Abstract
Nutrients and light are critical factors for sustained Centella asiatica L. Urban production under a controlled environment. The growth, triterpene glycosides, and antioxidant activities of C. asiatica grown under a controlled environment with different nutrient solution formulations (NFFs) and LED light intensities were investigated. Four different NSFs were tested on plant growth, bioactive compounds, and their activities in a conventional greenhouse. The results showed that the plants grown with Houghland and Arnon solution exhibited better growth performance, whereas the use of Resh’s Tropical Dry Summer solution led to increased bioactive compounds and their activities. Subsequently, Resh’s Tropical Dry Summer solution was selected to evaluate the effect of light intensity in a controlled environment. Plants were grown under three LED light intensities (110, 220, and 330 µmol/m2/s PPFD) compared with fluorescent and natural lights (45 and 326 µmol/m2/s PPFD, respectively). We found that light intensity had the strongest influence on growth, triterpene glycosides, and antioxidant activities. Significantly higher values of the most studied parameters were observed in plants grown under high light intensity compared to those grown under low light intensity. The optimal light intensity was 330 µmol/m2/s PPFD, representing an efficient approach for commercially producing this medicinal plant with a higher yield and medicinal properties in a controlled environment.
1. Introduction
The COVID-19 pandemic raised awareness among people about maintaining good health and consuming a diet containing foods that can boost immunity and help protect us from contracting disease [1]. This situation may enhance the global herbal medicine market, which is projected to grow from USD 201.06 billion in 2022 to USD 371.45 billion by 2030 [2]. Herbal medicines are now commonly and widely used all over the world due to their beneficial and therapeutic effects on human health [3]. Centella asiatica L. Urban is one of the common Apiaceae spices that has been widely used in Chinese and Indian traditional medicine for centuries in China, India, Sri Lanka, Indonesia, Malaysia, and Thailand, as well as South Africa and Madagascar [4,5,6,7]. It shows several biological activities such as wound healing, anti-diabetic, hepatoprotective, antinociceptive, neuroprotective, anti-inflammatory, and antioxidant properties [4,6,7]. These pharmacological properties are attributed to triterpenes, mostly asiaticoside, asiatic acid, madecassoside, and madecassic acid [6]. Madecassoside and asiaticoside, which co-occur in this plant, are the most abundant constituents than other saponins [8,9]. Moreover, several flavonoid derivatives, phenolic acids, polysaccharides, polyacetylenes, and sterols have been identified in this medicinal plant [4,9]. Generally, the plant materials collected from the wild and field production are of very poor quality in terms of purity and bioactive compounds, as well as being contaminated with pesticide residues, heavy metals, and unacceptable microbial loads [10]. Consequently, to assure the continuous availability of plant material with high concentrations of major bioactive compounds, a well-directed cultivation of C. asiatica is needed [7,10,11].
Crop production in a controlled environment provides a high-quality and stable plant product supply that is an alternative and complementary to field production [12,13]. Temperature, light, CO2, water, and nutrients are essential environmental conditions that affect plant growth and secondary metabolite accumulation in this farming system [7,12,14,15]. Nutrient solution formulations (NSFs) are one of the most important factors that influence growth performance and nutritional values [16]. An optimum NSF depends on plant species and variety, stage of plant growth, temperature, light intensity, and their interactions [11,17,18]. There are many available and well-known formulations that can be used. Some of these formulations used in preparing a nutrient solution have wide-scale applications, while others are designed for specific species and systems [11,17]. However, the results regarding the effects of nutrient supply on triterpene and its derivative accumulation in plants, obtained from in vitro or field studies, are contradictory [10,11,19]. Additionally, previous studies suggested that cultivating C. asiatica in hydroponic systems can be an effective platform to produce high biomass and triterpene glycoside content [10,20]. Thus, C. asiatica cultivated in this system produces clean and good-quality materials to be applied in the pharmaceutical, cosmetic, and food industries.
In addition, the light environment plays a significant role in influencing physiological changes and secondary metabolite accumulation in mature plants [21,22,23,24,25] and microgreens [26,27,28]. Plant growth performance and nutritional values depend on variations in the artificial lighting spectra, light intensities, and daily light integral (DLI) [22]. Light intensity affects photosynthesis, thereby inducing reactive oxygen species (ROS) production during photosynthetic electron fluxes [29]. ROS plays crucial roles in regulating primary and secondary metabolisms and can lead to the synthesis of important secondary metabolites [29,30]. The light-induced production of secondary metabolites can be exploited as a practical and effective strategy to produce high levels of bioactive compounds [30]. However, the light intensity should be optimized for specific crop species and cultivars of plants grown under a controlled environment [24]. Several studies have described the effects of light intensity on the growth and secondary metabolite accumulation of C. asiatica, but these results are not clear [31,32,33,34]. For example, Müller et al. [33] found that the light intensity did not affect growth and leaf yield, but flavonoids, anthocyanins, and saponins were enhanced under high-energy light, possibly due to their ability to defend themselves from oxidative damage. In contrast, Srithongkul et al. [31] reported that low light intensity was shown to reduce the total triterpene glycoside contents in three C. asiatica accessions from Thailand. Song et al. [34] found that the most suitable light intensity for C. asiatica growth under a controlled environment was 200 μmol/m2/s PPFD with 20% blue light, resulting in increased leaf dry weight, triterpene glycosides, and their antioxidant activities. However, asiaticoside and madecassoside exhibited opposite responses to light intensities. Although there has been an attempt to increase the growth and secondary metabolite production of C. asiatica, there are still some remaining points that have not been completely studied. Therefore, the aim of the current study was to investigate the effects of NSFs and LED light intensities on growth performance, triterpene glycoside, and antioxidant activities of C. asiatica grown under a controlled environment. The results from this study may be useful for extension cultivation strategies that are suitable for both growers and consumers.
2. Materials and Methods
2.1. Plant Material
Stock plants of C. asiatica, which contained the highest triterpene content and antioxidant activity in our preliminary study, were obtained from Chumphon province, Thailand (+9.94500, +99.07833, and 251 masl). Rooted cuttings with 1–2 nodes from stock plants were multiplied in a commercial substrate in a greenhouse with 50% shading at the Department of Agricultural Technology, Thammasat University, Thailand (+14.07450, +0.6094167, and 7.3 masl). A commercial substrate comprised loamy sand with a pH of 4.84, electrical conductivity of 0.94 dS/m, and concentrations of 0.31 mg/kg N, 110.95 mg/kg P, and 34.00 mg/kg K. The seedlings were irrigated daily using tap water to the field capacity level. After 4 weeks of growth, the uniform plantlets having four fully expanded leaves were selected to use in each experiment.
2.2. Establishment of Growth Conditions
2.2.1. Experiment I, Effect of Different NSFs
Experiments were conducted in a greenhouse (6 m × 20 m), located at the Department of Agricultural Technology, Thammasat University, Thailand, under 50% shading. This light transmission rate is the lower limit for commercial production of C. asiatica [35]. During the crop cycle, air temperature and air relative humidity were logged every 15 min by a HOBO data logger (Onset Computer Corporation, Bourne, MA, USA). In addition, photosynthetic photon flux density (PPFD) measurements were recorded using HOPOCOLOR (mod. OHSP-350P, Hangzhou Hopoo Light&Color Technology Co., Ltd., Hangzhou, China) (Supplementary Figure S1). The substrate culture system was designed so that the nutrient solution could be applied using an on-line drip irrigation system by dripping onto the plant’s root zone. Each experimental unit consisted of PP plastic pots (35 cm × 150 cm × 20 cm), which were purchased from a local agricultural store (Klong Luang, Thailand), and placed on the greenhouse table. The substate was perlite (with a particle average size 4–8 mm, Speedy Access Co., Ltd., Mueang Samut Prakan, Thailand). The nutrient solution was held in a 151 L HDPE plastic container and delivered to troughs by a submersible water pump (Sonic, mod. AP2500, 32-Watt pump; H2O Hydro Garden, Bangkok, Thailand), resulting in a flow of ~1 L/min per trough.
Four different NSFs, namely Enshi [36], Hoagland and Arnon [37], Resh Tropical Dry Summer [38], and Cooper [39], were arranged in a completely randomized design with three replicates. All chemical constituents of nutrient solution formulations are described in the Supplementary Table S1. An extensive literature review offered no findings about special NSFs for C. asiatica production. For this reason, other available NSFs that had been used before for leafy vegetable production for general purposes were used in the applications of this research.
On 2 November 2020 (4 days before sowing), the four NSFs were applied in each experimental unit substrate saturation. Consequently, 4-week-old uniform plantlets having four fully expanded leaves were transplanted, with 15 cm × 15 cm plant spacing. The nutrient solution pH was recorded daily and adjusted in the range of 5.6–5.8 by adding nitric acid.
2.2.2. Experiment II, Effect of LED Light Intensities
Experiments were performed in a controlled environment room with inner dimensions 4 m × 3 m × 3 m at the Faculty of Science and Technology, Thammasat University, Thailand. PP plastic melon pots were placed on a stainless-steel growing shelf with a single vertical layer. Each self-contained lamp was equipped with individual fluorescent and LED lamps that were separated by a corrugated plastic sheet to prevent light contamination. All chambers covered an area of approximately 1.20 m × 0.80 m and stood 1.80 m high. Growing conditions were as follows: a constant temperature of 25 ± 2 °C, 16 h photoperiod, 0.05% CO2 concentration, and relative air humidity (RH) of 60 ± 2%. Another set was left in the greenhouse with 50% shading and received an average 13 h photoperiod with a 30/24 °C (day/night) temperature for the natural light condition. Average air temperature, air relative humidity, and PPFD were also logged similarly to Experiment I. Moreover, the experimental units were also conducted similarly to Experiment I.
LED units were designed following Samuoliene et al. [26] and Tamulaitis et al. [40]. They comprised LEDs with emission wavelengths in the blue (I = 445 nm), red (I = 638 and 665 nm), and far-red (I = 735 nm) regions (1154 × 15.8 × 1.0-LM-20S1B, Grow Laboratory, Mueang Samut Prakan, Thailand). The irradiated area was approximately 0.60 m2. A range of irradiance intensities, expressed as PPFD, were set for each lighting unit (110 ± 1, 220 ± 1, and 330 ± 1 μmol/m2/s PPED, Supplementary Table S2). In this study, we used 220 μmol/m2/s as the normal irradiance intensity. The fluorescent light (45 ± 2 μmol/m2/s PPFD) and natural light (326 ± 3 μmol/m2/s PPFD) were used as the controls. The experimental treatments were arranged in a randomized complete block design with three replicates.
On 10 March 2021, 4-week-old uniform plantlets having four fully expanded leaves were also transplanted with 15 cm × 15 cm plant spacing, respectively. All experimental units were arranged and managed similarly to Experiment I. Resh Tropical Dry Summer solution, which was selected from Experiment I, was used in this experiment.
2.3. Plant Growth Measurements
The number of leaves per maternal plant (NL), maternal leaf width (LW), petiole length (PL), number of stolons per maternal plant (NS), and number of plantlets per stolon (NP) of five plants from each replication were measured at 5-day intervals between the 10th and 45th day of treatment application (DTA). Regarding the 45th DTA, Prasad et al. [10] suggested it was the optimal harvest date for achieving the highest biomass and triterpenoid glycoside content in C. asiatica cultivated under a hydroponic system. The NL was counted when the leaves became fully expanded. The second young, fully expanded leaf of the maternal plant was selected for measuring LW and PL. The fresh weight (FW) of the whole aboveground part (shoot) was measured at the end of the treatment, followed by the dry weight (DW) after frozen in liquid nitrogen and then drying by using lyophilization (GAMMA 1-16 LSC, Osterode, Germany). Following weighing, the dried samples were stored at −20 °C for future use.
Relative chlorophyll contents were measured via a nondestructive assay using the Soil and Plant Analyzer Development (SPAD) chlorophyll meter (SPAD-502; Konica Minolta, Tokyo, Japan). Measurements were also conducted at 5-day intervals using the second young fully expanded leaf of each plant.
2.4. Sample Preparation and Extraction
The procedure was carried out following previous study with some modifications [41]. Five grams of ground sample were extracted three times with 95% ethanol at a ratio of 1:3, v/v for 72 h and passed through filter paper. The combined extracts were dried using a hot air oven at 50 °C for 72 h, and the dried extract samples were kept at −20 °C for further analysis.
2.5. Determination of Triterpene Glycoside Content
LC–MS/MS analysis was carried out following previous study with some modifications to obtain linearity, accuracy, precision, recovery, and matrix effect [42]. LC–MS/MS (Shimadzu LCMS-8030 triple quadrupole mass spectrometer) was performed by electrospray ionization (ESI) mode, together with an HPLC system (Shimadzu, Kyoto, Japan). Gradient elution of triterpenes was performed on an InertSustain® C18 (3 µm, 150 × 2.1 mm) column coupled with a guard column. The mobile phase consisted of (solvent A) formic acid 0.2% (v/v) in deionized water (pH 2.5) and (solvent B) methanol at a flow rate of 0.2 mL/min and 37 °C column temperature. Elution occurred at a flow rate of 0.2 mL/min, with 20% methanol for 5 min, increasing to 90% methanol for 3 min, then decreasing to 20% methanol until 15 min. The mass spectrometer was equipped with an ESI source recorded in a negative ionization mode. To optimize the ESI conditions, we determined the following parameters: the block heater temperature and the curved desolvation line temperature were maintained at 200 °C and 250 °C, respectively. Flow rates were 3 L/min for nitrogen gas and 15 L/min for the nebulizer and the drying gas, respectively. Mass acquisitions were performed in multiple reaction mode (MRM). The detector was set to monitor mass-to-charge ratios of 973.4 → 503.5 for madecassoside, 957.4 → 469.2 for asiaticoside. The retention times were 8.77 and 8.96 min for madecassoside and asiaticoside, respectively. The calibration curves of madecassoside and asiaticoside exhibited a good linear relationship between concentrations and peak area ratios, with a correlation coefficient (R2) of 0.99. The lower limit of detection of all test compounds was estimated to be 0.1–0.5 μg/L with a signal to noise ratio of 5, and the recovery percentage of all test compounds was up to 93%.
2.6. Determination of Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Activities
The TPC was analyzed using the Folin–Ciocalteu colorimetric method described in Folin and Ciocalteu [43], whereas TFC followed from Kubola et al. [44]. A microplate reader (Power Wave XS, Biotek, CA, USA) was used to analyze the TFC and TPC at 510 and 765 nm absorbances, respectively. TPC was expressed as milligrams gallic acid equivalent per gram of dry weight (mg GAE/g DW). TFC was expressed as milligrams quercetin equivalent per gram of dry weight (mg QE/g DW).
The two most common antioxidant activity assays were conducted using 2,2′-azino bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. Antioxidant activity was analyzed using an ABTS and DPPH antioxidant activity assays followed from Re et al. [45] and Brand-Williams et al. [46], respectively. Absorbance was also measured in a microplate reader at 520 and 734 nm for DPPH and ABTS antioxidant activity assays, respectively. Trolox was used as the reference compound. The results are expressed in µmol of Trolox equivalents per g of dry weight (µmol TE/g DW).
2.7. Statistical Analysis
Statistical analysis was performed using Statistix program (ver. 10.0, Analytical Software, Tallahassee, FL, USA). Data were compared using one-way ANOVA at a significance level of p = 0.05, and mean comparisons were performed using LSD at 0.01 probability level. The graphs were then plotted by JMP® Statistical Software (ver. Free trial, SAS institute, Chicago, IL, USA).
3. Results
3.1. Effect of NSFs on Growth, Triterpene Glycoside, Antioxidants, and Antioxidant Activity of C. asiatica Grown in a Greenhouse
3.1.1. Growth Performance
The growth response of C. asiatica to different NSFs is shown in Figure 1. C. asiatica grown under Cooper solution demonstrated signs of irregular growth habits, including tip and leaf burns, and dying at 35th DTA. During the 10th–45th DTA, C. asiatica exhibited a significant difference in the NL on the 20th DTA, followed by a gradual increase thereafter. The highest NL was observed under Hoagland and Arnon solution (Figure 1a). The LW steadily increased from the 10th to 30th DTA, and then it gradually increased. The plants grown under Resh Tropical Dry Summer solution gave the highest LW at harvest (Figure 1b). The PL gradually increased throughout the experimental period, except for Resh Tropical Dry Summer solution, it was steady after the 35th DTA. The highest PL was observed under Enshi solution (Figure 1c). Stolon initiation began at the 20th DTA and showed significant differences in NS at the 35th DTA. The highest NS was observed under Hoagland and Arnon solution (Figure 1d). Plantlet initiation began at the 20th DTA, except those plants grown with Enshi solution began at the 25th DTA (Figure 1e). The plants showed a significant difference in NP at the 45th DTA. The highest NP was observed under Resh Tropical Dry Summer solution. The nutrient solutions showed significant differences in relative chlorophyll content throughout the experimental period (Figure 1f). The content gradually increased, and then there was a decline. The highest fresh and dry weights were observed under Hoagland and Arnon solution, whereas the lowest was observed under Enshi solution (Table 1).
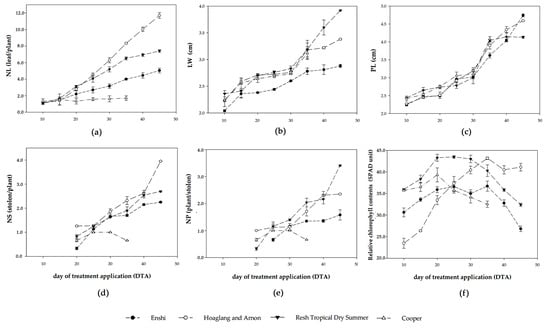
Figure 1.
Effect of NSFs on growth of C. asiatica grown in a greenhouse. (a) Number of leaves per maternal plant (NL); (b) maternal leaf width (LW); (c) petiole length (PL); (d) number of stolons per maternal plant (NS); (e) number of plantlets per stolon (NP); (f) relative chlorophyll content. The error bar represents the standard deviation of each value (n = 3).

Table 1.
Effect of nutrient solution formulations (NSFs) on fresh weight and dry weight in C. asiatica grown in a greenhouse at 45th DTA.
3.1.2. Triterpene Glycoside, Antioxidants, and Antioxidant Activity
Two major triterpene glycosides, i.e., asiaticoside and madecassoside, showed significant differences under different NSFs (Table 2). The plants grown under the Resh Tropical Dry Summer solution had the highest contents of asiaticoside and madecassoside (5.39 ± 0.37 and 26.78 ± 0.97 mg/g DW, respectively), whereas Hoagland and Arnon solution gave the lowest contents of both triterpenes (0.71 ± 0.07 and 3.70 ± 0.44 mg/g DW, respectively). Moreover, the plants grown under the Resh Tropical Dry Summer solution had the highest TPC, TFC, and antioxidant activities. Enshi solution gave the lowest TPC and TFC, whereas the lowest DPPH and ABTS antioxidant activities were obtained with the Hoagland and Arnon solution.

Table 2.
Effect of nutrient solution formulations (NSFs) on triterpene glycoside, antioxidants, and antioxidant activities in C. asiatica grown in a greenhouse.
Although Resh Tropical Dry Summer solution manifested lower C. asiatica biomass than Hoagland and Arnon solution, it yielded a higher triterpene glycoside content by 7.59 to 7.23-fold. Thus, this solution was chosen to evaluate the effect of light intensity.
3.2. Effect of LED Light Intensities on Growth, Triterpene Glycoside, Antioxidants, and Antioxidant Activity of C. asiatica Grown in a Controlled Environment
3.2.1. Growth Performance
The growth response of C. asiatica to different LED light intensities is shown in Figure 2 and Figure 3. During days 10th–45th DTA, NL appeared to gradually increase, whereas significant differences in NL appeared at 20th DTA (Figure 3a). The highest NL was observed under 330 µmol/m2/s PPFD, while fluorescent light and 220 µmol/m2/s PPFD yielded a lower NL than natural light and 110 µmol/m2/s PPFD. The LW and PL showed a gradual increase throughout the experimental period (Figure 3b,c). The stolon initiation of plants grown under LED light began at 10th DTA, whereas that of plants grown under fluorescent and natural lights began at 15th DTA (Figure 3d). Then, all plants rapidly emerged from stolon and the highest NS was observed under 220 and 330 µmol/m2/s PPFD. The plantlet initiation began at 10th DTA, except for plants grown under natural light, plantlet initiation began at 15th DTA (Figure 3e). The highest NP was observed under 220 and 330 µmol/m2/s PPFD, while growing under 110 µmol/m2/s PPFD and fluorescent light gave the lowest NP at harvest.

Figure 2.
The appearance and growth performance of C. asiatica grown under different light intensities at 45th DTA in a controlled environment with Resh’s Tropical Dry Summer solution. (a) Natural light; (b) fluorescent light; (c) 110 ± 1 μmol/m2/s PPFD; (d) 220 ± 1 µmol/m2/s PPFD; (e) 330 ± 1 µmol/m2/s PPFD.
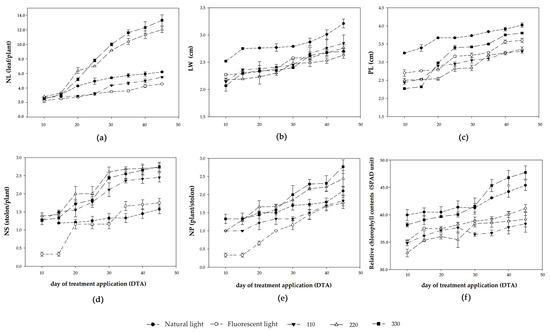
Figure 3.
Effect of LED light intensity on growth of C. asiatica grown in a controlled environment with Resh’s Tropical Dry Summer solution. (a) Number of leaves per maternal plant (NL); (b) maternal leaf width (LW); (c) petiole length (PL); (d) number of stolons per maternal plant (NS); (e) number of plantlets per stolon (NP); (f) relative chlorophyll content. The error bar represents the standard deviation of each value (n = 3).
The light intensities showed a significant difference in relative chlorophyll content throughout the experimental period (Figure 3f). The highest value of this trait was observed under natural light and 330 µmol/m2/s PPFD. The plants grown under 330 µmol/m2/s PPFD had the highest fresh and dry weights, while fluorescent light gave the lowest (Table 3).

Table 3.
Effect of light intensities on fresh weight and dry weight of C. asiatica grown in a controlled environment with Resh’s Tropical Dry Summer solution.
3.2.2. Triterpene Glycoside, Antioxidants, and Antioxidant Activity
The triterpene glycoside, antioxidants, and antioxidant activities showed significant differences under different LED light intensity treatments (Table 4). The highest levels of asiaticoside and madecassoside were found under 330 µmol/m2/s PPFD (8.12 ± 0.20 and 31.94 ± 0.68 mg/g DW, respectively), while the lowest levels of these triterpenes were recorded under fluorescent light. Plants grown under 330 µmol/m2/s PPFD had the highest TPC and TFC, whereas the lowest values were found under 110 µmol/m2/s PPFD and fluorescent light. Finally, the highest DPPH and ABTS antioxidant activities were found under 330 µmol/m2/s PPFD (51.37 ± 0.96 and 54.32 ± 0.61 mg TE/g DW, respectively), while plants grown under fluorescent light had the lowest antioxidant activities.

Table 4.
Effect of light intensities on triterpene glycoside, antioxidants, and antioxidant activities of C. asiatica grown in a controlled environment with Resh’s Tropical Dry Summer solution.
4. Discussion
Crop production in a controlled environment is increasingly feasible and may play a vital role in providing nutrition and choice to growing plants in urban settings. The key factors that influence crop growth and secondary metabolite accumulation in a controlled environment are temperature, humidity, substrate, nutrition, and light. This study summarized the effects of NSFs and LED light intensity on growth performance, bioactive compounds, and antioxidant activities of C. asiatica grown in a controlled environment. The management of mineral nutrients through selected nutrient solutions is a key pre-harvest factor that determines growth and quality of crops, as well as leading to the manipulation of target compounds, such as bioactive compounds and their activities. However, the extensive literature review offered no findings about special NSFs for C. asiatica production. Thus, we used four NSFs, which are used to grow other plant species and for general purposes, to grow C. asiatica under a substrate culture. The results showed a significant effect of NSFs on growth performance and biomass (Figure 1 and Table 1). All NSFs produced plants with different growth parameters, based on various stages of development- and plant-specific responses. The NL, NS, relative chlorophyll content, and fresh and dry weights were higher when C. asiatica was grown using Hoagland and Arnon solution, while growing under Resh Tropical Dry Summer led to higher LW, PL, and NP. The Hoagland and Arnon solution is a multipurpose NSF widely used in substrate culture; therefore, it can effectively nourish C. asiatica, resulting in high yields. This solution contained a higher amount of nitrogen (N) than the other NSFs used in this study (Supplementary Table S1). Among the mineral elements, N is considered an essential macronutrient for plants, significantly determining crop yield and quality [47,48]. This result was confirmed in plants grown in Hoagland and Arnon solution, as they had a higher leaf relative chlorophyll content, which is considered to reflect the absorption of nutrients by the plants [15,18,49]. On the other hand, the Cooper solution formulation showed an unexpectedly unfavorable result when C. asiatica was dried at 35 DTA. Incorrect incorporation of nutrient elements and their doses into nutrient solutions for plant nourishment can lead to various nutritional disorders. These disorders appear as physiological malfunctions within a plant, resulting in abnormal growth as a result of either a lack or an excess of mineral elements [50]. According to previous studies, the NSFs significantly influenced growth performance in fig trees [17], gerbera [18], and tomato [51].
The main chemical components responsible for the pharmacological activity of C. asiatica are triterpene glycosides or centellosides, mostly asiaticoside and madecassoside [6]. Our study confirmed that madecassoside was a predominant triterpene glycoside of C. asiatica because its content was higher than that of asiaticoside by 4.97-fold (Table 2 and Table 4), which was consistent with a previous study [52]. Furthermore, madecassoside, isolated from C. asiatica, exhibits greater medicinal properties [53], thereby confirming the importance of this triterpene. In addition, levels of both asiaticoside and madecassoside in C. asiatica in this study were higher than those in plants grown under both hydroponic systems [10] and on open fields [54]. Generally, it is known that the content of bioactive compounds and their activities may depend on many aspects such as genotype, growing conditions, and cultural practices. This study noted that both concentrations of bioactive compounds and their activities in C. asiatica varied with different NSFs (Table 2). The plants grown using the Resh Tropical Dry Summer solution, which contained a lower concentration of phosphorus (P), exhibited the highest contents of triterpene glycosides. Nevertheless, Hoagland and Arnon solution, which had a higher P concentration, increased C. asiatica growth performance, but its triterpene glycosides were low. Generally, P affects the accumulation of triterpenoid glycosides in C. asiatica by facilitating the creation of enzymes such as farnesyl pyrophosphate synthase (FPS) and farnesyl diphosphate (FPP). These enzymes function as building blocks within the isoprene units in the mevalonic acid pathway [55]. This result is consistent with many studies reporting that reduced nutrient availability leads to a higher concentration of triterpenes in C. asiatica [10,11,19,56]. The limited availability of essential macronutrients encourages the allocation of precursors from primary to secondary metabolism. Consequently, this process triggers the accumulation of triterpenoids, supporting the carbon/nutrient balance (CNB) and the growth differentiation balance (GDB) hypotheses [57,58]. Additionally, the observed solution notably affected the levels of total phenols and flavonoids, along with their activities. Decreasing macronutrient concentrations correlated with increased phenolic acids and flavonoids in crops, indicating that plant secondary metabolism was stimulated by low nutrient availability, consistent with previous studies [59,60]. These findings further substantiate that the Resh Tropical Dry Summer solution serves as a well-balanced macronutrient source, mitigating potential elemental antagonism and stress.
Management of light intensity within controlled environments is of major importance considering its implications for photosynthetic activity and the chemical composition of vegetables [24,30]. The results showed a significant effect of light intensity on C. asiatica growth performance and biomass (Figure 2 and Table 3). The different responses to light intensity varied with plant growth parameters and stages of development. The plants grown under 220 and 330 μmol/m2/s/PPFD showed better growth performance, including higher values of NL, NS, NP, and plant biomass. Higher light intensities promote photosynthesis rates and consequently enhance plant growth [61]. Moreover, C. asiatica grown under 330 µmol/m2/s PPFD exhibited high relative chlorophyll content, which is one of the essential factors affecting crop growth and yield. These results confirm that a high level of light intensity is necessary for adequate synthesis and activity of photosynthetic pigments, enhancing growth performance and biomass. In contrast, lower values for all parameters were observed in plants grown under fluorescent light. This might be due to low light intensity inhibiting plant growth and productivity by affecting gas exchange and the photosynthetic apparatus [23]. This finding aligns with similar results observed in other crops [62,63,64]. Li et al. [32] argued that C. asiatica treated with low light showed increased biomass and stolon length. Additionally, Müller et al. [32] found that light intensity did not significantly influence the growth and leaf yield of C. asiatica. Furthermore, plants grown under natural light exhibited lower fresh and dry weights, at 1.58 and 1.83-fold, respectively, compared to those cultivated in controlled environments at 330 µmol/m2/s. Our results support the notion that cultivating C. asiatica in controlled environments using LED light can be selectively advantageous for plant growth. The optimal light intensity, up to 330 µmol/m2/s, promotes growth performance and biomass. Utilizing this level of light intensity represents an efficient and promising strategy for achieving higher yields of this medicinal plant.
Light intensity affects the bioactive compounds in C. asiatica grown under LED light compared with the natural and fluorescent lighting needed to elucidate [34]. Considering our study, both triterpene glycosides, namely asiaticoside and madecassoside, were significantly affected by light intensity. We found significantly higher triterpene glycosides in C. asiatica grown under 330 μmol/m2/s PPFD, followed by 220 μmol/m2/s PPFD and natural light. This result indicated that higher light intensity promotes the accumulation of triterpene glycosides, according to Srithongkul et al. [31], who found increasing light intensities from 93.3 to 933.1 μmol/m2/s PPFD increased the total triterpene glycoside content in C. asiatica. However, Song et al. [34] argued that the highest asiaticoside content was obtained with 200 µmol/m2/s PPFD when compared to 150 μmol/m2/s PPFD, but madecassoside exhibited an opposite accumulation trend. Müller et al. [33] reported that the contents of glycosylated triterpenes (asiaticoside and madecassoside) and total triterpene glycoside and even the de novo synthesis of hydroxy triterpene (asiatic acid and madecassic acid) were enhanced under high-energy light, possibly due to their ability to protect the plant from oxidative damage. We assumed the various responses would be a result of the plant materials, bioactive compound extraction and analysis, and different blue, green, and/or red-light ratios used in LED lamps. The plants grown with 330 µmol/m2/s PPFD exhibited the highest TPC. This result agrees with previous reports [33,34,65] and was probably due to the increase in phenylalanine ammonia lyase and enzymes involved in the biosynthesis of phenolic acids [34,66]. These findings are contrary to Pennisi et al. [67], who reported that TPC increased with increasing light intensity up to 250 µmol/m2/s PPFD but began to decrease under 300 µmol m2/s PPFD. Similarly to TPC, the highest level of TFC was also obtained in the plant grown under 330 µmol/m2/s PPFD, which is consistent with Müller et al. [33], who found that a higher light energy induced flavonoid accumulation. Flavonoid accumulation under various lighting conditions correlated with elevated expression levels of CaABI5, CaMYB4, CaMYB12, and CaHYH transcription factors, which were associated with the expression of flavonoid biosynthetic genes under high-energy (blue) light. Conversely, the downregulation of CaERF38 might be linked to reduced expression levels of such genes under low-energy (red) light [68]. Both DPPH and ABTS antioxidant activities showed a significant response to light intensity, similarly to that in TPC and TFC. The highest DPPH and ABTS antioxidant activities were observed in plants grown under 330 µmol/m2/s PPFD. This result is in agreement with Pennisi et al. [67], who found that antioxidant activity increased with increasing light intensity up to 250 µmol/m2/s PPFD. On the other hand, Song et al. [34] found significantly higher antioxidant activity under low light intensity. From our study, the lowest levels of bioactive compounds and their activity were obtained in plants grown under fluorescent light; additionally, plants grown under 110 µmol/m2/s PPFD showed lower TPC and TFC. Contrary to previous studies, low light intensity may have various effects on different antioxidant activities, such as an increase in DPPH or a decrease in FRAP [23]. Low light could stimulate biosynthesis or accumulation of secondary metabolites, which is in parallel with poor growth, as suggested by the growth-defense tradeoffs [69]. Overall, the application of 330 μmol/m2/s PPFD was found to be necessary for cultivating C. asiatica in a controlled environment, ensuring optimal plant growth for economic viability and enhancing the production of bioactive compounds. Further investigation into the impact of nutrient solution concentrations and the light environment on the expression levels of biosynthetic genes associated with triterpenoid glycosides and other bioactive compounds in this plant are needed.
5. Conclusions
The results showed that responses to NSFs and light intensity varied with C. asiatica’s studied traits and stages of development. The plants grown in a conventional greenhouse with the Houghland and Arnon solution achieved superior growth, whereas the Resh Tropical Dry Summer solution yielded favorable bioactive compounds and their activities. Growth performance, triterpene glycosides, total phenolic and total flavonoid contents, and antioxidant activities increased with the increase in light intensity. Therefore, using 330 µmol/m2/s PPFD is recommended for C. asiatica grown in a controlled environment with the Resh Tropical Dry Summer solution to improve the crop yield and bioactive compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10010071/s1, Figure S1: Ambient temperature, humidity, and light intensity of the greenhouse during the cultivation period; Table S1: Chemical names of materials used to prepare nutrient solutions; Table S2: Combinations of light emitting diodes and photosynthetic photon flux densities (PPFD).
Author Contributions
Conceptualization, B.H.; methodology, B.H.; software, B.H.; validation, L.C., Y.J. and B.H.; formal analysis, L.C. and B.H.; investigation, L.C. and B.H.; resources, B.H.; data curation, L.C. and B.H.; writing—original draft preparation, L.C., Y.J. and B.H.; writing—review and editing, L.C., Y.J., P.R. and B.H.; visualization, P.R. and B.H.; supervision, P.R.; project administration, B.H.; funding acquisition, B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Science and Technology, Thammasat University, grant number SciGR 21/2565.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
Acknowledgement is also extended to the Department of Agricultural Technology, Faculty of Science and Technology, Thammasat University for providing research facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arshad, M.S.; Khan, U.; Sadiq, A.; Khalid, W.; Hussain, M.; Yasmeen, A.; Asghar, Z.; Rehana, H. Coronavirus disease (COVID-19) and immunity booster green foods: A mini review. Food Sci. Nutr. 2020, 8, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Fortune Business Insights. Available online: https://www.fortunebusinessinsights.com/herbal-medicine-market-106320 (accessed on 1 December 2023).
- Gomez, L.A. Growth evaluation and proximate analysis of Gotu Kola (Centella asiatica L.) in response to organic growing media combinations. Asian J. Fundam. Appl. Sci. 2021, 2, 65–76. [Google Scholar]
- Orhan, I.E.; Loizzo, M.R.; Khan, M.T.H. Therapeutic approaches to neuroprotective activity by complementary and alternative medicines. Evid.-Based Complement. Altern. Med. 2012, 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Maulidiani, A.F.; Khatib, A.; Shaari, K.; Lajis, N.H. Chemical characterization and antioxidant activity of three medicinal Apiaceae species. Ind. Crops Prod. 2014, 55, 238–247. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kharsyntiew, B.; Sharma, P.; Sahoo, U.K.; Sarangi, P.K.; Prus, P.; Imbrea, F. The effect of production and post-harvest processing practices on quality attributes in Centella asiatica (L.) Urban—A review. Agronomy 2023, 13, 1999. [Google Scholar] [CrossRef]
- Hashim, P.; Sidek, H.J.; Helan, M.; Sabery, A.; Palanisamy, U.D.; Ilham, M. Triterpene composition and bioactivities of Centella asiatica. Molecules 2011, 16, 1310–1322. [Google Scholar] [CrossRef]
- Long, H.S.; Stander, M.A.; Van Wyk, B.-E. Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. S. Afr. J. Bot. 2012, 82, 53–59. [Google Scholar] [CrossRef]
- Prasad, A.; Pragadheesh, V.S.; Mathur, A.; Srivastava, N.; Singh, M.; Mathur, A. Growth and centelloside production in hydroponically established medicinal plant-Centella asiatica (L.). Ind. Crops Prod. 2012, 35, 309–312. [Google Scholar] [CrossRef]
- Müller, V.; Lankes, C.; Zimmermann, B.F.; Noga, G.; Hunsche, M. Centelloside accumulation in leaves of Centella asiatica is determined by resource partitioning between primary and secondary metabolism while influenced by supply levels of either nitrogen, phosphorus or potassium. J. Plant Physiol. 2013, 170, 1165–1175. [Google Scholar] [CrossRef]
- Niu, G.; Masabni, J. Plant production in controlled environments. Horticulturae 2018, 4, 28. [Google Scholar] [CrossRef]
- Folta, K.M. Breeding new varieties for controlled environments. Plant Biol. 2019, 1, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Reza, M.N.; Chowdhury, M.; Chung, S.-O.; Choi, I.-S. A review on effect of ambient environment factors and monitoring technology for plant factory. Precis. Agric. Sci. Technol. 2021, 3, 83–98. [Google Scholar]
- Walters, K.J.; Currey, C.J. Effects of nutrient solution concentration and daily light integral on growth and nutrient concentration of several basil species in hydroponic production. HortScience 2018, 53, 1319–1325. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Orsini, F.; Barbieri, G. Nutrients influence on ready to eat sweet basil quality. Acta Hortic. 2006, 718, 523–530. [Google Scholar] [CrossRef]
- Kilinc, S.S.; Ertan, E.; Seferoğlu, S. Effects of different nutrient solution formulations on morphological and biochemical characteristics of nursery fig trees grown in substrate culture. Sci. Hortic. 2007, 113, 20–27. [Google Scholar] [CrossRef]
- Şirin, U. Effects of different nutrient solution formulations on yield and cut flower quality of gerbera (Gerbera jamesonii) grown in soilless culture system. Afr. J. Agric. Res. 2011, 6, 4910–4919. [Google Scholar]
- Devkota, A.; Dall’Acqua, S.; Comai, S.; Innocenti, G.; Jha, P.K. Centella asiatica (L.) urban from Nepal: Quali-quantitative analysis of samples from several sites, selection of high terpene containing populations for cultivation. Biochem. Syst. Ecol. 2010, 38, 12. [Google Scholar] [CrossRef]
- Shawon, M.R.A.; Azad, M.O.K.; Ryu, B.R.; Na, J.K.; Choi, K.Y. The electrical conductivity of nutrient solution influenced the growth, centellosides content and gene expression of Centella asiatica in a hydroponic system. Agriculture 2023, 13, 2236. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.Y.; Yu, J.; Liu, H.; Cao, Z.y.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. Var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of daily light integral and LED spectrum on growth and nutritional quality of hydroponic spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Sutulienė, R.; Laužikė, K.; Pukas, T.; Samuolienė, G. Effect of light intensity on the growth and antioxidant activity of sweet basil and lettuce. Plants 2022, 11, 1709. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Yang, S.; Xu, J.; Wang, H.; Zhang, Y.; Cui, J.; Zhang, H.; Jin, H.; Lu, P.; He, L.; et al. Effects of light intensity on growth and quality of lettuce and spinach cultivars in a plant factory. Plants 2023, 12, 3337. [Google Scholar] [CrossRef]
- Veremeichik, G.N.; Grigorchuk, V.P.; Makhazen, D.S.; Subbotin, E.P.; Kholin, A.S.; Subbotina, N.I.; Bulgakov, D.V.; Kulchin, Y.N.; Bulgakov, V.P. High production of flavonols and anthocyanins in Eruca sativa (Mill) Thell plants at high artificial LED light intensities. Food Chem. 2023, 408, 135216. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaityte, A.; Jankauskiene, J.; Virsile, A.; Sirtautaus, R.; Novickovas, A.; Sakalauskaite, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Harakotr, B.; Srijunteuk, S.; Rithichai, P.; Tabunhan, S. Effects of Light-Emitting Diode light irradiance levels on yield, antioxidants and antioxidant capacities of indigenous vegetable microgreens. Sci. Technol. Asia 2019, 24, 59–66. [Google Scholar]
- Jones-Baumgardt, C.; Llewellyn, D.; Zheng, Y. Different microgreen genotypes have unique growth and yield responses to intensity of supplemental PAR from Light-emitting Diodes during winter greenhouse production in Southern Ontario, Canada. HortScience 2020, 55, 156–163. [Google Scholar] [CrossRef]
- Borbély, P.; Gasperl, A.; Pálmai, T.; Ahres, M.; Asghar, M.A.; Galiba, G.; Müller, M.; Kocsy, G. Light intensity- and spectrum-dependent redox regulation of plant metabolism. Antioxidants 2022, 11, 1311. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Sritongkul, J.; Srilaong, V.; Uthairatanakij, A.; Kanlayanarat, S.; Chalermglin, P. Effect of light intensity on chemical composition of asiatic pennywort (Centella asiatica L. Urban). Acta Hortic. 2009, 837, 87–93. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Wei, Q.; Li, Q.; Lei, N. Effects of transgenerational plasticity on morphological and physiological properties of stoloniferous herb Centella asiatica subjected to high/low light. Front. Plant Sci. 2018, 9, 1640. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Albert, A.; Winkler, J.B.; Lankes, C.; Noga, G.; Hunsche, M. Ecologically relevant UV-B dose combined with high PAR intensity distinctly affect plant growth and accumulation of secondary metabolites in leaves of Centella asiatica L. Urban. J. Photochem. Photobiol. B Biol. 2013, 127, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Bhandari, S.R.; Shin, Y.K.; Lee, J.G. The influence of red and blue light ratios on growth performance, secondary metabolites, and antioxidant activities of Centella asiatica (L.) Urban. Horticulturae 2022, 8, 601. [Google Scholar] [CrossRef]
- Srithongkul, J.; Kanlayanarat, S.; Srilaong, V.; Uthairatanakij, A.; Chalermglin, P. Effects of light intensity on growth and accumulation of triterpenoids in three accessions of Asiatic pennywort (Centella asiatica (L.) Urb.). J. Food Agric. Environ. 2011, 9, 360–363. [Google Scholar]
- Hori, H. Gravel Culture of Vegetables and Ornamentals; Yokendo: Tokyo, Japan, 1966; pp. 60–79. [Google Scholar]
- Hoagland, D.R.; Arnon, D.L. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 12. [Google Scholar]
- Resh, M.H. Hydroponics for Food Production; Woodbridge Press Publishing Company: Santa Barbara, CA, USA, 1978; 277p. [Google Scholar]
- Cooper, A.J. Crop production in recirculating nutrient solution. Sci. Hortic. 1975, 3, 251–258. [Google Scholar] [CrossRef]
- Tamulaitis, G.; Duchovskis, P.; Bliznikas, Z.; Breive, K.; Ulinskaite, R.; Brazaityte, A.; Novičkovas, A.; Žukauskas, A. High power light-emitting diode based facility for plant cultivation. J. Phys. D Appl. Phys. 2005, 38, 3182–3187. [Google Scholar] [CrossRef]
- Jaiarree, N. Biological Activities of Dioscorea birmanica Prain and Burkill Extract and Its Active Ingredients. Ph.D Thesis, Thammasat University, Pathum Thani, Thailand, 2010. [Google Scholar]
- Hengjumrut, P.; Anukunwithaya, T.; Tantisira, M.H.; Tantisira, B.; Khemawoot, P. Comparative pharmacokinetics between madecassoside and asiaticoside presented in a standardised extract of Centella asiatica, ECa 233 and their respective pure compound given separately in rats. Xenobiotica 2018, 48, 18–27. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophan determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S.; Meeso, N. Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chem. 2011, 126, 972–981. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Leb. Wiss Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kolega, S.; Miras-Moreno, B.; Buffagni, V.; Lucini, L.; Valentinuzzi, F.; Maver, M.; Mimmo, T.; Trevisan, M.; Pii, Y.; Cesco, S. Nutraceutical profiles of two hydroponically grown sweet basil cultivars as affected by the composition of the nutrient solution and the inoculation with Azospirillum brasilense. Front. Plant Sci. 2020, 11, 596000. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, S.P.; Sujatha, S.; Smitha, G.R.; Suryanarayana, M.A.; Kalaivanan, D. Biomass accumulation, bioactive compounds and nutrient uptake in Centella asiatica (L.) in relation to organic nutrition in open-field and shade. Ind. Crops Prod. 2022, 176, 114352. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower, 7th ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Ma, L.; Zhang, J.; Ren, R.; Fan, B.; Hou, L.; Li, J. Effects of different organic nutrient solution formulations and supplementation on tomato fruit quality and aromatic volatiles. Arch. Agron. Soil Sci. 2020, 67, 563–575. [Google Scholar] [CrossRef]
- Prasad, A.; Yadav, K.S.; Yadav, N.P.; Mathur, A.; Sreedhar, R.V.; Lal, R.K.; Mathur, A.K. Biomass and centellosides production in two elite Centella asiatica germplasms from India in response to seasonal variation. Ind. Crops Prod. 2016, 94, 711–720. [Google Scholar] [CrossRef]
- Tan, S.C.; Bhattamisra, S.K.; Chellappan, D.K.; Candasamy, M. Actions and therapeutic potential of madecassoside and other major constituents of Centella asiatica: A review. Appl. Sci. 2021, 11, 8475. [Google Scholar] [CrossRef]
- Puttarak, P.; Panichayupakaranant, P. Factors affecting the content of pentacyclic triterpenes in Centella asiatica raw materials. Pharm. Biol. 2012, 50, 1508–1512. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef]
- Vinolina, N.S.; Sigalingging, R. Growth and secondary metabolites production of Centella asiatica (L.) Urb. cultivated at different phosphate application rates in acid soil. Trends Sci. 2022, 19, 5820. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin, F.S.; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The Dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Juszczuk, I.M.; Wiktorowska, A.; Malusá, E.; Rychter, A.M. Changes in the concentration of phenolic compounds and exudation induced by phosphate deficiency in bean plants (Phaseolus vulgaris L.). Plant Soil 2004, 267, 41–49. [Google Scholar] [CrossRef]
- Sakamoto, M.; Komatsu, Y.; Suzuki, T. Nutrient deficiency affects the growth and nitrate concentration of hydroponic radish. Horticulturae 2021, 7, 525. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Dong, C.; Fu, Y.; Liu, C.; Liu, H. Low light intensity effects on the growth, photosynthetic characteristics, antioxidant capacity, yield and quality of wheat (Triticum aestivum L.) at different growth stages in BLSS. Adv. Space Res. 2014, 53, 1557–1566. [Google Scholar] [CrossRef]
- Wu, X.; Khan, R.; Gao, H.; Liu, H.; Zhang, J.; Ma, X. Low light alters the photosynthesis process in cigar tobacco via modulation of the chlorophyll content, chlorophyll fluorescence, and gene expression. Agriculture 2021, 11, 755. [Google Scholar] [CrossRef]
- Yamuangmorn, S.; Jumrus, S.; Jamjod, S.; Sringarm, K.; Arjin, C.; Prom-u-thai, C. Responses of purple rice variety to light intensities and soil zinc application on plant growth, yield and bioactive compounds synthesis. J. Cereal Sci. 2022, 106, 103495. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.; Ghasemzadeh, A.; Ibrahim, M.H. Light intensity effects on production and antioxidant activity of flavonoids and phenolic compounds in leaves, stems and roots of three varieties of Labisia pumila Benth. Aust. J. Crop Sci. 2013, 7, 1016–1023. [Google Scholar]
- Kumari, R.; Singh, S.; Agrawal, S.B. Effects of supplemental ultraviolet-B radiation on growth and physiology of Acorus calamus L. (sweet flag). Acta Biol. Crac. Ser. Bot. 2009, 51, 19–27. [Google Scholar]
- Pennisi, G.; Orsini, F.; Landolfo, M.; Pistillo, A.; Crepaldi, A.; Nicola, S.; Fernández, J.A.; Marcelis, L.F.M.; Gainquinto, G. Optimal photoperiod for indoor cultivation of leafy vegetables and herbs. Eur. J. Hortic. Sci. 2020, 85, 329–338. [Google Scholar] [CrossRef]
- Nawae, W.; Yoocha, T.; Narong, N.; Paemanee, A.; Ketngamkum, Y.; Romyanon, K.; Toojinda, T.; Tangphatsornruang, S.; Pootakham, W. Transcriptome sequencing revealed the influence of blue light on the expression levels of light-stress response genes in Centella asiatica. PLoS ONE 2021, 16, e0260468. [Google Scholar] [CrossRef] [PubMed]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.A.; Taulavuori, E.; Julkunen-Tiitto, R. Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).