation of phenolic compounds [12,13]. In plant tissues, the two starting substrates of the shikimic acid pathway derive from the glycolysis pathway and phosphopentose pathway, including phosphoenol pyruvate and erythrose-4-phosphate [15]. Hence, the precursors needed for the production of phenolic compounds are produced from sugars [19]. Some studies have provided evidence that enhancing the availability of sugars in wounded tissues can help to increase the accumulation of phenolic compounds. It was shown that the application of exogenous amylolytic enzymes improved the levels of reducing sugars, thereby increasing the accumulation of chlorogenic acid isomers in wounded potato tubers [20]. Similar results were observed in methyl jasmonate-treated fresh-cut pitaya fruit [21]. Recently, we have found that sucrose was the predominant sugar in carrots, and the accumulation of phenolic compounds after wounding was accompanied by the decomposition of sucrose [22]. The invertase (INV), including acid INV (AI) and neutral INV (NI), can hydrolyze sucrose into glucose and fructose and display crucial functions in response to abiotic stress [23]. To our knowledge, the potential role of invertase in wound-induced accumulation of phenolic compounds has not been elucidated.
The objectives of this study were to explore the defense response mechanism of INV under mechanical injury and to understand the possible role of INV in influencing the biosynthesis of phenolics in carrots. Thus, we investigated changes in the TSP content, the soluble sugar content, and the transcript levels and activities of the INVs in wounded carrots under different cutting styles. Furthermore, the effects of the activator and inhibitor of the INV on the biosynthesis of phenolics were also evaluated.
2. Materials and Methods
2.1. Plant Material and Treatment
Carrots (Daucus carota L. cv. Sanhongqicun) were purchased in the local market (Jinan, China) and transported to the laboratory within an hour. Carrots with uniform color and shape (the total length was 19–23 cm and the diameter of the middle part was 3.5–4.5 cm) and without mechanical injuries were selected, washed and air dried.
In the first experiment, the skin of the carrots was peeled off prior to cutting. Subsequently, to obtain two different wounding intensities, the carrot sticks were cut into slices (thickness: 5 mm) and cubes (5 mm × 5 mm × 5 mm) with a sharp stainless steel knife. After cutting, the wounded samples with uniform style were randomly placed in transparent polypropylene containers with plastic covers on them. To keep the samples in contact with the outside environment, 10 little holes with a diameter of 0.5 cm were punched and uniformly ranked on the cover. For each container, 150 g wounded samples were included. Finally, the polypropylene containers were stored in a constant temperature and humidity chamber for 48 h. The storage condition was set as 20 °C, 90% relative humidity and no light. The intact samples with no cutting treatment were used as a control and stored in the same conditions as the wounded samples. Sampling was performed at different periods of storage time (0, 12, 24, 36 and 48 h) for all stored samples. During each sampling time, three containers of each treatment were taken out and the samples were frozen with liquid nitrogen and kept in polyethylene bags at −80 °C. The frozen tissues were used for the determination of TSP content, antioxidant capacity, the activities of INVs, soluble sugar content and quantitative real-time PCR (qRT-PCR). Three biological replicates were performed for each treatment, and each replicate contained three whole carrots.
In the second experiment, cubed carrots as described in the first experiment were used to evaluate the effects of activator and inhibitor of the INV on the biosynthesis of phenolics in wounded carrots. Two chemical reagents of exogenous invertase (INV) and concanavalin A (ConA) were used to enhance the activities of INVs, while the iodoacetamide (IAM) and silver nitrate (AgNO3) were used to inhibit the activities of INVs. The cubed carrots immersed in distilled water were used as control. Whole carrots were used to evaluate the impact of soaking treatment. The concentrations of these four reagents were determined according to the previous research [24] and our preliminary experiments.
The different treatments in the carrot cubes were as follows:
- (1)
- Whole: carrots with uniform color and shape and without mechanical injuries were selected, washed and air dried.
- (2)
- CK: the carrot cubes were immersed in the distilled water for 3 min and then air dried.
- (3)
- INV: the carrot cubes were immersed in the 0.5 g L−1 INV solution for 3 min and then air dried.
- (4)
- ConA: the carrot cubes were immersed in the 0.2 g L−1 ConA solution for 3 min and then air dried for 15 min.
- (5)
- IAM: the carrot cubes were immersed in the 1 mmol L−1 IAM solution for 3 min and then air dried.
- (6)
- AgNO3: the carrot cubes were immersed in the 5 mmol L−1 AgNO3 solution for 3 min and then air dried.
After treatment, the storage condition and sampling method of carrot cubes were the same in the first experiment. Sampling time was performed at different storage stages of 0, 1 and 2 d. The frozen tissues were used for the analysis of different physiochemical parameters.
2.2. Total Soluble Phenolics (TSP) Contents
Total soluble phenolics (TSP) content was analyzed according to the method by Singleton et al. [25], with slight modifications. Five grams of frozen sample were homogenized with 25 mL cold methanol, and the homogenate was transferred to a 50 mL covered centrifuge tube. After keeping overnight at 4 °C in the dark, the tube was centrifuged at 12,000× g for 20 min and the supernatant was collected to determine the level of TSP. The reaction mixture contained 0.4 mL of supernatant, 1.6 mL of distilled water, 1 mL of Folin-Ciocalteu reagent and 1 mL of 7.5% (m/v) anhydrous sodium carbonate (Na2CO3). After incubation at 25 °C for 2 h, the absorbance of the mixture was measured at 765 nm and the TSP content was expressed as milligrams of chlorogenic acid (CA) per kilogram.
2.3. Antioxidant Capacity Analysis
The same supernatant prepared for the determination of TSP content was utilized for antioxidant capacity analysis. The 2,2-diphenyl-1-picryl-hydrazyl (DPPH) free radical scavenging activity was used to reflect the antioxidant capacity of carrot tissue. According to the method by Thaipong et al. [26], 2.9 mL of 0.12 m mol L−1 DPPH solution (dissolved in methanol) was added to 0.1 mL TSP extracts and the mixture was incubated at 25 °C for 30 min with no light. Then, the absorbance was measured at 525 nm and the result was expressed as % DPPH inhibition. The value of % DPPH inhibition was calculated with the following formula:
where ‘A0’ was the absorbance of the DPPH solution (control), and ‘A1’ was the absorbance of the test sample.
% DPPH inhibition = [(A0 − A1)/A0] × 100
2.4. Measurement of Soluble Sugars Content
The fructose, glucose and sucrose of carrot were extracted and measured in accordance with the methods of Han et al. [22], with slight modifications. First, 2 g of frozen tissues were ground in 5 mL of 80% (v/v) ethanol solution and centrifuged at 12,000× g for 20 min. The supernatant was used to assay the contents of soluble sugars. The identification and quantification of soluble sugars were achieved by a high-performance liquid chromatography (HPLC) system (Shimadzu LC-20A, Kyoto, Japan) equipped with an evaporative light scattering detector (Shimadzu ELSDLT II, Kyoto, Japan). Twenty microliters of filtered supernatant were injected into the HPLC system and separated on a 4.6 mm × 250 mm, 5 μm, hydrophilic interaction chromatography (HILIC) column (Shodex Asahipak NH2P-50 4E, Tokyo, Japan) eluted with mobile phase A (acetonitrile) and phase B (water) at a constant flow rate of 1 mL/min. The oven temperature was maintained at 40 °C. The concentrations of soluble sugars were calculated using standard calibration curves generated by pure standards.
2.5. AI and NI Activities
A mass of 2.0 g frozen tissues was ground and extracted by 5 mL of 100 mM cold phosphate potassium buffer (pH 6.5) containing 2.5 mmol L−1 dithiothreitol (DTT), 5 mmol L−1 MgCl2, 1 mmol L−1 EDTA and 0.1% Tritonx-100 (v/v). The homogenate was centrifuged at 13,000× g for 20 min at 4 °C and the clear supernatant was used for assays of these two enzymes. The activities of AI and NI were assayed according to the method of Wang et al. [27].
2.6. Gene Expression (Quantitative Real-Time PCR, qRT-PCR)
The total RNA in sampled carrot tissues was isolated using a Plant Total RNA Extraction Kit (TaKaRa: 9769) according to the corresponding protocol. The cDNA was synthesized using a RT-PCR Kit (TaKaRa: RR036A) following the manufacturer’s instructions. Relative gene expression levels of targeted genes were determined by qRT-PCR using SYBR® Premix Ex TaqTM (TaKaRa: RR420A). The qRT-PCR analysis was carried out using the Applied Biosystems 7500 Real-time PCR System (Waltham, MA, USA). The reaction procedure was initiated with a preliminary step of 10 min at 95 °C, followed by 40 cycles of 95 °C for 15 s and 60 °C for 34 s. Ten genes encoding the invertase were analyzed by qRT-PCR. Relative gene expression was calculated using the 2−ΔΔCt method. The targeted genes and their sequences of primers are listed in Supplementary Table S1.
2.7. Statistical Analysis
The two independent experiments were performed with a completely randomized design. Statistics analysis was conducted using SPSS software (V. 23, IBM CO., New York, NY, USA) and one-way analysis of variance (ANOVA). Results were represented as mean ± standard deviation (SD) of three replicates, and the significant differences were set as p < 0.05 using Fisher’s least significant differences (LSD) tests.
3. Results
3.1. Effects of Different Cutting Styles on TSP Content and DPPH Inhibition of Carrots
The changes in TSP content and DPPH inhibition for whole, sliced and cubed carrots were evaluated. As shown in Figure 1A, only a slight increase in TSP content was observed in whole carrots with storage duration. The application of cutting induced a dramatic increase in TSP content in both sliced and cubed carrots. After 24 h of storage, the TSP value in cubed carrots was much higher (p < 0.05) than that of the control, followed by sliced carrots. At the end of storage, the cubed carrots showed the highest level of TSP, which was 5.5 times higher than that of the control. During storage, DPPH inhibition of carrot tissues presented the same pattern as the TSP (Figure 1B). Compared with the control, DPPH inhibition of wounded carrots was much higher after 24 h. The DPPH inhibition of sliced and cubed carrots peaked at 48 h, which was 5.5 and 6.1 times higher than that of the control, respectively.
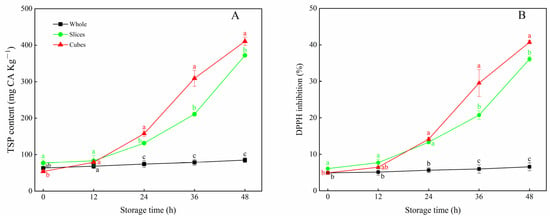
Figure 1.
TSP content (A) and DPPH inhibition (B) of whole, sliced and cubed carrots stored at 20 °C for 48 h. Each value is presented as the mean ± SD, the different lowercase letters indicate significant differences (p < 0.05) among whole, sliced and cubed carrots at each time point.
3.2. Effects of Different Cutting Styles on Soluble Sugar Contents of Carrots
As shown in Figure 2A,B, the fructose and glucose contents generally increased in all carrot tissues with the storage time. The fructose contents in sliced and cubed carrots were significantly higher (p > 0.05) than that of whole carrots after 24 h of storage. Similarly, after 24 h, the glucose content in wounded carrots was significantly higher (p < 0.05) than that observed in the control, except for the sliced carrots at 48 h.
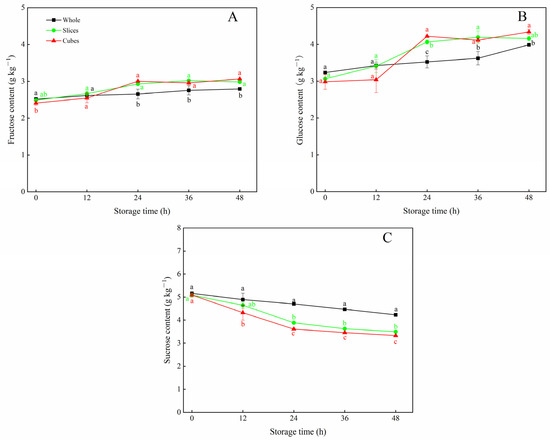
Figure 2.
Fructose content (A), glucose content (B) and sucrose content (C) of whole, sliced and cubed carrots stored at 20 °C for 48 h. Each value is presented as the mean ± SD, the different lowercase letters indicate significant differences (p < 0.05) among whole, sliced and cubed carrots at each time point.
As shown in Figure 2C, the sucrose content in whole carrots decreased gradually with prolonged storage time. However, after 24 h, the application of cutting resulted in a further loss of the sucrose content, and a significant difference (p < 0.05) was observed between whole and wounded carrots. Especially at 24 h of storage, compared with the control, the sucrose content in the sliced and cubed carrots was 17.4% and 23.3% lower, respectively. At the end of storage, only approximately 60% of the sucrose content was retained in both sliced and cubed carrots, as compared with the initial value.
3.3. Effects of Different Cutting Styles on AI and NI Activities of Carrots
As shown in Figure 3, the AI and NI activities of whole, sliced and cubed carrots displayed a similar upward pattern during the entire storage period. For whole carrots, the AI and NI activities increased slowly during storage. Cutting treatment markedly promoted the activities of AI and NI in carrots. After 12 h of storage, the average values of AI and NI in sliced and cubed carrots were much higher (p < 0.05) than in untreated carrots, although an evident decline was observed between 24 h and 36 h. At the end of storage, the maximum values of AI and NI activities were both observed in cubed carrots, which were 1.5 and 1.8 times higher than those of the control, respectively.
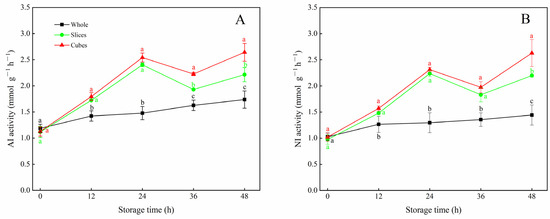
Figure 3.
AI activity (A) and NI activity (B) of whole, sliced and cubed carrots stored at 20 °C for 48 h. Each value is presented as the mean ± SD, the different lowercase letters indicate significant differences (p < 0.05) among whole, sliced and cubed carrots at each time point.
3.4. Effects of Different Cutting Styles on Relative Expression Levels of the Genes Related to INV in Carrots
The relative expression levels of 10 genes encoding invertase in whole, sliced and cubed carrots are presented in Figure 4. Initially, compared with the control, cutting treatment induced an immediate upregulation in 3 genes (LOC108216856, LOC108218454, LOC108222818), while it caused the decline in 4 genes (LOC108206477, LOC108207625, LOC108218494, LOC108222817). With prolonged storage time, an evident increase in relative expression level was observed in most of these genes in sliced and cubed carrots compared to whole carrots, including LOC108204362, LOC108206477, LOC108218454, LOC108218455, LOC108218494, LOC108222817 and LOC108222818. For instance, the expression level of LOC108218494 in wounded carrots, especially the cubed carrots, had a sharp increase during the first 12 h, and thereafter kept at a high level in the subsequent storage. After 12 h, the expression level of LOC108218494 in wounded carrots was significantly higher (p < 0.05) than that of the control. Particularly, at some measuring points, the expression levels of LOC108218454, LOC108218455 and LOC108222817 in cubed carrots were tens or even hundreds of times higher than that of the control. In addition, it was also observed that the expression levels of LOC108204365 and LOC108216856 in wounded carrots were relatively lower than in whole carrots after 24 h.
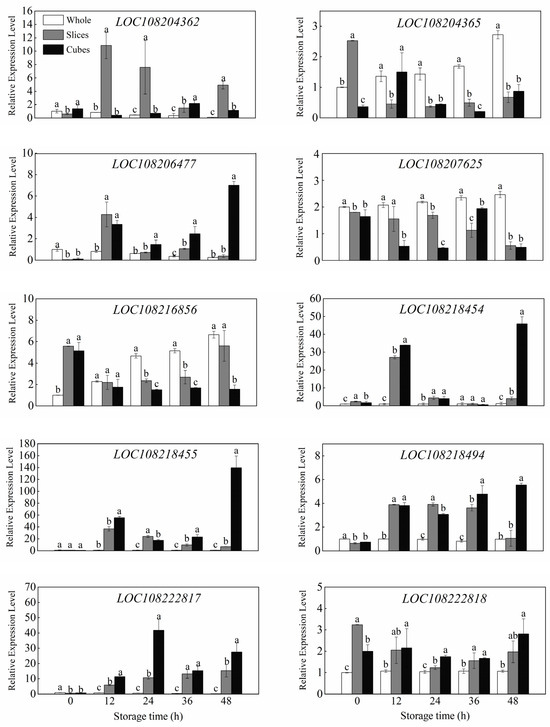
Figure 4.
Relative expression levels of 10 genes related to INV in whole, sliced and cubed carrots stored at 20 °C for 48 h. Each value is presented as the mean ± SD, the different lowercase letters indicate significant differences (p < 0.05) among whole, sliced and cubed carrots at each time point.
3.5. Effects of INV Activators and Inhibitors on TSP Content and DPPH Inhibition of Cubed Carrots
As shown in Figure 5A, a small loss of TSP content was observed in all cubed carrots after water immersion. Cutting treatment triggered the accumulation of TSP content in carrot tissue through storage time; however, the production of phenolic compounds was even higher in cubed carrots with additional use of INV activators, including exogenous INV and ConA. In comparison with exogenous INV, the promotion effect of ConA was more evident. After 2 days of storage, ConA treatment brought a 21.6% increase in TSP content compared to the control. In contrast, the synthesis of phenolic compounds was retarded in cubed carrots with the application of INV inhibitors, including AgNO3 and IAM. The TSP content in AgNO3- and IAM-treated samples decreased by 43.6% and 20.8%, respectively, as compared with the control. Correspondingly, the DPPH inhibition during storage showed a similar pattern as observed for TSP content (Figure 5B). As compared with the control, except for the starting point, the cubed carrots treated with INV activators displayed higher values of DPPH inhibition, while the cubed carrots treated with INV inhibitors showed lower values of DPPH inhibition.
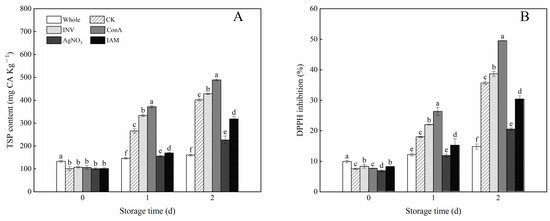
Figure 5.
Effects of INV activators and inhibitors on TSP content (A) and DPPH inhibition (B) of cubed carrots stored at 20 °C for 2 days. Each value is presented as the mean ± SD, the different lowercase letters indicate significant differences (p < 0.05) among different treatments at each time point.
3.6. Effects of INV Activators and Inhibitors on AI and NI Activities of Cubed Carrots
As shown in Figure 6, the AI and NI activities in the intact carrots increased slightly with the storage time; however, cutting treatment pronouncedly improved the values of AI and NI activities during the entire storage period. As two activators of INV, the use of exogenous INV and ConA brought an obvious increase in both AI and NI activities in cubed carrots. After day 1, the ConA-treated samples exhibited the highest AI and NI activities among all treatments. In contrast, as two inhibitors of INV, the application of AgNO3 and IAM greatly suppressed the increasing trend of AI and NI activities in cubed carrots. In comparison with IAM, the inhibition effect of AgNO3 was more evident. At the end of storage, the AI and NI activities decreased by 47.8% and 40.3% in AgNO3-treated samples, respectively, as compared with the control.
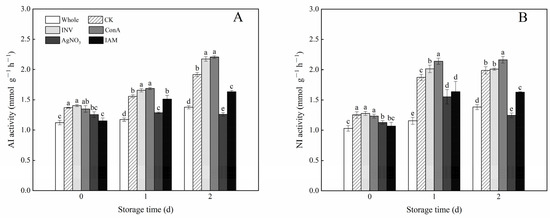
Figure 6.
Effects of INV activators and inhibitors on AI activity (A) and NI activity (B) of cubed carrots stored at 20 °C for 2 days. Each value is presented as the mean ± SD, the different lowercase letters indicate significant differences (p < 0.05) among different treatments at each time point.
3.7. Effects of INV Activators and Inhibitors on Soluble Sugar Contents of Cubed Carrots
As shown in Figure 7, the soluble sugar contents in all cubed carrots experienced an immediate decline after water immersion. Thereafter, the variation trend of reducing sugars and sucrose was diametrically opposite. The fructose and glucose contents in control, INV- and ConA-treated cubed carrots increased continuously with prolonged storage. Cubed carrots treated with ConA at either day 1 or day 2 showed a level of glucose content higher than that of the control. In contrast, the increasing trend of fructose and glucose contents in cubed carrots was impeded by the inhibitor of INV. At day 1, the lowest value of glucose content was observed in AgNO3-treated samples. At day 2, the lowest values of fructose and glucose contents were both observed in AgNO3- and IAM-treated samples. With regard to sucrose, a high decomposition rate was observed in cubed carrots that were treated with the INV activators. After 2 days of storage, the levels of sucrose in INV- and ConA-treated samples decreased by 0.3 and 0.2 times, respectively, with respect to their initial values. However, the sucrose contents in AgNO3- and IAM-treated samples changed slightly, and only limited decrements were observed in these two treatments.
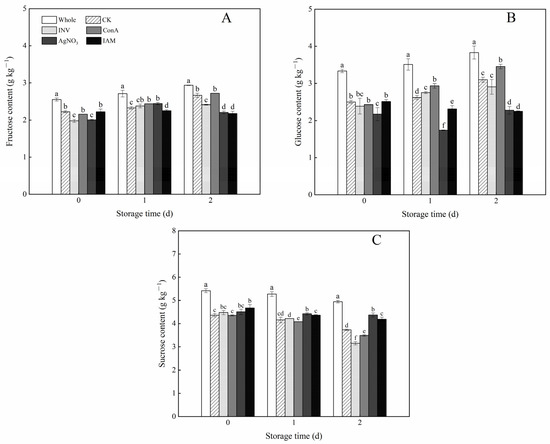
Figure 7.
Effects of INV activators and inhibitors on fructose content (A), glucose content (B) and sucrose content (C) of cubed carrots stored at 20 °C for 2 days. Each value is presented as the mean ± SD, the different lowercase letters indicate significant differences (p < 0.05) among different treatments at each time point.
4. Discussion
In the past few years, the application of postharvest abiotic stresses (e.g., wounding, UV-light irradiation and cold plasma) to produce antioxidant phenolic compounds in horticultural crops has been extensively studied [17,28,29]. Among these abiotic stresses, wounding is established as a simple and effective strategy to induce the accumulation of the individuals and the total amount of phenolic compounds. Due to its observed high enhancement of phenolic compounds, wounded carrots showed great possibilities of becoming a functional food or a functional food ingredient [30]. As a type of secondary metabolite, the synthesis of phenolic compounds depends on the availability of L-phe, the main amino acid derived from sugars [31]. Previously, the accumulation of phenolic compounds in some wounded tissues presented a clear positive correlation with the utilization of sugars [20,21]. This study has gone further to understand the effects of wounding on the activities of invertases and the physiological role of invertases in regulating wound-induced accumulation of phenolic compounds in carrot tissue.
Wounding induced a tremendous increase in TSP contents in carrot tissues (Figure 1A). Regarding the effect of wounding intensity on the production of phenolic compounds, the cubed carrots showed higher levels of TSP as compared to sliced carrots. Our data confirmed previous findings that increased wounding intensity could speed up the biosynthesis of phenolics in wounded carrots [8]. As an important category of antioxidant substances, polyphenols have been recognized as the main contributor influencing the antioxidant capacity of fruits and vegetables [32]. Accordingly, the inhibitory value of DPPH, which reflects the antioxidant capacity, exhibited a similar pattern with TSP content (Figure 1B). In plants, the biosynthesis of secondary metabolites is closely related to primary metabolism. Much evidence has indicated that the intermediate metabolites produced by carbohydrate metabolism can be used as raw materials for the synthesis of polyphenols. For example, pitaya fruit subjected to wounding stress showed a higher content of total phenolics as compared with the intact fruit during 48 h of storage, accompanied by the elevated loss of fructose and glucose [21]. Furthermore, the additional use of methyl jasmonate could synergistically strengthen these two processes of the production of phenolics and the decomposition of soluble sugars [21]. In the present research, sucrose was found to be the predominant sugar in carrot fruit, in accordance with our previous research [22]. The application of wounding inevitably caused a decrease in sucrose, but also resulted in an increase in glucose, especially after 24 h (Figure 2). When wounding occurs, plant cells can absorb sugars produced at the site of wounding [33], and sugars are known to be utilized in many metabolic processes like respiration as well as in the synthesis of amino acids [34]. In the particular case of wounded carrots, the rapid degradation of sucrose could produce essential precursors and serve as substrates for the biosynthesis of phenolic compounds. With regard to glucose, the increased concentrations in wounded tissues might be attributed to the rate of production from sucrose being higher than the utilization rate.
Among sucrose metabolizing enzymes, INVs, are specifically responsible for the irreversible hydrolyzation of sucrose into glucose and fructose [35]. Based on the optimum pH, solubility and subcellular localization, invertases can be further subdivided into three types: cell wall-bound invertases (CWIN), vacuole invertases (VIN) and cytoplasmic invertases (CIN) [36]. CWIN and VIN accumulate in the extracellular space and the vacuole, respectively, and have an acidic pH optimum (3.5~5.5), which are referred to as acid invertases; CIN accumulates in the cytoplasm and has a neutral or alkalic pH optimum (6.5~8.0), which can be classified as alkaline/neutral invertases. Both VIN and CIN are soluble invertases, while CWIN is insoluble invertase [37,38]. An increasing body of evidence indicates that invertases, as well as their related genes, cannot only participate in the processes of plant development and growth, but also play a principal role in regulating responses to abiotic stresses in plants. For example, as Yu et al. [39] reported, during low-temperature storage at 5 °C, AI transcript levels in peach fruit with chilling injury increased about 1400 fold as compared with fruit before storage, and was associated with the promotion of AI activity and a reduction in sucrose content. Similarly, under drought, salinity and phytohormone (ABA) conditions, almost all of the CsINV (Camellia sinensis Invertase) genes were up-regulated after 5 days of treatment, suggesting all of these genes may act as key modulators in preventing damage in the tea plant [40]. In this study, wounding stress greatly activated AI and NI activities in carrot tissues (Figure 3), and this observation matched the variation trend of sucrose and reducing sugars. In addition, it is important to know which gene plays the central role in controlling invertase activities under wounding stress. As shown in Figure 4, a total of 10 invertase genes were expressed in the carrot tissue. According to the interpretation obtained from the website of NCBI (https://www.ncbi.nlm.nih.gov, accessed on 8 January 2023), four genes (LOC108204362, LOC108204365, LOC108218454, LOC108218455) were predicted to encode CWIN and four genes (LOC108216856, LOC108218494, LOC108222817 and LOC108222818) were predicted encoding VIN or CIN. Wounding stress could up-regulate the majority of the invertase genes in most of the measuring points, except for LOC108204365, LOC108207625 and LOC108216856, which were shown to be down-regulated during storage. Specifically, the expression levels of LOC108218454, LOC108218455 and LOC108222817 increased remarkably after wounding, and their values were tens or even hundreds of times higher than the whole carrots, especially in cubed carrots, which suggested that these genes may play a vital role in regulating sucrose metabolism to endure wounding stress. Moreover, it is worth noting that both LOC108218454 and LOC108218455 were characterized as CWIN genes, and this result was in agreement with previous research that the mRNA levels of CWIN in carrots increased dramatically after wounding [41].
In order to obtain direct evidence about the regulatory effect of invertase on the production of secondary metabolites in wounded tissues, cubed carrots were utilized to assay the impact of INV activator or inhibitor on concentrations of soluble sugars and phenolic compounds during storage. In a previous study [24], the enzymatic properties of INVs were examined in bamboo suspension cells during cultivation, and the effects of INV activator or inhibitor were related to chemical reagent types and concentrations. They found that treatment with bovine serum albumin, ConA and urease enhanced AI activity to a different extent, while aniline, q-chloromercuribenzoic acid, IAM, pyridoxine and metal ions including Cu2+, Hg2+ and Ag+ could inhibit the activities of AI and NI to relatively low levels. In this study, based on the preliminary experiments, ConA was selected as the INV activator, while IAM and AgNO3 were selected as INV inhibitors. In addition, considering the direct effect of INV, exogenous INV was used as another activator. Our results showed that the application of ConA or exogenous INV significantly reduced the sucrose content and accelerated the TSP content (Figure 5 and Figure 7), suggesting that the higher availability of soluble sugars could result in a higher level of synthesis of phenolic compounds. In contrast, treatment with AgNO3 or IAM distinctly suppressed the degradation of sucrose, and relatively low TSP contents were observed in AgNO3- and IAM-treated carrot tissues.
5. Conclusions
In summary, wounding stress caused an obvious increase in TSP content and antioxidant capacity in carrot tissues, and a positive correlation was observed between the two processes of biosynthesis of phenolic compounds and degradation of sucrose. The accelerated metabolism of sucrose could be attributed to the activated sucrose-cleaving enzymes of INVs, including AI and NI, which were closely related to the up-regulated expressions of most encoding genes. Most importantly, our results presented here strongly suggested that activated INV could result in a higher level of synthesis of phenolic compounds, whereas inhibited INV brought an adverse impact on the production of phenolic compounds. Our findings provide new insights into the regulation role of INV on the wound-induced accumulation of phenolic compounds in carrots, which may be helpful in using wounded plants to produce more phenolic antioxidants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10010072/s1. Table S1. Primers of the 10 genes encoding the INV in carrot. Table S2. FPKM values of DEGs associated with the biosynthesis of phenolic profiles. Figure S1. The relative expression levels of 10 selected DEGs.
Author Contributions
Conceptualization and methodology, C.H.; formal analysis, X.R. and M.Z.; investigation, X.R., M.L., M.Z. and R.Z.; writing—original draft, X.R. and M.Z.; writing—review and editing, M.L. and C.H.; funding acquisition, M.L. and C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Nos. 32001751 and 31901754), the Innovation Pilot Project of Integration of Science, Education and Industry of Qilu University of Technology (Grant No. 2022PY021) and the Fundamental Research Project of Pilot Project of Integration of Science, Education, and Industry (2022JBZ01-08).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lahlou, A.; Chileh-Chelh, T.; Lyashenko, S.; Rincón-Cervera, M.Á.; Rodríguez-García, I.; López-Ruiz, R.; Urrestarazu, M.; Guil-Guerrero, J.L. Arecaceae fruits: Fatty acids, phenolic compounds and in vitro antitumor activity. Food Biosci. 2022, 50, 102181. [Google Scholar] [CrossRef]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Sen, S.; Kalita, P.; Chakraborty, R. Evaluation of hypolipidemic, antioxidant, atherogenic index and cardiac risk suppressing effects of unpolished maniki madhuri rice extract and HPLC analysis of phenolics compounds. J. Cereal Sci 2022, 108, 103581. [Google Scholar] [CrossRef]
- Viacava, F.; Santana-Gálvez, J.; Heredia-Olea, E.; Pérez-Carrillo, E.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Sequential application of postharvest wounding stress and extrusion as an innovative tool to increase the concentration of free and bound phenolics in carrots. Food Chem. 2020, 307, 125551. [Google Scholar] [CrossRef]
- Li, X.A.; Li, M.L.; Ji, N.N.; Jin, P.; Zhang, J.H.; Zheng, Y.H.; Zhang, X.H.; Li, F.J. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT-Food Sci. Technol. 2019, 115, 108447. [Google Scholar] [CrossRef]
- Guan, Y.G.; Hu, W.Z.; Jiang, A.L.; Xu, Y.P.; Zhao, M.R.; Yu, J.X.; Ji, Y.R.; Sarengaowa; Yang, X.Z.; Feng, K. The effect of cutting style on the biosynthesis of phenolics and cellular antioxidant capacity in wounded broccoli. Food Res. Int. 2020, 137, 109565. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.Z.; Guan, Y.G.; Ji, Y.R.; Yang, X.Z. Effect of cutting styles on quality, antioxidant activity, membrane lipid peroxidation, and browning in fresh-cut potatoes. Food Biosci. 2021, 44, 101435. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Heredia, J.B.; Cisneros-Zevallos, L. The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biol. Technol. 2019, 51, 242–249. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Martinez-Hernandez, G.B.; Rodriguez, S.; Cao, C.M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front Plant Sci. 2015, 6, 837. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Moreno, A.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Glyphosate-induced production of shikimic acid and phenolic antioxidants in wounded carrot tissue. J. Agric. Food Chem. 2012, 60, 11378–11386. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, J.; Jin, P.; Li, X.A.; Wang, L.; Zheng, Y.H. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Physiological role of reactive oxygen species, ethylene, and jasmonic acid on UV light induced phenolic biosynthesis in wounded carrot tissue. Postharvest Biol. Technol. 2021, 172, 111388. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Umbarger, H.E. Amino acid biosynthesis and its regulation. Annu. Rev. Biochem. 1978, 47, 533–606. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Effect of exogenous amylolytic enzymes on the accumulation of chlorogenic acid isomers in wounded potato tubers. J. Agric. Food Chem. 2014, 62, 7671–7675. [Google Scholar] [CrossRef]
- Li, X.A.; Li, M.L.; Wang, J.; Wang, L.; Han, C.; Jin, P.; Zheng, Y.H. Methyl jasmonate enhances wound-induced phenolic accumulation in pitaya fruit by regulating sugar content and energy status. Postharvest Biol. Technol. 2018, 137, 106–112. [Google Scholar] [CrossRef]
- Han, C.; Jin, P.; Li, M.L.; Wang, L.; Zheng, Y.H. Physiological and transcriptomic analysis validates previous findings of changes in primary metabolism for the production of phenolic antioxidants in wounded carrots. J. Agric. Food Chem. 2017, 65, 7159–7167. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.Z.; Zhu, Z.; Wang, W.H.; Cai, J.H.; Chen, Y.; Li, L.; Tian, S.P. A tomato vacuolar invertase inhibitor mediates sucrose metabolism and Influences fruit ripening. Plant Physiol. 2016, 172, 1596–1611. [Google Scholar] [CrossRef]
- Liu, C.C.; Huang, L.C.; Chang, C.T.; Sung, H.Y. Purification and characterization of soluble invertases from suspension-cultured bamboo (Bambusa edulis) cells. Food Chem. 2006, 96, 621–631. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.F.; Gong, Y.F.; Zhu, Y.; Wang, H.F.; Zhang, X.L.; Yu, D.D.; Yu, F.; Qiu, Z.Y.; Lu, H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol. Technol. 2013, 86, 53–61. [Google Scholar] [CrossRef]
- Zhen, W.N.; Tu, Y.; Lin, Z.H.; Xu, X.X.; Fu, M.R.; Han, C. Comparative transcriptome analysis reveals the molecular mechanism of UV-B irradiation in promoting the accumulation of phenolic compounds in wounded carrot. Horticulturae 2022, 8, 896. [Google Scholar] [CrossRef]
- Li, M.L.; Li, X.A.; Han, C.; Ji, N.N.; Jin, P.; Zheng, Y.H. Physiological and metabolomic analysis of cold plasma treated fresh-cut strawberries. J. Agric. Food Chem. 2019, 67, 4043–4053. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A. Transformation of carrots into novel food ingredients and innovative healthy foods. Appl. Food Res. 2023, 3, 100303. [Google Scholar] [CrossRef]
- Yoshida, S. Biosynthesis and conversion of aromatic amino acids in plants. Annu. Rev. Plant Physiol. 1969, 20, 41–62. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Linask, J.; Laties, G.G. Multiphasic absorption of glucose and 3-O-methyl glucose by aged potato slices. Plant Physiol. 1973, 51, 289–294. [Google Scholar] [CrossRef]
- Dai, Z.W.; Léon, C.; Feil, R.; Lunn, J.E.; Delrot, S.; Gomeès, E. Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry (Vitis vinifera L.), a non-climacteric fleshy fruit. J. Exp. Bot. 2013, 64, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.B.; Wu, L.; Busse, J.S.; Whitty, B.R.; Hamernik, A.J.; Jansky, S.H.; Buell, C.R.; Bethke, P.C.; Jiang, J.M. Suppression of the vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiol. 2010, 154, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Tang, G. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. 1999, 4, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; González, M.C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Vargas, W.; Pontis, H.; Salerno, G. Differential expression of alkaline and neutral invertases in response to environmental stresses: Characterization of an alkaline isoform as a stress response enzyme in wheat leaves. Planta 2007, 226, 1535–1545. [Google Scholar] [CrossRef]
- Yu, L.N.; Liu, H.X.; Shao, X.F.; Yu, F.; Wei, Y.Z.; Ni, Z.M.; Xu, F.; Wang, H.F. Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol. Technol. 2016, 113, 8–16. [Google Scholar] [CrossRef]
- Qian, W.J.; Yue, C.; Wang, Y.C.; Cao, H.L.; Li, N.N.; Wang, L.; Hao, X.Y.; Wang, X.C.; Xiao, B.; Yang, Y.J. Identification of the invertase gene family (INVs) in tea plant and their expression analysis under abiotic stress. Plant Cell Rep. 2016, 35, 2269–2283. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Chrispeels, M.J. cDNA cloning of carrot extracellular beta-fructosidase and its expression in response to wounding and bacterial infection. Plant Cell 1990, 2, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).