Abstract
Agronomic biofortification of crops is a promising approach for the accumulation of Si in plant organs and can be achieved through the application of Si-containing fertilizers in the nutrient solution (NS) using a soilless technique. In the present research, a local variety of Cucumis melo L. called Carosello and two tomato hybrids, ‘Alfa 200’ (TA) and ‘Versus’ (TV), were cultivated in a floating system with three levels of Si (0, 50, and 100 mg·L−1) in the NS with the aim to study the Si translocation/accumulation in leaves, stems, and roots of these genotypes. In general, by adding Si to the NS, Si accumulation in plants increased. Regarding Si translocation, it was found that Carosello exhibited a better translocation capacity than tomato hybrids, and Si movement from roots to shoots was very much dependent on tomato genotypes. With the highest Si content in the NS, TA had a similar Si concentration in leaves and stems, while TV showed a greater Si concentration in leaves. In conclusion, Carosello landrace is confirmed as a good Si accumulator, while the tomato is confirmed as a species with low Si accumulation capacity. Nevertheless, the effectiveness of Si biofortification in tomatoes is very much dependent on the genotype.
1. Introduction
Biofortification is a process that entails enhancing the nutritional quality of fruits and vegetables through the introduction of essential micronutrients [1], and it can be achieved using various methods, including conventional breeding, genetic engineering, and agronomic practices [2]. Moreover, non-essential but beneficial elements can also be interesting targets of agronomic biofortification. One of these is silicon (Si), due to its beneficial effects on human health [3] and its role in biotic and abiotic stress tolerance and plant physiology [4,5]. According to essentiality criteria for mineral elements [6], Si has not been accepted as an essential element for plants, with few exceptions, such as horsetail [7,8]. However, Si can be considered an ‘agronomically essential’ element that could improve the yields and quality of several crops [9,10]. Studies have shown that Si can have beneficial effects on plant health, disease resistance, and nutrient uptake [11,12,13]. Si supplementation is also proven to potentially benefit species that are known to be low accumulators or Si excluders, such as the tomato [14,15]. Although the tomato species has been considered a typical Si excluder [13], recent studies have revealed the genotypic-dependent accumulation of Si in some tomato tissues, leading to a potentiality for tomato biofortification with Si [16,17]. The scientific literature reports that Si-fed tomato plants can improve the tolerance to salinity [18,19,20,21], water stresses [22,23], and some pathogens [24,25]. In addition, it can play a role in balancing other minerals for plant nutrition and phytotoxicity [26,27]. The ability of different plant species to accumulate Si varies significantly, ranging from 0.1 to 10% of the plant dry weight [28,29]. This is partially attributed to differences in the presence, density, and distribution of the Si protein transportation system, from the environment to roots and from roots to other tissues [29,30]. Silicon accumulation is more commonly observed in monocotyledonous plants, although it is not exclusive to them [31,32]. Certain plant families, such as Poaceae, Equisetaceae, and Cyperaceae, exhibit high Si accumulation, with Si comprising around 4% of their dry weight [33]. The Cucurbitaceae and Commelinaceae families show intermediate Si accumulation, ranging from 2 to 4% of Si. In contrast, most other plant species, such as the tomato, demonstrate poor Si accumulation [32,33].
The Cucurbitaceae family, which includes vegetable crops like the cucumber (Cucumis sativus L.), zucchini (Cucurbita pepo L.), and melon (C. melo), are known to accumulate Si to a greater extent compared to other dicotyledonous crops. In addition, this family, and particularly cucumber, has been studied as a model for Si transporters in dicots [34], and great attention has been given to the positive effect of Si on abiotic stresses and combating powdery mildew in cucumber crops [35,36,37,38,39,40]. For example, the study conducted by Liang et al. [41] investigated the effects of Si biofortification on cucumber plants. The researchers found that Si supplementation significantly increased Si concentration in cucumber fruits and enhanced their resistance to powdery mildew, a common fungal disease.
Local varieties of cucurbits can also benefit from Si. The Mediterranean melon landrace Carosello is typically grown in the Puglia region (Southern Italy), and its fruits are picked and consumed while still unripe, as an alternative to cucumbers due to their quality [42,43]. Although Carosello is generally cultivated using traditional techniques, A significant interest in soilless cultivation has been recently growing among local farmers, due to the growing demand from consumers and encouraging research results on the application of soilless systems [42,43]. Furthermore, by reason of the long period of continuous progressive harvesting and the lack of specific registered chemical ingredients, the management of the key disease cucumber powdery mildew is critical. Carosello was successfully enriched by Si, and its accumulation in leaves reduced the incidence of powdery mildew and improved fruit yield [40]. The beneficial effect of Si on powdery mildew and other fungal pathogens is also reported for other melon varieties [44,45,46,47].
In the last decade, numerous scientific research papers have been published concerning the potential of augmenting micronutrients within plants by employing soilless methodologies as a means of biofortification [48,49,50,51,52,53]. Within this framework, the floating system enables the effective monitoring of Si uptake by plants through the nutrient solution [54], and over the last decade it has become popular due to the simplicity of nutrient and water management. In comparison to most of the other soilless systems, this water culture system enables the increase of plant density, the uniformity of growth, and the automation of agricultural practices. For these reasons, the floating system is particularly appreciated for plant physiology studies, and is the most suitable hydroponic system for short-cycle cultivation, such as horticultural seedling production [55,56,57].
Starting with (i) the study conducted by Buttaro et al. [40], who demonstrated the good translocation capacity of Si in Carosello, studying the incidence of powdery mildew in plants fertigated with Si-containing NS is crucial given, and (ii) the fact that the tomato is one of the main vegetables cultivated in soilless greenhouses worldwide, with a low capacity of Si translocation/accumulation [58]. The aim of this research is to study the Si accumulation capacity of two tomato genotypes in plant organs with three doses of Si supplemented to NS, using Carosello as a ‘control species’.
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
The research activity was carried out from October to December 2021 in an unheated plastic (polymethachrylate) greenhouse (≈700 m2) at the Experimental Farm—‘La Noria’—of the Institute of Sciences of Food Production (ISPA-CNR) in Mola di Bari (BA), Southern Italy (41°03′ N, 17°04′ E; 24 m a.s.l.).
Two tomato (Solanum lycopersicum L.) hybrids (Esasem), called ‘Alfa 200’ (TA) and ‘Versus’ (TV), and a local variety of unripe melon (Cucumis melo L.) called ‘Carosello striato’ [42], were sown (15 October 2021) in cell trays, with 228 cells filled with peat (Brill 3 Special, Brill Substrates, Georgsdorf, Germany). To reduce seedlings overlapping and competing, only half of the cells were sown, in alternating rows (114 cells). The seedlings placed at the edge of the tray were not used for the purposes of the test, but were considered as ‘edge seedlings’. To facilitate germination, the trays were placed at a constant temperature of 20 °C and covered with a black film to maintain the humidity around the seeds. Ten days after sowing (DAS), the hypocotyl of the three genotypes emerged, the black plastic film was removed, the trays with two-leaved seedlings were moved to a floating hydroponic system, and treatment was started (Figure 1). This consisted of the use of three genotypes and three levels (0, 50, 100 mg·L−1) of Si in the nutrient solution (NS), supplied through the root. The NS was composed of N (75 mg L−1), P (25 mg L−1), K (150 mg L−1), Ca (75 mg L−1), and Mg (25 mg L−1). Regarding Si, potassium metasilicate (K2SiO3) was added to the NS at different concentrations to set up two levels of Si dosing, and a control without adding any K2SiO3. The tested concentrations of additional Si were 0, 50, and 100 mg L−1. It is noted that the natural Si concentration in NS and the control treatment was already 2 mg·L−1, as a result of traces in the fertilizer salts used. In any case, potassium, supplemented by K2SiO3, was considered in the formulation of the NS, and the additional K introduced as K2SiO3 was balanced by reducing K2SO4 in the control treatment without Si. The pH value was between 6.0–6.5, and the EC was between 1.1–1.3 mS∙cm−1 for the duration of the experiment. Every two days, for each bench, the NS was discharged and recharged to avoid anoxia in the root zone. Furthermore, NS was reintegrated according to seedling adsorption. The daily mean temperature and relative humidity of the air inside the greenhouse were 20 °C and 72%, respectively. The Daily Light Integral (DLI) had a mean value of 14.5 mol∙m−2·day−1 and consisted of natural light only.

Figure 1.
Details of the floating cultivation system: from initial set up after sowing (A,B) to seedling rooting (C) and growth (D).
2.2. Biometric Parameters
Biometric parameters were calculated at the end of the trial, 42 DAS, when the sixth leaf of the tomato seedling was longer than two centimeters and the first inflorescence of the tomato seedling was visible. The number of leaves, as well as leaf, stem, and root fresh weight (FW) and respective dry weight (DW) were measured. To measure DW, leaves, stems, and roots were separated and oven-dried at 65 °C until a constant weight was reached. To follow, the dry matter (DM) content was derived from DW and FW, and expressed as g·100 g−1 FW.
2.3. Silicon Content
To determine Si tissue concentration, samples of dried tissues were ashed in a muffle furnace (A024, Matest spa, Treviolo (BG), Italy) at 550 °C and digested with HCl (1 mol·L−1) in boiling Milli-Q water (99.5 ± 0.5 °C) for 30 min. The resulting solution was filtered, diluted, and analyzed using a spectroscopy method to determine Si content, as reported in [59]. The standards were made from a 115 mg∙L−1 Si stock solution (expressed as Si), and standard concentrations ranged from 0.72 to 5.75 mg·L−1. The quantification of Si in vegetables was determined by interpolation with a calibration curve, obtained in advance, with an R2 = 0.9993.
2.4. Experimental Design and Statistical Analyses
The experimental design used was a split-plot with three replications. Each ‘bench’ (675 × 540 × 100 mm; Kartell Labware, Noviglio (MI), Italy) represented the elementary unit, containing one tray per genotype and the NS with one of the three tested levels of Si.
All data were subjected to variance analysis. Statistical analysis was performed using the GLM (General Linear Model) procedure using SAS software (SAS Software, Cary, NC, USA, www.sas.com), and all experimental factors were considered as fixed for this purpose. According to the research objectives, the means were compared using orthogonal contrasts with one degree of tolerance. Namely, two contrasts were performed between the three plant organs: (i) shoot (leaves and stems) vs. roots, and (ii) leaves vs. stems. Two contrasts were performed between the three genotypes: (i) tomato vs. Carosello, and (ii) TA vs. TV. Lastly, for the Si concentration (‘Si’ factor), being interested in studying the relationship between the increase of Si in the NS and the accumulation of silicon in the plants, the relationship with the response variables was assessed by partitioning the sums of the squares into components that were associated with linear and quadratic terms.
3. Results and Discussion
3.1. Biometric Parameters
Generally, Carosello accumulated less DM in the leaves, stems, and roots compared to the two tomato hybrids (Table 1), and the different Si content in the NS did not influence the fresh biomass production (Table 1). Instead, by increasing the Si content in the NS, the leaves’ DM of the three tested genotypes increased linearly (Table 1). Furthermore, the Si content in the NS did not influence the number of leaves of each genotype (Table 1). Starting from the aboveground organs, the leaves’ FW did not vary between plant species; however, comparing the tomato genotypes, it was almost 17% higher in TV than TA (Table 1). The leaves’ DM content was 24.9% higher for the young tomato plants compared to Carosello (Table 1). Furthermore, on average, the leaves’ DM content linearly increased with the increasing of Si content in the NS (Table 1). Conversely, stem FW was not affected by either the genotype or the Si concentration in the NS (average value of 0.18 g·plant−1) (Table 1). Furthermore, the stem DM content was, on average, 23% more in tomato plants than in Carosello; between tomato genotypes, its value was almost 8% higher in TV than TA (Table 1). The average root FW was 0.22 g·plant−1, and tomato plants accumulated 44% more DM in roots compared to Carosello plants (Table 1). The results obtained during our research activity are in line with those obtained by Karimi and Zare [56], who indicate that a 5 mg·L−1 potassium silicate solution, added twice to the growing medium as Si pretreatment before transplantation, increased the dry mass of leaves of a melon hybrid. Furthermore, as shown in the study by Gomes et al. [60], by increasing the Si content in the NS up to 5 mg·L−1, the leaves’ DM of a landrace of C. melo L. (that is well known to be a good Si translocator) increased. Interestingly, the same trend was found for the tomato, which is considered to be a species with low or absent capacity for Si translocation.

Table 1.
Average number of leaves; leaf, stem, and root fresh weight (FW) and dry matter (DM) ± standard error of young plants of Carosello (Cucumis melo L.) and two tomato genotypes fertigated with three silicon (Si) concentrations in the nutrient solution. Si levels refer to additional Si added to the nutrient solution having a Si level of 2 mg·L−1.
3.2. Silicon Content
Table 2 reports the average Si content found in leaves, stems, and roots of young plants of Carosello and two tomato genotypes. Overall, the roots of the three genotypes accumulated 64% more Si than shoots (Table 2). Focusing on the epigeal level, Si content was 22% higher in leaves compared to stems (Table 2).

Table 2.
Average silicon (Si) content of stems, leaves, and roots ± standard error of young plants of Carosello (Cucumis melo L.) and two tomato genotypes fertigated with three different silicon concentrations in nutrient solution (NS). Si levels refer to additional Si added to the nutrient solution having a Si level of 2 mg·L−1.
At a cellular level, three types of Si uptake and transport mechanisms are suggested in relation to water-based Si uptake, from the higher to the lower uptake level: active, passive, and rejective [61,62]. Takahashi et al. [63] described (i) active, as faster/higher Si uptake than water uptake, resulting in Si depletion in NS; (ii) passive, as similar in magnitude to water uptake without consequences on Si concentration in the NS; and (iii) rejective, as slower than water uptake, leading to a tendency to exclude Si from their tissues, consequently increasing Si concentration in the uptake solution over time. According to this classification, the tomato is considered rejective [64,65,66]. Instead, in species with a high capacity to translocate Si, the Si translocation from roots to shoots is faster than the Si uptake, and consequently, they have lower Si content in the roots than in the shoot. Due to this, the different Si content found in leaves, stems, and roots (Table 2) confirm that Carosello and tomato are not hyper or high Si-accumulators.
To better evaluate the Si accumulation/translocation of the three genotypes at different Si dosages in the NS, a comparison was made between the Carosello and the two tomato genotypes, and it was observed that Si accumulation in the leaves and stems of Carosello was higher than the two tomato genotypes by approximately 3.6 times and 5 times, respectively (Figure 2a). Furthermore, by increasing the Si concentration in the NS up to 100 mg·L−1, Si accumulation in Carosello shoots and in tomato shoots and roots followed a linear increase, whereas a linear decreasing trend for Carosello roots was observed (Figure 2a). From the comparison between leaves and stems, it emerged that by increasing the Si content of the NS from 0 to 100 mg·L−1, Si accumulation climbed from 438 to 1772 mg·kg−1 DW for Carosello leaves, and from 151 to 1365 mg·kg−1 DW for Carosello stems (Figure 2b); similarly, the Si content in tomato leaves rose from 69.4 to 380 mg·kg−1 DW, and in tomato stems from 49.3 to 229 mg·kg−1 DW (Figure 2b). The rate of Si accumulation was higher, as shown in the following, in ascending order: Carosello leaves > Carosello stems > tomato leaves > tomato stems. The results displayed in Figure 2 are in line with previous research on Si translocation in plants, and on the lower capability of tomatoes with regards to root-to-shoot translocation of Si, compared to cucumbers (model species for the studies on Si and Cucurbitaceae family) [67]. In fact, Liang et al. [68] found that the Si uptake in cucumber is concentration-independent and supported by an active metabolic process, while Do Nascimento [69] described a Si supply concentration-dependent effect in melon, as the Si increment in melon shoots followed the increment in applied Si-containing fertilizer. The results obtained in the present research show a concentration-dependent effect on Si accumulation in the plant of Carosello (local variety of melon) which is in line with the results described by Do Nascimento [69], and with the results obtained by Buttaro et al. [40], who demonstrated that by adding 3.6 mM (101.16 mg·L−1) of Si in the NS, the incidence of powdery mildew infection on the leaves of the plants of Carosello decreased. Furthermore, although the tomato is not considered a good Si translocator in the epigeal organs, Figure 2 shows that the Si content of the epigeal organs increased by increasing the concentration of Si in the NS. This could be explained by considering that the Si uptake relies on the passive transport of uncharged Si(OH)4, which is a concentration-dependent process present in all plant species, regardless of their accumulation ability [70]. Furthermore, the Mitani and Jian [36] study suggests that a transporter-mediated component is present in cucumber and tomato, but the density of the transporters is higher in the former, and the presence of an active component is excluded in the tomato [67]. In Figure 2a it can be seen that by adding Si in the NS, the Si content in Carosello roots decreased, while it rose in the tomato genotype’s roots. As well, during the experiment conducted by Sonneveld and Vogt [71], it was shown that by increasing the Si content in the NS to over 84 mg·L−1, the Si concentration in cucumber roots decreased. This depletion can only be explained by precipitation, which is unlikely at these low concentrations [72], or by active uptake. Our hypothesis regarding these results is that since Carosello is a ‘good accumulator genotype’ with high Si content in the NS, the roots-to-shoots Si translocation is faster than in the two tomato hybrids, further confirming that the tested tomato genotypes are poor Si accumulators.
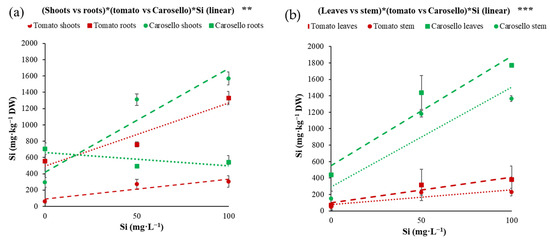
Figure 2.
(a) Silicon (Si) accumulation in shoot and roots’ dry weight (DW) of young plants of Carosello (Cucumis melo L.) and tomato (average of two cultivars) fertigated with three silicon (Si) concentrations in nutrient solution. Red lines and green lines represent, respectively, the linear trends of silicon accumulation for tomato and Carosello, while the average values of leaves and stems are differentiated by indicators with a different shape. Si levels refer to additional Si added to the nutrient solution having a Si level of 2 mg·L−1. Vertical bars represent ± standard errors of mean values. Significance: ** for p < 0.01. (b) Silicon (Si) accumulation in leaves and stems of young plants of Carosello (Cucumis melo L.) and tomato (average of two genotypes) fertigated with three silicon concentrations in a nutrient solution, referred to as dry weight (DW). Red lines and green lines represent, respectively, the linear trends of silicon accumulation for tomato and Carosello plants, while the average values of leaves and stems have differently shaped indicators. Si levels refer to additional Si added to the nutrient solution having a Si level of 2 mg·L−1. Vertical bars represent ± standard errors of mean values. Significance: *** for p < 0.001.
Comparing the different tomato genotype’s capacity for Si translocation, it was observed that TV shoots had an average accumulation of 100 mg·kg−1 DW of Si more than TA, while the Si content of roots of both tomato genotypes (TA and TV) was, on average, 881 mg·kg−1 of DW (Figure 3a). Instead, Figure 3b shows the linear Si accumulation in the leaves and stems of the two tomato genotypes (TA vs. TV). In greater detail, by increasing the Si content of the NS from 0 to 100 mg·L−1, the Si accumulation of TA leaves escalated from 14.9 to 240 mg·kg−1 DW, while in TA stems it went up from 69.5 to 264 mg·kg−1 DW. On the other hand, by increasing the Si content of the NS from 0 to 100 mg·L−1, the Si accumulation rose from 124 to 520 mg·kg−1 DW in TV leaves, and from 29.1 to 194 mg·kg−1 DW in TV stems. Therefore, a different attitude towards Si accumulation/translocation in epigeal organs was observed between the two tomato genotypes (Figure 3b). With the highest Si content in the NS, TA accumulated a similar amount of Si in the leaves and stems, whereas TV showed greater Si accumulation in leaves rather than in stems (Figure 3b). These results underline that the translocation capacity of Si is not only species-dependent, but also variety-dependent. In conclusion, in our experiment the tomato was confirmed to be a species with a lower capacity of Si accumulation in the epigeal organs compared to Carosello; nevertheless, it was highlighted that TV has a higher capacity of Si translocation in the epigeal organs compared to TA, and this could be mainly attributed to the better capacity of TV of translocating Si from the stem to the leaves, compared to TA (Figure 3b).
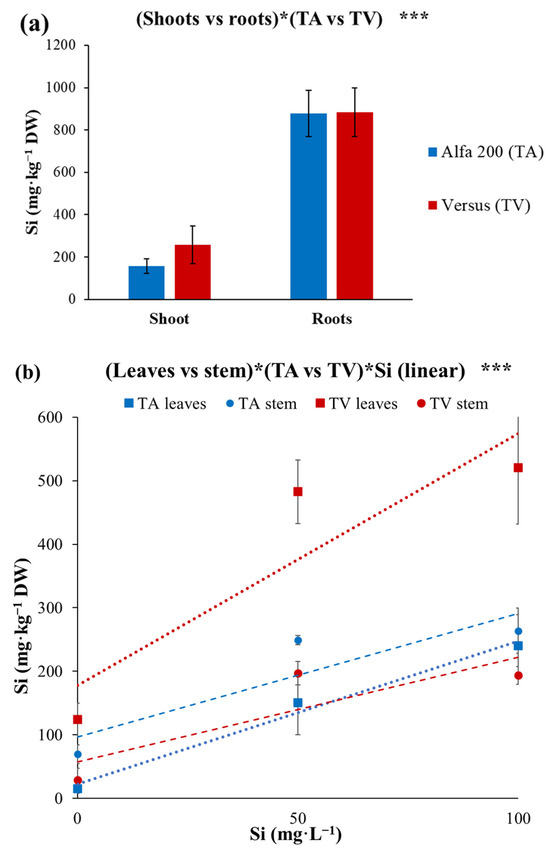
Figure 3.
(a) Silicon (Si) content of shoot and roots’ dry weight (DW) of young plants of two tomato genotypes: TA and TV. Vertical bars represent ± standard errors of mean values. Significance: *** for p < 0.001. (b) Silicon (Si) accumulation in leaves and stems of young plants of two genotypes of tomato, TA (Tomato ‘Alfa 200’) and TV (Tomato ‘Versus’), fertigated with three silicon concentrations in a nutrient solution, referred to as dry weight (DW). Red and blue lines represent, respectively, the linear trends of silicon accumulation for TV and TA, while the average values of leaves and stems have differently shaped indicators. Si levels refer to additional Si added to the nutrient solution having a Si level of 2 mg·L−1. Vertical bars represent ± standard errors of mean values. Significance: *** for p < 0.001.
4. Conclusions
This study demonstrated that the Si translocation ability of tomato plants depends on the genotype. It was observed that the ‘Versus’ (TV) genotype exhibited a higher capacity for Si translocation than the ‘Alfa 200’ (TA) genotype. The Apulian melon landrace called Carosello showed a greater Si assimilation/translocation roots-to-shoots capacity compared to the two tomato genotypes. By increasing the supplemental Si content in the NS up to 100 mg·L−1, the accumulation of leaf dry matter in young Carosello and tomato plants was promoted. In the near future, the influence of environmental factors on the silicon translocation of further tomato genotypes will be evaluated.
Author Contributions
Conceptualization, A.S., O.D.P., F.S. and P.S.; methodology, A.S., O.D.P., M.D., F.S. and P.S.; validation, F.S. and P.S.; formal analysis, A.S., O.D.P. and M.D.; investigation, A.S., O.D.P., M.D., F.S. and P.S.; resources, F.S. and P.S.; data curation, F.S. and P.S.; writing—original draft preparation, A.S. and O.D.P.; writing—review and editing, M.D., F.S. and P.S.; visualization, F.S. and P.S.; supervision, F.S. and P.S.; project administration, F.S. and P.S.; funding acquisition, F.S. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Rural Development Program of the Apulia Region (Italy) 2014–2020, Submeasure 16.2 (Support for pilot projects and the development of new products, practices, processes, and technologies, and the transfer and dissemination of the results obtained by the Operational Groups), in the framework of the SOILLESS GO project, project code (CUP) B97H20000990009. Paper no. 21.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Agritech National Research Center, European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)–MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4–D.D. 1032 17/06/2022, CN00000022). We thank Nicola Gentile for providing assistance during the greenhouse experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bouis, H.E. Micronutrient fortification of plants through plant breeding: Can it improve nutrition in man at low cost? Proc. Nutr. Soc. 2003, 62, 403–411. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Perez-Granados, A.M.; Vaquero, M.P. Silicon, aluminium, arsenic and lithium: Essentiality and human health implications. J. Nutr. Health Aging 2002, 6, 154–162. [Google Scholar] [PubMed]
- Deshmukh, R.K.; Ma, J.F.; Bélanger, R.R. Editorial: Role of silicon in plants. Front. Plant Sci. 2017, 8, 1858. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.M.R.; de Mello Prado, R.; Barreto, R.F. Silicon and sodium attenuate potassium deficiency in Eruca sativa Mill. Food Chem. 2024, 432, 137225. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.; Stout, P. The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol 1939, 14, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Lewin, J.; Reimann, B.E.F. Silicon and Plant Growth. Annu. Rev. Plant Physiol. 1969, 20, 289–304. [Google Scholar] [CrossRef]
- Martos-García, I.; Fernández-Escobar, R.; Benlloch-González, M. Silicon is a non-essential element but promotes growth in olive plants. Sci. Hortic. 2024, 323, 112541. [Google Scholar] [CrossRef]
- Yan, G.C.; Nikolic, M.; Ye, M.J.; Xiao, Z.X.; Liang, Y.C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Hosseini-Nasr, F.; Etesami, H.; Alikhani, H.A. Silicon Improves Plant Growth-Promoting Effect of Nodule Non-Rhizobial Bacterium on Nitrogen Concentration of Alfalfa Under Salinity Stress. J. Soil Sci. Plant Nutr. 2023, 23, 496–513. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Viciedo, D.; Oliveira, K.S.; de Mello Prado, R.; Habermann, E.; Martínez, C.A.; de Moura Zanine, A. Silicon uptake and utilization on Panicum maximum grass modifies C:N:P stoichiometry under warming and soil water deficit. Soil Tillage Res. 2024, 235, 105884. [Google Scholar] [CrossRef]
- Heine, G.; Tikum, G.; Horst, W.J. Silicon nutrition of tomato and bitter gourd with special emphasis on silicon distribution in root fractions. J. Plant Nutr. Soil Sci. 2005, 168, 600–606. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- López-Pérez, M.C.; Pérez-Labrada, F.; Ramírez-Pérez, L.J.; Juárez-Maldonado, A.; Morales-Díaz, A.B.; González-Morales, S.; García-Dávila, L.R.; García-Mata, J.; Benavides-Mendoza, A. Dynamic modeling of silicon bioavailability, uptake, transport, and accumulation: Applicability in improving the nutritional quality of tomato. Front. Plant Sci. 2018, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Zellner, W.L. Drastic differences in silicon concentrations between roots and leaves of 10 different Solanum lycopersicum L. varieties. HortScience 2021, 56, 838. [Google Scholar] [CrossRef]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Hu, Y.; Han, W.; Gong, H. Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol. Plant. 2015, 37, 71. [Google Scholar] [CrossRef]
- Hoffmann, J.; Berni, R.; Hausman, J.F.; Guerriero, G. A review on the beneficial role of silicon against salinity in non-accumulator crops: Tomato as a model. Biomolecules 2020, 10, 1284. [Google Scholar] [CrossRef]
- Hernández-Salinas, M.; Valdez-Aguilar, L.A.; Alia-Tejacal, I.; Alvarado-Camarillo, D.; Cartmill, A.D. Silicon enhances the tolerance to moderate NaCl-salinity in tomato grown in a hydroponic recirculating system. J. Plant Nutr. 2022, 45, 413–425. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.; Fernandes, A.P.G.; Bokor, B.; Vaculík, M.; Heuvelink, E.; Carvalho, S.M.P.; Vasconcelos, M.W. The effect of silicon on the antioxidant system of tomato seedlings exposed to individual and combined nitrogen and water deficit. Ann. Appl. Biol. 2023, 184, 50–60. [Google Scholar] [CrossRef]
- Ali, N.; Schwarzenberg, A.; Yvin, J.C.; Hosseini, S.A. Regulatory role of silicon in mediating differential stress tolerance responses in two contrasting tomato genotypes under osmotic stress. Front. Plant Sci. 2018, 9, 1475. [Google Scholar] [CrossRef] [PubMed]
- Kiirika, L.M.; Stahl, F.; Wydra, K. Phenotypic and molecular characterization of resistance induction by single and combined application of chitosan and silicon in tomato against Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2013, 81, 1–12. [Google Scholar] [CrossRef]
- Fan, X.Y.; Lin, W.P.; Liu, R.; Jiang, N.H.; Cai, K.Z. Physiological response and phenolic metabolism in tomato (Solanum lycopersicum) mediated by silicon under Ralstonia solanacearum infection. J. Integr. Agric. 2018, 17, 2160–2171. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Guneri, M.; Ashraf, M. Mitigation effects of silicon on tomato plants bearing fruit grown at high boron levels. J. Plant Nutr. 2011, 34, 1985–1994. [Google Scholar] [CrossRef]
- Hu, A.Y.; Xu, S.N.; Qin, D.N.; Li, W.; Zhao, X.Q. Role of silicon in mediating phosphorus imbalance in plants. Plants 2021, 10, 51. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Zhao, X.; Jin, X.; Hou, L.; Shi, Y.; Ahammed, G.J. Silicon compensates phosphorus deficit-induced growth inhibition by improving photosynthetic capacity, antioxidant potential, and nutrient homeostasis in tomato. Agronomy 2019, 9, 733. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Silicon in Agriculture: From Theory to Practice; Springer Science + Business Media Dordrecht, Ed.; Springer: Dordrecht, The Netherlands, 2015; ISBN 9789401799782. [Google Scholar]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological, biochemical and chemical studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Santamaria, P.; Serio, F. Silicon biofortification of leafy vegetables and its bioaccessibility in the edible parts. J. Sci. Food Agric. 2016, 96, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Jian, F.M. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Fawe, A.; Menzies, J.G.; Chérif, M.; Bélanger, R.R. Silicon and disease resistance in dicotyledons. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndorfe, G.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001; pp. 159–169. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Abiotic and Biotic Plant Stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef]
- Buttaro, D.; Bonasia, A.; Minuto, A.; Serio, F.; Santamaria, P. Effect of silicon in the nutrient solution on the incidence of powdery mildew and quality traits in Carosello and barattiere (Cucumis melo L.) grown in a soilless system. J. Hortic. Sci. Biotechnol. 2009, 84, 300–304. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, W.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef]
- Somma, A.; Palmitessa, O.D.; Leoni, B.; Signore, A.; Renna, M.; Santamaria, P. Extraseasonal production in a soilless system and characterisation of landraces of Carosello and Barattiere (Cucumis melo L.). Sustainability 2021, 13, 11425. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Durante, M.; Leoni, B.; Montesano, F.; Renna, M.; Serio, F.; Somma, A.; Santamaria, P. Enhancement of a landrace of Carosello (Unripe Melon) through the Use of light-emitting diodes (LED) and nutritional characterization of the fruit placenta. Sustainability 2021, 13, 11464. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; Tanaka, F.A.O.; Amorim, L.; Camargo, L.E.A. Effect of potassium silicate on epidemic components of powdery mildew on melon. Plant Pathol. 2012, 61, 323–330. [Google Scholar] [CrossRef]
- Pozo, J.; Urrestarazu, M.; Morales, I.; Sánchez, J.; Santos, M.; Dianez, F.; Álvaro, J.E. Effects of silicon in thenutrient solution for three horticultural plant families on the vegetative growth, cuticle, and protection against Botrytis cinerea. HortScience 2015, 50, 1447–1452. [Google Scholar] [CrossRef]
- Preston, H.A.F.; Do Nascimento, C.W.A.; Preston, W.; de Souza Nunes, G.H.; Loureiro, F.L.C.; Mariano, R. de L.R. Silicon slag increases melon growth and resistance to bacterial fruit blotch. Acta Sci.-Agron. 2021, 43. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Jeong, B.R. Foliar Silicon Spray before Summer Cutting Propagation Enhances Resistance to Powdery Mildew of Daughter Plants. Int. J. Mol. Sci. 2022, 23, 3803. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; D’Imperio, M.; Maggi, S.; Serio, F. Soilless biofortification, bioaccessibility, and bioavailability: Signposts on the path to personalized nutrition. Front. Nutr. 2022, 9, 2425. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; McNaughton, S.A.; Jugdaohsingh, R.; Anderson, S.H.C.; Dear, J.; Khot, F.; Mowatt, L.; Gleason, K.L.; Sykes, M.; Thompson, R.P.H.; et al. A provisional database for the silicon content of foods in the United Kingdom. Br. J. Nutr. 2005, 94, 804–812. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium biofortification impacts the nutritive value, polyphenolic content, and bioactive constitution of variable microgreens genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M.; D’imperio, M.; Santamaria, P.; Serio, F. Iodine biofortification of four brassica genotypes is effective already at low rates of potassium iodate. Nutrients 2019, 11, 451. [Google Scholar] [CrossRef]
- D’imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Serio, F.; Santamaria, P. Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Voogt, W.; Holwerda, H.T.; Khodabaks, R. Biofortification of lettuce (Lactuca sativa L.) with iodine: The effect of iodine form and concentration in the nutrient solution on growth, development and iodine uptake of lettuce grown in water culture. J. Sci. Food Agric. 2010, 90, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Tüzel, Y.; Gül, A.; Tüzel, I.H.; Öztekin, G.B. Different soilless culture systems and their management. J. Agric. Food Environ. Sci. 2019, 73, 7–12. [Google Scholar] [CrossRef]
- Karimi, S.; Zare, N. Silicon pretreatment at the transplanting stage, a tool to improve the drought tolerance and subsequent growth of melons in the field. Silicon 2023, 15, 4921–4929. [Google Scholar] [CrossRef]
- Abul-Soud, M.A.; Emam, M.S.A.; Hawash, A.M.H. The potential of soilless culture systems in producing tomato and cucumber under greenhouse conditions. Int. J. Plant Soil Sci. 2021, 33, 67–85. [Google Scholar] [CrossRef]
- Torres Pineda, I.; Lee, Y.D.; Kim, Y.S.; Lee, S.M.; Park, K.S. Review of inventory data in life cycle assessment applied in production of fresh tomato in greenhouse. J. Clean. Prod. 2021, 282, 124395. [Google Scholar] [CrossRef]
- Montesano, F.F.; D’Imperio, M.; Parente, A.; Cardinali, A.; Renna, M.; Serio, F. Green bean biofortification for Si through soilless cultivation: Plant response and Si bioaccessibility in pods. Sci. Rep. 2016, 6, 31662. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.A.L.; Araújo, R.H.C.R.; Nóbrega, J.S.; de Fátima, R.T.; Santos, M.S.; Santos, A.S.; Teodósio, A.E.M.d.M.; Oliveira, C.J.A. Application of Silicon to Alleviate Irrigation Water Salinity in Melon Growth. J. Exp. Agric. Int. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Kaur, H.; Greger, M. A review on si uptake and transport system. Plants 2019, 8, 81. [Google Scholar] [CrossRef]
- Takahashi, E.; Ma, J.F.; Miyake, Y. The possibility of silicon as an essential element for higher plants. Comments Agric. Food Chem. 1990, 2, 99–122. [Google Scholar]
- Sun, H.; Duan, Y.; Mitani-Ueno, N.; Che, J.; Jia, J.; Liu, J.; Guo, J.; Ma, J.F.; Gong, H. Tomato roots have a functional silicon influx transporter but not a functional silicon efflux transporter. Plant Cell Environ. 2020, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Yamaji, N.; Ago, Y.; Iwasaki, K.; Ma, J.F. Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J. 2011, 66, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fei, S.; Xu, Y.; He, Y.; Zhu, Z.; Liu, Y. The structure, function and expression analysis of the nodulin 26-like intrinsic protein subfamily of plant aquaporins in tomato. Sci. Rep. 2022, 12, 9180. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Nikolic, N.; Liang, Y.; Kirkby, E.A.; Römheld, V. Germanium-68 as an adequate tracer for silicon transport in plants. Characterization of silicon uptake in different crop species. Plant Physiol. 2007, 143, 495–503. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Römheld, V. Silicon uptake and transport is an active process in Cucumis sativus. New Phytol. 2005, 167, 797–804. [Google Scholar] [CrossRef]
- do Nascimento, C.W.A.; de Souza Nunes, G.H.; Preston, H.A.F.; da Silva, F.B.V.; Preston, W.; Loureiro, F.L.C. Influence of Silicon Fertilization on Nutrient Accumulation, Yield and Fruit Quality of Melon Grown in Northeastern Brazil. Silicon 2020, 12, 937–943. [Google Scholar] [CrossRef]
- Raven, J.A. Silicon transport at the cell and tissue level. In Silicon in Agriculture; Datnoff, L.E., Korndorfer, G.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001; pp. 41–55. [Google Scholar]
- Voogt, W.; Sonneveld, C. Silicon in horticultural crops grown in soilless culture. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndorfer, G.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001; Volume 6, pp. 115–131. [Google Scholar]
- Iler, R.K. The Chemistry of Silica; Wiley-Interscience: Chichester, UK, 1979; ISBN 978-0471024040. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).