Obtaining Spatial Variations in Cabernet Sauvignon (Vitis vinifera L.) Wine Flavonoid Composition and Aromatic Profiles by Studying Long-Term Plant Water Status in Hyper-Arid Seasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site, Plant Materials, and Weather Information
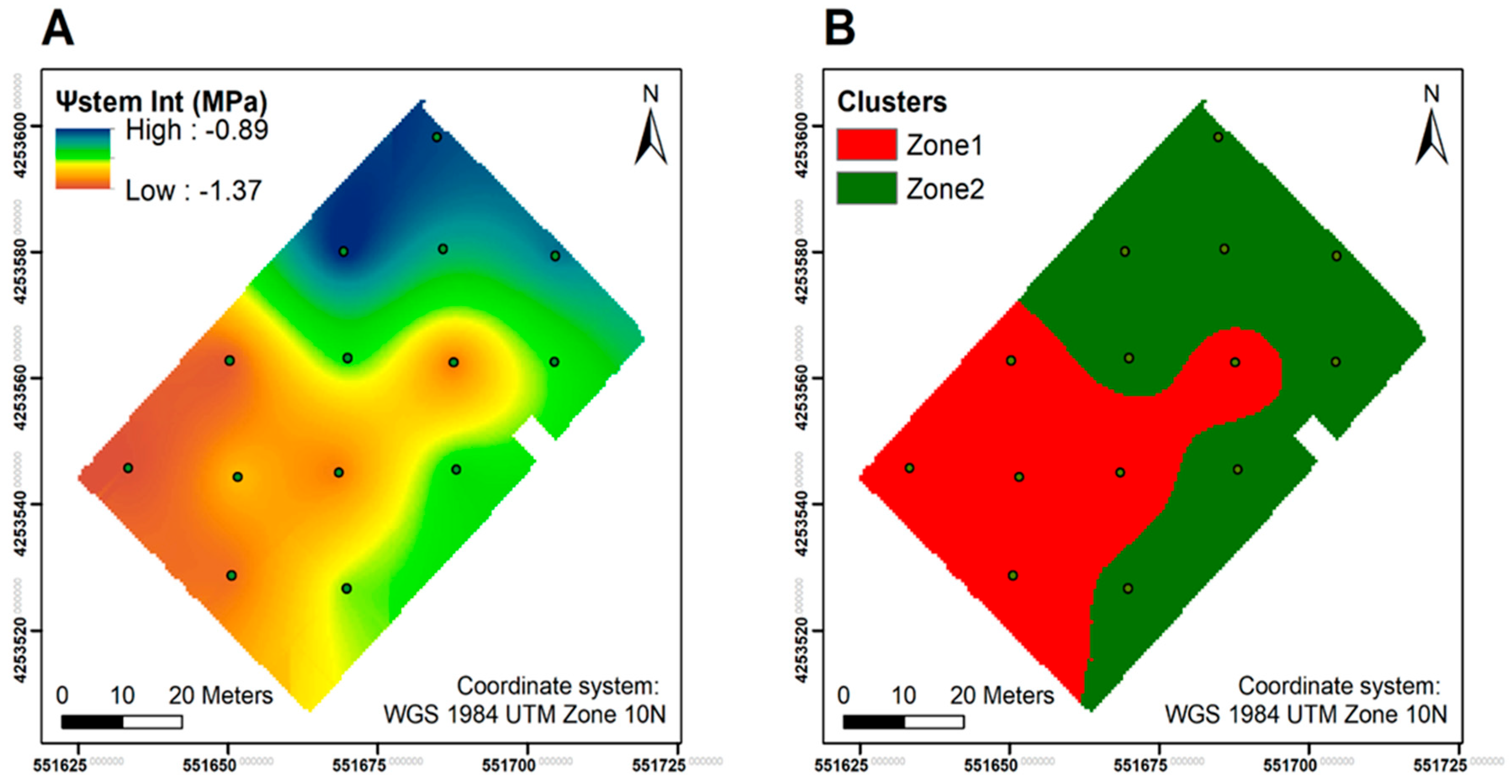
2.2. Experimental Design and Vineyard Delineation
2.3. Berry Sampling and Berry Primary Metabolite Assessment at Harvest
2.4. Extraction of Berry Skin Flavonoids at Harvest
2.5. Winemaking Procedures
2.6. Berry Skin and Wine Chemical Composition Assessment
2.7. Chemicals
2.8. Statistical Analysis
3. Results
3.1. Weather at Experiment Site
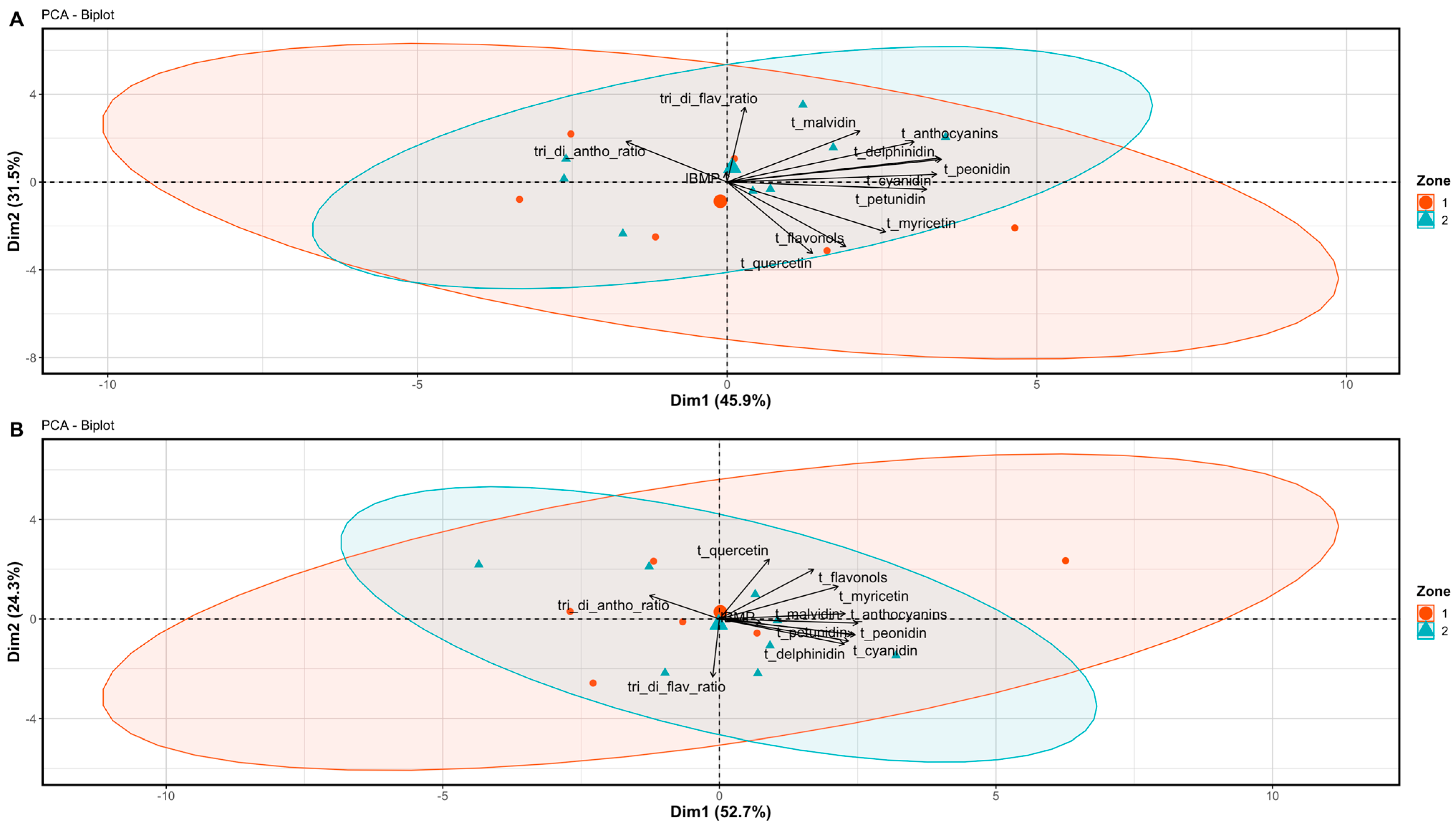
3.2. Berry and Wine Chemical Profiles
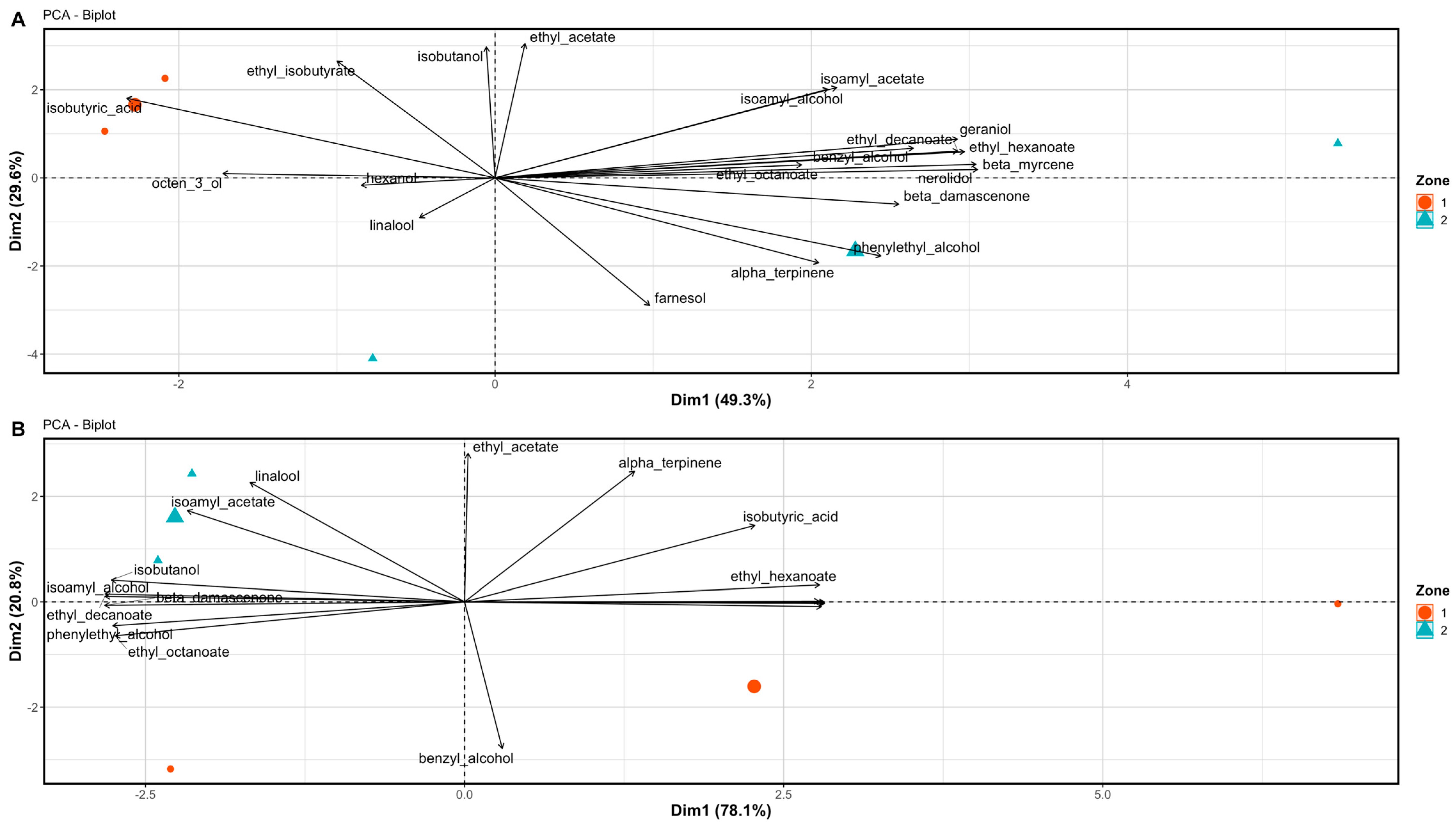
3.3. Wine Aromatic Profiles
4. Discussion
4.1. The Potential of Differential Harvest in Managing Spatial Variability in Plant Physiology
4.2. The Potential of Differential Harvest in Managing Spatial Variability in Berry and Wine Chemistry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 2003, 9, 110–121. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Alonso-Alonso, O.; Gallo-Montesdeoca, A.; Díaz-Romero, C.; Darias-Martín, J. Influence of copigmentation and phenolic composition on wine color. J. Food Sci. Technol. 2016, 53, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Diago, A.; Dizy, M.; Fernández-Zurbano, P. Taste and mouthfeel properties of red wines proanthocyanidins and their relation to the chemical composition. J. Agric. Food Chem. 2013, 61, 8861–8870. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Hilbert, G.; Luquin, J.; Goicoechea, N.; Antolín, M.C. Flavonoid and amino acid profiling on Vitis vinifera L. cv Tempranillo subjected to deficit irrigation under elevated temperatures. J. Food Compos. Anal. 2017, 62, 51–62. [Google Scholar] [CrossRef]
- Torres, N.; Yu, R.; Martinez-Luscher, J.; Girardello, R.C.; Kostaki, E.; Oberholster, A.; Kaan Kurtural, S. Shifts in the phenolic composition and aromatic profiles of Cabernet Sauvignon (Vitis vinifera L.) wines are driven by different irrigation amounts in a hot climate. Food Chem. 2022, 371, 131163. [Google Scholar] [CrossRef]
- Gambetta, G.; Kurtural, S.K. Global warming and wine quality: Are we close to the tipping point? OENO One 2021, 55, 353–361. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef]
- Picard, M.; Van Leeuwen, C.; Guyon, F.; Gaillard, L.; De Revel, G.; Marchand, S. Vine water deficit impacts aging bouquet in fine red Bordeaux wine. Front. Chem. 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Du, X.; Shellie, K.; Ross, C.; Qian, M.C. Volatile compounds and sensory attributes of wine from cv. Merlot (Vitis vinifera L.) grown under differential levels of water deficit with or without a kaolin-based, foliar reflectant particle film. J. Agric. Food Chem. 2010, 58, 12890–12898. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Liu, M.; Tu, T.; Zhao, X.; Yue, X.; Zhang, J.; Fang, Y.; Meng, J. Effect of regulated deficit irrigation on fatty acids and their derived volatiles in ‘Cabernet Sauvignon’ grapes and wines of Ningxia, China. Food Chem. 2018, 245, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Brillante, L.; Martínez-Lüscher, J.; Kurtural, S.K. Applied water and mechanical canopy management affect berry and wine phenolic and aroma composition of grapevine (Vitis vinifera L., cv. Syrah) in Central California. Sci. Hortic. 2018, 227, 261–271. [Google Scholar] [CrossRef]
- Arnó, J.; Rosell, J.R.; Blanco, R.; Ramos, M.C.; Martínez-Casasnovas, J.A. Spatial variability in grape yield and quality influenced by soil and crop nutrition characteristics. Precis. Agric. 2012, 13, 393–410. [Google Scholar] [CrossRef]
- Trought, M.C.T.; Bramley, R.G.V. Vineyard variability in Marlborough, New Zealand: Characterising spatial and temporal changes in fruit composition and juice quality in the vineyard. Aust. J. Grape Wine Res. 2011, 17, 79–89. [Google Scholar] [CrossRef]
- Yu, R.; Brillante, L.; Martínez-Lüscher, J.; Kurtural, S.K. Spatial variability of soil and plant water status and their cascading effects on grapevine physiology are linked to berry and wine chemistry. Front. Plant Sci. 2020, 11, 790. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.P.; Balda, P.; Zorer, R.; Meggio, F.; Morales, F.; Tardáguila, J. Assessment of vineyard water status variability by thermal and multispectral imagery using an unmanned aerial vehicle (UAV). Irrig. Sci. 2012, 30, 511–522. [Google Scholar] [CrossRef]
- Romero, M.; Luo, Y.; Su, B.; Fuentes, S. Vineyard water status estimation using multispectral imagery from an UAV platform and machine learning algorithms for irrigation scheduling management. Comput. Electron. Agric. 2018, 147, 109–117. [Google Scholar] [CrossRef]
- Brillante, L.; Martínez-Lüscher, J.; Yu, R.; Plank, C.M.; Sanchez, L.; Bates, T.L.; Brenneman, C.; Oberholster, A.; Kurtural, S.K. Assessing spatial variability of grape skin flavonoids at the vineyard scale based on plant water status mapping. J. Agric. Food Chem. 2017, 65, 5255–5265. [Google Scholar] [CrossRef]
- Williams, L.E.; Ayars, J.E. Grapevine water use and the crop coefficient are linear functions of the shaded area measured beneath the canopy. Agric. For. Meteorol. 2005, 132, 201–211. [Google Scholar] [CrossRef]
- Yu, R.; Kurtural, S.K. Proximal sensing of soil electrical conductivity provides a link to soil-plant water relationships and supports the identification of plant water status zones in vineyards. Front. Plant Sci. 2020, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.J. Water stress integral—A link between short-term stress and long-term growth. Tree Physiol. 1988, 4, 315–323. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol profile is a reliable indicator to assess canopy architecture and the exposure of red wine grapes to solar radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.M.; Thorngate, J.H.; Matthews, M.A.; Guinard, J.-X.; Ebeler, S.E. Yield effects on 2-methoxy-3-isobutylpyrazine concentration in Cabernet Sauvignon using a solid phase microextraction gas chromatography/mass spectrometry method. J. Agric. Food Chem. 2004, 52, 5431–5435. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Doyle, C.L.; Matthews, M.A.; Williams, L.E.; Ebeler, S.E. 2-Methoxy-3-isobutylpyrazine in grape berries and its dependence on genotype. Phytochemistry 2010, 71, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Cauduro Girardello, R.; Rich, V.; Smith, R.J.; Brenneman, C.; Heymann, H.; Oberholster, A. The impact of grapevine red blotch disease on Vitis vinifera L. Chardonnay grape and wine composition and sensory attributes over three seasons. J. Sci. Food Agric. 2020, 100, 1436–1447. [Google Scholar] [CrossRef]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A.; Charrad, M.M. Package ‘nbclust’. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar]
- Team, R.C.; Team, M.R.C.; Suggests, M.; Matrix, S. Package “Stats”. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Kassambara, A.; Mundt, F. Package ‘factoextra’. In Extract and Visualize the Results of Multivariate Data Analyses; The R Foundation: Indianapolis, IN, USA, 2017; p. 76. [Google Scholar]
- Cillis, D.; Pezzuolo, A.; Gasparini, F.; Marinello, F.; Sartori, L. Differential harvesting strategy: Technical and economic feasibility. In Proceedings of the 6th International Conference on Trends in Agricultural Engineering, Prague, Czech Republic, 7–9 September 2016. [Google Scholar]
- Ferrer, M.; Echeverría, G.; Pereyra, G.; Gonzalez-Neves, G.; Pan, D.; Mirás-Avalos, J.M. Mapping vineyard vigor using airborne remote sensing: Relations with yield, berry composition and sanitary status under humid climate conditions. Precis. Agric. 2020, 21, 178–197. [Google Scholar] [CrossRef]
- Campos, J.; Llop, J.; Gallart, M.; García-Ruiz, F.; Gras, A.; Salcedo, R.; Gil, E. Development of canopy vigour maps using UAV for site-specific management during vineyard spraying process. Precis. Agric. 2019, 20, 1136–1156. [Google Scholar] [CrossRef]
- Bramley, R.G.V.; Ouzman, J.; Boss, P.K. Variation in vine vigour, grape yield and vineyard soils and topography as indicators of variation in the chemical composition of grapes, wine and wine sensory attributes. Aust. J. Grape Wine Res. 2011, 17, 217–229. [Google Scholar] [CrossRef]
- Satorra, J.A.; Casasnovas, J.A.M.; Ribes-Dasi, M.; Polo, J.R.R. Clustering of grape yield maps to delineate site-specific management zones. Span. J. Agric. Res. 2011, 3, 721–729. [Google Scholar]
- Morari, F.; Castrignanò, A.; Pagliarin, C. Application of multivariate geostatistics in delineating management zones within a gravelly vineyard using geo-electrical sensors. Comput. Electron. Agric. 2009, 68, 97–107. [Google Scholar] [CrossRef]
- González-Fernández, A.B.; Rodríguez-Pérez, J.R.; Ablanedo, E.S.; Ordoñez, C. Vineyard zone delineation by cluster classification based on annual grape and vine characteristics. Precis. Agric. 2017, 18, 525–573. [Google Scholar] [CrossRef]
- Yu, R.; Brillante, L.; Torres, N.; Kurtural, S.K. Proximal Sensing of Vineyard Soil and Canopy Vegetation Provide Direction in Identifying Vineyard Spatial Variability in Plant Physiology and Berry Chemistry. OENO One 2021, 55, 315–333. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Torres, N.; Yu, R.; Martínez-Lüscher, J.; Kostaki, E.; Kurtural, S.K. Application of Fractions of Crop Evapotranspiration Affects Carbon Partitioning of Grapevine Differentially in a Hot Climate. Front. Plant Sci. 2021, 12, 75. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; De Freitas, V.; Zamora, F. Influence of the heterogeneity of grape phenolic maturity on wine composition and quality. Food Chem. 2011, 124, 767–774. [Google Scholar] [CrossRef]
- Tisseyre, B.; Ojeda, H.; Carillo, H.; Deis, L.; Heywang, M. Precision viticulture and water status: Mapping the predawn water potential to define within vineyard zones. In Proceedings of the 7 Fruit, Nut and Vegetable Production Engineering Symposium, Montpellier, France, 12–16 September 2005. [Google Scholar]
- Acevedo-Opazo, C.; Tisseyre, B.; Guillaume, S.; Ojeda, H. The potential of high spatial resolution information to define within-vineyard zones related to vine water status. Precis. Agric. 2008, 9, 285–302. [Google Scholar] [CrossRef]
- Yu, R.; Zaccaria, D.; Kisekka, I.; Kurtural, S.K. Soil apparent electrical conductivity and must carbon isotope ratio provide indication of plant water status in wine grape vineyards. Precis. Agric. 2021, 22, 1333–1352. [Google Scholar] [CrossRef]
- Yu, R.; Fidelibus, M.W.; Kennedy, J.A.; Kurtural, S.K. Precipitation before Flowering Determined Effectiveness of Leaf Removal Timing and Irrigation on Wine Composition of Merlot Grapevine. Plants 2021, 10, 1865. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, O.; Brunetti, C.; Egipto, R.; Pinheiro, C.; Genebra, T.; Gori, A.; Lopes, C.M.; Tattini, M.; Chaves, M.M. Grape ripening is regulated by deficit irrigation/elevated temperatures according to cluster position in the canopy. Front. Plant Sci. 2016, 7, 1640. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Fernández-Fernández, J.I.; Martinez-Cutillas, A. Physiological thresholds for efficient regulated deficit-irrigation management in winegrapes grown under semiarid conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Intrigliolo, D.S. Grape Composition under Abiotic Constrains: Water Stress and Salinity. Front. Plant Sci. 2017, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Cook, M.G.; Yacco, R.S.; Watrelot, A.A.; Gambetta, G.; Kennedy, J.A.; Kurtural, S.K. Effects of Leaf Removal and Applied Water on Flavonoid Accumulation in Grapevine (Vitis vinifera L. cv. Merlot) Berry in a Hot Climate. J. Agric. Food Chem. 2016, 64, 8118–8127. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Plank, C.M.; Brillante, L.; Cooper, M.L.; Smith, R.J.; Al-Rwahnih, M.; Yu, R.; Oberholster, A.; Girardello, R.; Kurtural, S.K. Grapevine Red Blotch Virus May Reduce Carbon Translocation Leading to Impaired Grape Berry Ripening. J. Agric. Food Chem. 2019, 67, 2437–2448. [Google Scholar] [CrossRef]
- Bindon, K.A.; Madani, S.H.; Pendleton, P.; Smith, P.A.; Kennedy, J.A. Factors affecting skin tannin extractability in ripening grapes. J. Agric. Food Chem. 2014, 62, 1130–1141. [Google Scholar] [CrossRef]
- Zsófi, Z.; Villangó, S.; Pálfi, Z.; Tóth, E.; Bálo, B. Texture characteristics of the grape berry skin and seed (Vitis vinifera L. cv. Kékfrankos) under postveraison water deficit. Sci. Hortic. 2014, 172, 176–182. [Google Scholar] [CrossRef]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J. Agric. Food Chem. 2006, 54, 5077–5086. [Google Scholar] [CrossRef]
- Savoi, S.; Herrera, J.C.; Carlin, S.; Lotti, C.; Bucchetti, B.; Peterlunger, E.; Castellarin, S.D.; Mattivi, F. From grape berries to wines: Drought impacts on key secondary metabolites. OENO One 2020, 54, 569–582. [Google Scholar] [CrossRef]
| Year | Month | Precipitation (mm) | GDD (°C) | Air Max Temperature (°C) | Air Min Temperature (°C) |
|---|---|---|---|---|---|
| 2019 | Jan | 248.5 | - | 15.9 | 4.7 |
| Feb | 422.2 | - | 12.9 | 2.7 | |
| Mar | 145.6 | 48.5 | 17.5 | 4.9 | |
| Apr | 12.5 | 229.5 | 23.3 | 8.8 | |
| May | 88.9 | 397.0 | 22.4 | 8.4 | |
| Jun | 0.0 | 702.7 | 29.2 | 11.2 | |
| Jul | 0.2 | 1029.2 | 29.9 | 11.1 | |
| Aug | 0.0 | 1393.7 | 31.2 | 12.3 | |
| Sep b | 1.5 | 1681.5 | 29.4 | 9.7 | |
| Oct | 0.2 | 1859.0 | 26.6 | 4.9 | |
| 2020 | Jan | 58.5 | - | 15.4 | 3.5 |
| Feb | 1.0 | - | 20.6 | 3.7 | |
| Mar | 29.8 | 31.6 | 17.6 | 4.4 | |
| Apr | 25.9 | 182.1 | 23.0 | 7.1 | |
| May | 26.1 | 414.8 | 26.2 | 8.8 | |
| Jun | 0.2 | 713.6 | 29.5 | 10.4 | |
| Jul | 0.2 | 1027.1 | 30.2 | 10.1 | |
| Aug | 1.6 | 1388.9 | 31.8 | 12.3 | |
| Sep b | 0.3 | 1726.3 | 31.4 | 11.1 | |
| Oct | 0.3 | 1903.8 | 29.7 | 8.1 |
| 2019 | 2020 | Year | Zoning | Year × Zoning | |||||
|---|---|---|---|---|---|---|---|---|---|
| Zone 1 ± SD b | Zone 2 ± SD | Pr (>F) | Zone 1 ± SD | Zone 2 ± SD | Pr (>F) | ||||
| Glucoside | |||||||||
| Delphinidin-3-glucoside | 21.61 ± 3.41 | 22.94 ± 3.86 | ns | 21.81 ± 3.31 | 22.83 ± 3.90 | ns | ns | ns | ns |
| Cyanidin-3-glucoside | 1.82 ± 0.45 | 1.88 ± 0.45 | ns | 1.42 ± 0.28 | 1.79 ± 0.52 | ns | ns | ns | ns |
| Petunidin-3-glucoside | 15.11 ± 2.03 | 15.98 ± 2.18 | ns | 16.89 ± 2.43 | 17.03 ± 2.49 | ns | ns | ns | ns |
| Peonidin-3-glucoside | 9.30 ± 1.16 | 9.57 ± 1.35 | ns | 9.09 ± 1.56 | 9.91 ± 1.59 | ns | ns | ns | ns |
| Malvidin-3-glucoside | 91.61 ± 5.97 | 98.73 ± 8.32 | ns | 118.02 ± 12.41 | 113.25 ± 9.65 | ns | <0.001 *** | ns | ns |
| Total glucosides | 139.46 ± 12.34 | 149.11 ± 15.21 | ns | 167.23 ± 19.02 | 164.81 ± 17.36 | ns | 0.002 ** | ns | ns |
| Acetylated | |||||||||
| Delphinidin-3-acetyl-glucoside | 4.22 ± 0.69 | 4.24 ± 0.66 | ns | 4.52 ± 0.82 | 4.40 ± 0.80 | ns | ns | ns | ns |
| Cyanidin-3-acetyl-glucoside | 0.51 ± 0.14 | 0.45 ± 0.17 | ns | 1.15 ± 0.14 | 1.14 ± 0.11 | ns | <0.001 *** | ns | ns |
| Petunidin-3-acetyl-glucoside | 4.64 ± 0.59 | 4.66 ± 0.54 | ns | 5.27 ± 0.79 | 4.98 ± 0.73 | ns | ns | ns | ns |
| Peonidin-3-acetyl-glucoside | 3.10 ± 0.17 a | 2.84 ± 0.23 b | 0.034 * | 3.10 ± 0.63 | 3.07 ± 0.50 | ns | ns | ns | ns |
| Malvidin-3-acetyl-glucoside | 48.18 ± 3.69 | 48.37 ± 2.99 | ns | 64.55 ± 6.70 | 57.53 ± 6.23 | ns | <0.001 *** | ns | ns |
| Total acetylated | 60.70 ± 4.57 | 60.56 ± 3.67 | ns | 78.58 ± 8.65 | 71.12 ± 7.77 | ns | <0.001 *** | ns | ns |
| Coumarylated | |||||||||
| Delphinidin-3-p-coumaroyl-glucoside | 1.76 ± 0.30 | 1.90 ± 0.29 | ns | 1.97 ± 0.44 | 1.78 ± 0.46 | ns | ns | ns | ns |
| Cyanidin-3-p-coumaroyl-glucoside | 0.43 ± 0.06 | 0.44 ± 0.05 | ns | 0.98 ± 0.29 | 0.81 ± 0.20 | ns | <0.001 *** | ns | ns |
| Petunidin-3-p-coumaroyl-glucoside | 1.90 ± 0.24 | 1.94 ± 0.27 | ns | 2.16 ± 0.30 | 2.05 ± 0.20 | ns | ns | ns | ns |
| Peonidin-3-p-coumaroyl-glucoside | 2.96 ± 0.34 | 2.87 ± 0.19 | ns | 2.82 ± 0.36 | 2.91 ± 0.23 | ns | ns | ns | ns |
| Malvidin-3-p-coumaroyl-glucoside | 22.43 ± 1.45 | 23.45 ± 1.68 | ns | 27.99 ± 2.67 | 26.35 ± 1.75 | ns | <0.001 *** | ns | ns |
| Total coumarylated | 29.48 ± 2.05 | 30.59 ± 2.23 | ns | 35.89 ± 2.77 | 33.90 ± 2.47 | ns | <0.001 *** | ns | ns |
| 3′5′-hydroxylated | 18.13 ± 2.03 | 18.05 ± 2.13 | ns | 18.55 ± 3.07 | 19.63 ± 2.78 | ns | ns | ns | ns |
| 3′4′5′-hydroxylated | 211.51 ± 15.71 | 222.20 ± 17.82 | ns | 263.16 ± 27.97 | 250.20 ± 23.09 | ns | <0.0001 *** | ns | ns |
| 3′4′5′/3′5′-hydroxylated ratio | 11.72 ± 0.76 | 12.41 ± 1.28 | ns | 14.30 ± 0.87 a | 12.86 ± 1.18 b | 0.028 * | 0.003 ** | 0.017 * | ns |
| Total anthocyanins | 229.65 ± 17.43 | 240.25 ± 19.03 | ns | 281.70 ± 30.96 | 269.83 ± 25.25 | ns | <0.0001 *** | ns | ns |
| 2019 | 2020 | Year | Zoning | |||||
|---|---|---|---|---|---|---|---|---|
| Zone 1 ± SD b | Zone 2 ± SD | Pr (>F) | Zone 1 ± SD | Zone 2 ± SD | Pr (>F) | |||
| Myricetin-3-galactoside | 2.95 ± 0.56 | 2.77 ± 0.20 | ns | 2.74 ± 0.34 | 2.71 ± 0.17 | ns | ns | ns |
| Myricetin-3-glucoside | 0.95 ± 0.17 | 0.90 ± 0.07 | ns | 0.79 ± 0.12 | 0.74 ± 0.07 | ns | <0.001 *** | ns |
| Quercetin-3-galactoside | 0.45 ± 0.18 | 0.38 ± 0.08 | ns | 0.48 ± 0.14 | 0.49 ± 0.07 | ns | ns | ns |
| Quercetin-3-glucoside | 2.80 ± 1.01 | 2.41 ± 0.54 | ns | 2.32 ± 0.58 | 2.36 ± 0.38 | ns | ns | ns |
| Laricetin-3-glucoside | 0.68 ± 0.10 | 0.62 ± 0.09 | ns | 0.71 ± 0.12 | 0.66 ± 0.04 | ns | ns | ns |
| Kaempferol-3-glucoside | 0.69 ± 0.07 | 0.65 ± 0.08 | ns | 0.51 ± 0.15 | 0.47 ± 0.11 | ns | <0.001 *** | ns |
| Isorhamnetin-3-glucoside | 1.29 ± 0.09 | 1.30 ± 0.06 | ns | 0.58 ± 0.20 | 0.51 ± 0.07 | ns | <0.001 *** | ns |
| Syringetin-3-glucoside | 0.64 ± 0.04 | 0.67 ± 0.05 | ns | 0.79 ± 0.10 | 0.70 ± 0.06 | ns | <0.001 *** | ns |
| 3′5′-hydroxylated | 4.54 ± 1.15 | 4.09 ± 0.61 | ns | 3.38 ± 0.90 | 3.36 ± 0.52 | ns | 0.005 ** | ns |
| 3′4′5′-hydroxylated | 5.22 ± 0.84 | 4.96 ± 0.30 | ns | 5.03 ± 0.66 | 4.81 ± 0.29 | ns | ns | ns |
| 3′4′5′/3′5′-hydroxylated ratio | 1.18 ± 0.15 | 1.23 ± 0.15 | ns | 1.53 ± 0.22 | 1.47 ± 0.25 | ns | <0.001 *** | ns |
| Total flavonols | 10.45 ± 1.97 | 9.70 ± 0.84 | ns | 9.13 ± 1.60 | 8.83 ± 0.56 | ns | 0.033 * | ns |
| 2019 | 2020 | Year | Zoning | Year × Zoning | |||||
|---|---|---|---|---|---|---|---|---|---|
| Zone 1 ± SD b | Zone 2 ± SD | Pr (>F) | Zone 1 ± SD | Zone 2 ± SD | Pr (>F) | ||||
| Glucoside | |||||||||
| Delphinidin-3-glucoside | 7.41 ± 2.46 b | 14.91 ± 2.56 a | <0.001 *** | 21.51 ± 0.87 a | 19.85 ± 0.36 b | 0.001 ** | <0.001 *** | <0.001 *** | <0.001 *** |
| Cyanidin-3-glucoside | 0.62 ± 0.10 b | 0.90 ± 0.11 a | <0.001 *** | 1.35 ± 0.22 | 1.16 ± 0.01 | ns | <0.001 *** | ns | <0.001 *** |
| Petunidin-3-glucoside | 16.54 ± 2.81 b | 8.90 ± 2.91 a | <0.001 *** | 27.99 ± 1.43 a | 24.43 ± 0.21 b | <0.001 *** | <0.001 *** | 0.030 * | <0.001 *** |
| Peonidin-3-glucoside | 4.33 ± 1.31 b | 7.48 ± 0.77 a | <0.001 *** | 15.05 ± 1.36 | 13.41 ± 1.27 | ns | <0.001 *** | ns | <0.001 *** |
| Malvidin-3-glucoside | 107.90 ± 31.80 b | 184.00 ± 23.99 a | <0.001 *** | 421.82 ± 13.45 a | 352.50 ± 1.12 b | <0.001 *** | <0.001 *** | ns | <0.001 *** |
| Total glucosides | 129.16 ± 38.54 b | 223.82 ± 30.17 a | <0.001 *** | 487.73 ± 17.34 a | 411.35 ± 0.72 b | <0.001 *** | <0.001 *** | ns | <0.001 *** |
| Acetylated | |||||||||
| Delphinidin-3-acetyl-glucoside | 5.81 ± 0.22 | 5.92 ± 0.62 | ns | 7.49 ± 0.42 a | 6.12 ± 0.20 b | <0.001 *** | <0.001 *** | 0.001 ** | <0.001 *** |
| Cyanidin-3-acetyl-glucoside | 1.23 ± 0.41 | 0.93 ± 0.11 | ns | 5.03 ± 0.37 a | 4.05 ± 0.03 b | <0.001 *** | <0.001 *** | <0.001 *** | 0.001 ** |
| Petunidin-3-acetyl-glucoside | 2.90 ± 1.02 b | 5.31 ± 0.75 a | <0.001 *** | 9.52 ± 0.09 a | 7.41 ± 0.08 b | <0.001 *** | <0.001 *** | ns | <0.001 *** |
| Peonidin-3-acetyl-glucoside | 0.68 ± 0.10 | 0.77 ± 0.06 | ns | 2.04 ± 0.46 | 1.57 ± 0.26 | ns | <0.001 *** | ns | 0.022 * |
| Malvidin-3-acetyl-glucoside | 44.64 ± 14.44 b | 76.84 ± 10.57 a | 0.001 ** | 192.79 ± 4.51 a | 166.39 ± 0.93 b | <0.001 *** | <0.001 *** | ns | <0.001 *** |
| Total acetylated | 55.27 ± 15.33 b | 89.77 ± 10.75 a | 0.001 ** | 216.88 ± 4.93 a | 185.54 ± 0.92 b | 0.001 ** | <0.001 *** | ns | <0.001 *** |
| Coumarylated | |||||||||
| Delphinidin-3-p-coumaroyl-glucoside | 2.04 ± 0.63 b | 3.66 ± 0.58 a | 0.001 ** | 6.36 ± 0.43 a | 5.67 ± 0.07 b | 0.002 ** | <0.001 *** | 0.029 ** | <0.001 *** |
| Cyanidin-3-p-coumaroyl-glucoside | 1.35 ± 0.16 | 1.54 ± 0.32 | ns | 2.44 ± 0.20 | 2.26 ± 0.09 | ns | <0.001 *** | ns | 0.048 * |
| Petunidin-3-p-coumaroyl-glucoside | 0.66 ± 0.21 b | 0.99 ± 0.24 a | 0.030 * | 2.40 ± 0.28 a | 2.10 ± 0.01 b | 0.030 * | <0.001 *** | ns | 0.002 ** |
| Peonidin-3-p-coumaroyl-glucoside | 0.47 ± 0.16 b | 0.96 ± 0.20 a | <0.001 *** | 2.37 ± 0.30 | 2.33 ± 0.01 | ns | <0.001 *** | 0.011 * | 0.004 ** |
| Malvidin-3-p-coumaroyl-glucoside | 7.64 ± 2.87 b | 16.01 ± 2.69 a | <0.001 *** | 46.13 ± 4.02 a | 42.12 ± 0.59 b | 0.036 * | <0.001 *** | ns | <0.001 *** |
| Total coumarylated | 12.16 ± 3.68 b | 23.14 ± 4.01 a | <0.001 *** | 59.70 ± 5.23 a | 54.49 ± 0.75 b | 0.037 * | <0.001 *** | ns | <0.001 *** |
| Total anthocyanins | 196.59 ± 57.53 b | 336.74 ± 44.92 a | <0.001 *** | 764.31 ± 27.51 a | 651.38 ± 2.38 b | <0.001 *** | <0.001 *** | ns | <0.001 *** |
| 3′5′-hydroxylated | 8.69 ± 1.38 b | 12.57 ± 1.39 a | <0.001 *** | 28.29 ± 2.18 a | 24.78 ± 1.08 b | 0.005 ** | <0.001 *** | ns | <0.001 *** |
| 3′4′5′-hydroxylated | 187.90 ± 56.18 b | 324.17 ± 43.55 a | <0.001 *** | 736.02 ± 25.33 a | 626.60 ± 1.30 b | <0.001 *** | <0.001 *** | ns | <0.001 *** |
| 3′4′5′/3′5′-hydroxylated ratio | 21.23 ± 3.25 b | 25.74 ± 0.79 a | 0.008 ** | 26.09 ± 1.11 | 25.32 ± 1.05 | ns | 0.008 ** | 0.022 * | 0.002 ** |
| 2019 | 2020 | Year | Zoning | Year × Zoning | |||||
|---|---|---|---|---|---|---|---|---|---|
| Zone 1 ± SD b | Zone 2 ± SD | Pr (>F) | Zone 1 ± SD | Zone 2 ± SD | Pr (>F) | ||||
| Myricetin-3-galactoside | 1.80 ± 0.13 b | 2.07 ± 0.12 a | 0.003 ** | 3.02 ± 0.01 a | 2.20 ± 0.07 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| Myricetin-3-glucoside | 10.41 ± 0.12 b | 11.77 ± 0.38 a | <0.001 *** | 21.20 ± 0.28 a | 16.16 ± 0.28 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| Quercetin-3-galactoside | 0.82 ± 0.05 | 0.81 ± 0.05 | ns | 0.80 ± 0.03 a | 0.60 ± 0.03 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| Quercetin-3-glucoside | 6.42 ± 0.26 a | 4.20 ± 0.06 b | <0.001 *** | 7.31 ± 0.15 a | 6.01 ± 0.42 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| Laricetin-3-glucoside | 2.99 ± 0.08 | 2.94 ± 0.03 | ns | 3.83 ± 0.10 a | 2.95 ± 0.06 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| Kaempferol-3-glucoside | 0.50 ± 0.03 a | 0.35 ± 0.11 b | 0.011 * | 0.34 ± 0.03 a | 0.27 ± 0.04 b | 0.004 ** | <0.001 *** | <0.001 *** | ns |
| Isorhamnetin-3-glucoside | 3.48 ± 0.30 | 3.45 ± 0.93 | ns | 3.51 ± 0.44 a | 2.03 ± 0.13 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| Syringetin-3-glucoside | 4.68 ± 0.13 | 4.59 ± 0.28 | ns | 7.24 ± 0.35 a | 5.94 ± 0.10 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| 3′5′-hydroxylated | 10.72 ± 0.58 a | 8.46 ± 0.93 b | <0.001 *** | 11.62 ± 0.55 a | 8.64 ± 0.32 b | <0.001 *** | ns | <0.001 *** | ns |
| 3′4′5′-hydroxylated | 19.88 ± 0.25 b | 21.37 ± 0.42 a | <0.001 *** | 35.29 ± 0.73 a | 27.25 ± 0.52 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| 3′4′5′/3′5′-hydroxylated ratio | 1.86 ± 0.08 b | 2.55 ± 0.27 a | <0.001 *** | 3.05 ± 0.21 | 3.16 ± 0.06 | ns | <0.001 *** | <0.001 *** | <0.001 *** |
| Total flavonols | 31.09 ± 0.82 | 30.18 ± 0.98 | ns | 47.25 ± 0.21 a | 36.15 ± 0.88 b | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| 2019 | 2020 | Year | Zoning | Year × Zoning | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zone 1 ± SD b | Zone 2 ± SD | Pr (>F) | Zone 1 ± SD | Zone 2 ± SD | Pr (>F) | |||||
| Extension subunits | EGC c | 113.36 ± 59.63 | 352.75 ± 134.59 | ns | 212.44 ± 40.81 b | 281.84 ± 6.16 a | 0.002 ** | ns | 0.014 * | ns |
| C c | 40.78 ± 21.46 | 55.12 ± 7.47 | ns | 66.67 ± 4.63 b | 73.55 ± 5.87 a | 0.048 * | <0.001 *** | ns | ns | |
| EC c | 153.43 ± 84.15 b | 501.50 ± 184.02 a | 0.004 *** | 210.72 ± 11.34 b | 285.71 ± 10.37 a | <0.001 *** | ns | 0.017 * | ns | |
| ECG c | 9.73 ± 5.48 b | 18.63 ± 6.32 a | 0.033 * | 6.06 ± 1.35 | 6.06 ± 0.44 | ns | 0.002 ** | ns | ns | |
| Terminal subunits | C | 61.15 ± 38.43 | 125.73 ± 52.74 | 0.043 * | 97.02 ± 19.54 b | 136.99 ± 15.45 a | 0.003 ** | ns | 0.017 ** | ns |
| EC | 9.45 ± 5.62 | 9.90 ± 2.64 | ns | 12.79 ± 5.18 | 12.46 ± 2.39 | ns | ns | ns | ns | |
| ECG | 4.56 ± 2.71 | 4.78 ± 1.27 | ns | 6.18 ± 2.50 | 6.02 ± 1.15 | ns | ns | ns | ns | |
| Total proanthocyanidins | 467.87 ± 291.46 b | 1068.42 ± 371.27 a | 0.015 * | 611.88 ± 44.67 b | 802.63 ± 22.04 a | <0.001 *** | ns | 0.017 ** | ns | |
| mDP c | 5.27 ± 0.51 b | 7.13 ± 1.73 a | 0.042 * | 6.64 ± 0.98 b | 12.71 ± 2.67 a | <0.001 *** | <0.001 *** | <0.001 *** | 0.003 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Torres, N.; Kurtural, S.K. Obtaining Spatial Variations in Cabernet Sauvignon (Vitis vinifera L.) Wine Flavonoid Composition and Aromatic Profiles by Studying Long-Term Plant Water Status in Hyper-Arid Seasons. Horticulturae 2024, 10, 68. https://doi.org/10.3390/horticulturae10010068
Yu R, Torres N, Kurtural SK. Obtaining Spatial Variations in Cabernet Sauvignon (Vitis vinifera L.) Wine Flavonoid Composition and Aromatic Profiles by Studying Long-Term Plant Water Status in Hyper-Arid Seasons. Horticulturae. 2024; 10(1):68. https://doi.org/10.3390/horticulturae10010068
Chicago/Turabian StyleYu, Runze, Nazareth Torres, and Sahap Kaan Kurtural. 2024. "Obtaining Spatial Variations in Cabernet Sauvignon (Vitis vinifera L.) Wine Flavonoid Composition and Aromatic Profiles by Studying Long-Term Plant Water Status in Hyper-Arid Seasons" Horticulturae 10, no. 1: 68. https://doi.org/10.3390/horticulturae10010068
APA StyleYu, R., Torres, N., & Kurtural, S. K. (2024). Obtaining Spatial Variations in Cabernet Sauvignon (Vitis vinifera L.) Wine Flavonoid Composition and Aromatic Profiles by Studying Long-Term Plant Water Status in Hyper-Arid Seasons. Horticulturae, 10(1), 68. https://doi.org/10.3390/horticulturae10010068







