Abstract
Citrus fruits have a distinctive flavor and can convey health benefits because of their unique phytochemicals. Phytochemical profiles are influenced by many factors, including variety and environmental growing conditions; however, the effect of the cultivation methods on the phytochemical profile of Satsuma mandarin (Citrus unshiu) has received little attention. In this study, we examined the relationships between the cultivation conditions, sensory quality, and phytochemical profiles of C. unshiu cultivated using four methods: open field, greenhouse, film mulching, and tunnel farming. The soil water content differed significantly between the cultivation methods and showed a strong positive correlation with sourness, bitterness, and astringency and a strong negative correlation with sweetness. The metabolites of C. unshiu were not associated with the soil water content but with the soil mineral content, including nitrogen (N+), phosphorus (P+), and potassium (K+). The soil P+ and K+ content was positively correlated with most secondary metabolites. The relative abundance of sugars did not differ significantly between the cultivation methods; however, the sweetness was higher under film mulching than under the other cultivation methods because of the suppression of sweetness by bitter compounds. We did not investigate the effect of other growing conditions, such as sunlight; however, the results improve our understanding of the effect of cultivation methods on the quality of C. unshiu and may inform crucial decisions concerning citrus cultivation.
1. Introduction
Citrus fruits, which are among the most important fruit crops globally [1], have a distinctive flavor profile and have attracted research attention with regard to their potential health benefits, including improved gut health, obesity management, diabetes mitigation, and cardiovascular disease prevention [1,2,3]. These flavor attributes and health-promoting properties are attributed to phytochemicals, including sugars, organic acids, flavonoids, limonoids, and terpenes [4]. The phytochemical profiles of citrus fruits depend on numerous factors, including the variety [5], growing region [6], postharvest treatment [7], storage conditions [8], and environmental growing conditions [2]. Factors such as the cultivation method, lighting conditions, fertilization, temperature, and soil moisture markedly affect the phytochemical profiles of citrus fruits [9,10,11]. In particular, cultivation methods such as open field, film mulching, greenhouse, and tunnel farming have been shown to influence the quality of many fruits, including oranges [12], melons [13], strawberries [14], and red bayberries [15]. Greenhouse, film mulching, and tunnel farming increase the sugar content in red bayberries [15], oranges [12], strawberries [16], and blueberries [17] and improve the phytochemical profiles of strawberries [14] compared to open-field cultivation. Variations in environmental factors during fruit growth, such as the temperature and humidity, influenced the activity of numerous enzymes associated with sucrose–phosphate and secondary metabolite synthesis [15,18]. These alterations ultimately resulted in shifts in the sugar content, acidity, and phytochemical profiles of fruits. So far, however, the effects of the various cultivation methods on the flavor quality and phytochemical profile of Satsuma mandarin (Citrus unshiu) are unclear, even though this crop has a significant market presence owing to its consumer-friendly traits, such as its easy peeling and seedlessness, as well as its high productivity [19].
Recently, “omics” approaches such as transcriptomics, proteomics, and metabolomics have been used to elucidate the factors associated with fruit quality and the phytochemical profiles of various fruits, including strawberries [20], oranges [21], melons [22], and mangoes [23]. In particular, metabolomics, which utilizes nuclear magnetic resonance spectroscopy and mass spectrometry (MS), has been used to compare the phytochemical profiles of citrus fruits between varieties [6], growing regions [24], fruit development [5], and storage [25]. However, metabolomics has rarely been applied to assess the effects of cultivation conditions on citrus quality.
Therefore, to investigate the effects of cultivation methods on the sensory quality and phytochemical profiles of C. unshiu, we examined the phytochemical profiles of C. unshiu cultivated using four methods: open field, greenhouse, film mulching, and tunnel farming. To this end, we employed gas chromatography (GC)-MS, ultra-performance liquid chromatography-quadrupole-time-of-flight (UPLC-Q-TOF) MS, and high-performance liquid chromatography (HPLC). Moreover, a citrus metabolomic pathway associated with the cultivation methods is proposed here, and the correlations of the phytochemical profiles and sensory and soil characteristics are discussed. Our results help to understand the effect of cultivation methods on C. unshiu quality and provide useful information for C. unshiu cultivation.
2. Materials and Methods
2.1. Citrus Samples
The greenhouse was covered with two layers of semi-transparent polyvinyl chloride (PVC), while the tunnel farming used PVC only on the roof. The film mulching involved applying white high-density polyethylene fiber to the entire surface of the citrus farm. The citrus trees were cultivated using conventional methods, maintaining a planting distance of 2.5–3 m, and a standardized fertilizer composition (N+, 21%; P+, 17%; K+, 17%). The open field, film mulching, and tunnel farming relied on natural conditions (Figure S1), excluding tunnel farming, which involved irrigation. The greenhouse and tunnel farming had controlled irrigation (germination–flowering stage: 10 t/1000 m2/4–5 day; fruit development stage:1 t/1000 m2/7–10 day). The greenhouse conditions also included temperature control (germination-flowering stage: 24–25 °C/day, 16–18 °C/night; fruit development stage: 30–31 °C/day, 22–24 °C/night). C. unshiu fruits cultivated using four cultivation methods, including open field (n = 104 farms, December 2021), greenhouse (n = 17 farms, June 2021), film mulching (n = 25 farms, December 2021), and tunnel farming (n = 21 farms, January 2022), were purchased from the Citrus Agricultural Cooperative Federation in Jeju, Korea. Three kilograms (n = 28–32 fruits per farm) of C. unshiu fruits, randomly selected from the entire farm, were used. After peeling, the flesh samples were randomly mixed and immediately used for measurement of the soluble solids content (SSC), titratable acidity (TA), sensory evaluation, and analysis of volatile terpenes. The remaining samples were crushed, freeze-dried, and stored at −80 °C until use.
2.2. Determination of Water, pH, and Minerals in Soil
Using the Obreza method [26], soil samples were collected from six open-field farms, eight greenhouse farms, five film-mulching farms, and eight farms using tunnel farming. The water content was determined through oven-drying at 105 °C for 24 h. The soil was mixed with water at a ratio of 1:5 (g/w), and the pH of the mixed samples was measured using a pH meter. For the soil mineral analysis, soil samples were digested using nitric acid, filtered, and adjusted to 100 mL using distilled water. The P+ and K+ contents of the soil were measured using an inductively coupled plasma-optical emission spectroscopy device (OPTIMA 8300DV, PerkinElmer, Waltham, MA, USA), and the N+ content was measured using a macro elemental analyzer (vario MACRO cube, Elementar, Langenselbold, Germany).
2.3. SSC and Titratable Acidity
The squeezed samples were centrifuged at 14,000× g and 4 °C for 15 min to measure the SSC and TA. The SSC (%) in the supernatant was assessed using a handheld refractometer (Daihan Scientific Co., Wonju, Republic of Korea). For the TA determination, 1 mL of the supernatant, 1 mL distilled water, and 200 μL 1% phenolphthalein were mixed and subsequently titrated with 0.1 N NaOH to the endpoint (red color). The TA value is expressed as the percentage of citric acid on a fresh-weight basis.
2.4. Sensory Evaluation
Sensory evaluation was carried out by nine trained panelists aged 24–34 years, following approval by the Human Research Ethics Committee of Gyeongsang National University (approval number GIRB-G22-Y-0063). All the panelists had been trained for more than 1 year in citrus sensory evaluation, and they discussed a series of taste reference solutions, including sucrose (8%) for sweetness, citric acid (0.3%) for sourness, quinine (0.0025%) for bitterness, and tannins (0.2%) for astringency. Purified water was provided between evaluations to eliminate any residual taste from the tongue. Each taste intensity was marked on a 15 cm line scale with 0.5 cm anchors, labeled “very weak” on the left and “very strong” on the right [27].
2.5. Citrus Metabolomic Analysis
2.5.1. GC-MS Analysis
Non-volatile and volatile citrus metabolomics analyses based on GC-MS were carried out as described in our previous study, with some modifications [27]. Non-volatile metabolites from the lyophilized samples were extracted with 70% methanol containing dicyclohexyl phthalate as an internal standard (IS). After drying, the metabolites in the dried sample were derivatized using 70 μL pyridine containing methoxyamine hydrochloride (20 mg/mL) at 37 °C for 90 min, followed by treatment with 70 μL N,O-bis(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane at 70 °C for 30 min. The derivatized samples (1 µL) were injected into the GC-2010 plus system (Shimadzu, Kyoto, Japan), equipped with a DB-5ms capillary column (30 m × 0.25 mm, 0.25 μm; Agilent Technologies, Santa Clara, CA, USA) with a split ratio of 1:50. The gas flow rate and injector temperature were maintained at 1 mL/min and 200 °C, respectively. The oven gradient program was set as follows: maintain at 70 °C for 2 min; increase to 150 °C at 5 °C/min, to 210 °C at 3 °C/min, and to 320 °C at 8 °C/min; and hold for 8 min at 320 °C.
To analyze the volatile terpenes, the sample was extracted using methyl tert-butyl ether containing 2-methyl-1-pentanol as an IS. Thereafter, 1 μL of the extract was injected into the GC system (Shimadzu), equipped with a DB-WAX column (30 m × 0.25 mm, 0.25 μm; Agilent Technologies) with a split ratio of 1:10. The injector temperature was set to 250 °C. The oven gradient program was set as follows: maintain at 40 °C for 3 min; increase to 160 °C at 8 °C/min, to 240 °C at 10 °C/min; and hold for 3 min at 240 °C.
The eluted non-volatile and volatile metabolites were detected using a TQ 8030 MS (Shimadzu) with electron ionization at 70 eV, an ion source temperature of 200 °C, and an interface temperature of 250 °C. Data were collected in the mass range of m/z 45–550.
2.5.2. UPLC-Q-TOF MS Analysis
Metabolites extracted with 70% methanol containing terfenadine as an IS were analyzed using a UPLC-Q-TOF MS device (XevoTM G2-S; Waters, Milford, MA, USA) equipped with an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 m; Waters). The column was equilibrated with water containing 0.1% formic acid (FA), and the metabolites were eluted with a gradient of acetonitrile containing 0.1% FA. The eluted metabolites were detected using Q-TOF-MS in the positive electrospray ionization mode. The optimized MS conditions were 800 L/h desolvation gas flow, 400 °C desolvation temperature, 100 °C ion source temperature, 3 kV capillary voltage, and 40 V sampling cone voltage. Data were collected in the range of 100–1500 m/z. The MS/MS spectra were obtained using a collision energy ramp from 10 to 30 eV or 20 to 40 eV. The QC sample was analyzed between the sets of samples [27].
2.5.3. Vitamin C Quantification Using HPLC
Vitamin C was extracted from the lyophilized samples using 5% aqueous meta-phosphoric acid. The extract was analyzed using an HPLC device (Shimadzu) equipped with a Triart C18 column (250 × 4.6 mm, I.D., 5 μm; YMC Co., Ltd., Kyoto, Japan). Separation was performed using 0.1% phosphoric acid at a flow rate of 1 mL/min with isocratic elution, and vitamin C was detected at 254 nm [27].
2.5.4. Data Processing
The collected MS datasets obtained via GC-MS and UPLC-Q-TOF MS were aligned and normalized using the IS. The metabolites were provisionally identified using an online database (Wiley 9 and NIST 11 for GC-MS; UNIFI software (version 1.9.2, Waters Corp.) for UPLC-Q-TOF MS) and authentic standards.
2.6. Statistical Analysis
Multivariate statistical analysis of the processed MS datasets was conducted using SIMCA-P+ v.16 (Umetrics, Umea, Sweden) and visualized using partial least squares discriminant analysis (PLS-DA). Statistical differences were analyzed using a one-way analysis of variance, with Duncan’s test at p < 0.05, using SPSS software (v.27.0; SPSS Inc., Chicago, IL, USA). The Pearson’s correlation coefficients were calculated and visualized using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Soil Characteristics
The soil characteristics, including the water and mineral content and pH, were compared between the four cultivation methods (Table 1). The lowest soil water content was observed with film mulching (20.37%), whereas the soil water content of the open- field, greenhouse, and tunnel farming was 36.59%, 32.66%, and 25.89%, respectively. The soil pH of the greenhouse (pH 4.53) and tunnel farming (pH 4.6) plots was significantly lower than that of the open field (pH 6.05) and film mulching (pH 5.17) plots. With regard to the mineral content, tunnel farming produced the highest N+ content (8.21 mg/g dry soil), which in the other samples ranged from 5.22 to 7.15 mg/g dry soil. Greenhouse soil had the highest content of P+ (4.59 mg/g dry soil) and K+ (4.45 mg/g dry soil), whereas no significant difference was observed between the other samples with regard to the content of P+ (1.93–2.32 mg/g dry soil) and K+ (2.53–3.45 mg/g dry soil).

Table 1.
Contents of water and minerals in the soil samples with different cultivation methods.
3.2. SSC, TA, SSC/TA Ratio, and Sensory Characteristics
The SSC, TA, and SSC/TA ratios of citrus samples cultivated using different methods were compared (Figure 1). Greenhouse, film mulching, and tunnel farming produced significantly higher SSC than open-field farming, and their values (9.9–11.5%) were 139–144% higher than those of open-field farming. In contrast, the TA value of film mulching (1.0%) was lower than that of the other methods. The variations in the SS and TA values depending on the cultivation method resulted in differences in the SSC/TA ratio. The ratio of film mulching (11.08) was approximately two-fold higher than that of open-field farming, whereas the ratios for tunnel (8.84) and greenhouse farming (7.12) were 1.7- and 1.3-fold higher, respectively.
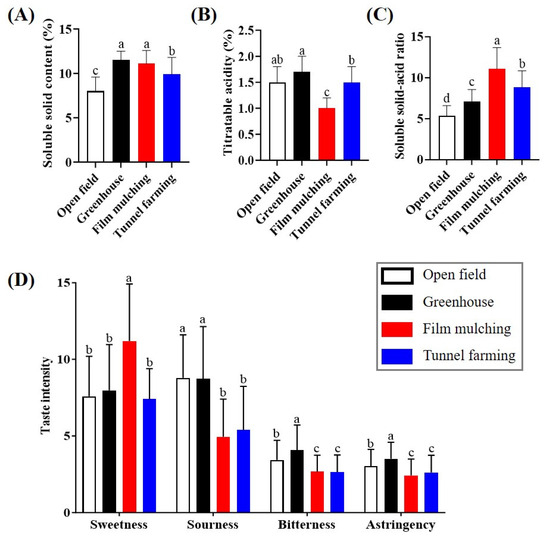
Figure 1.
Soluble solids content (A), titratable acidity (B), soluble solids content–acid ratio (C), and sensory quality (D) of Citrus unshiu produced using different cultivation methods. Different letters in each column indicate significant differences according to Duncan’s test (p < 0.05).
Sensory evaluation of C. unshiu produced using different cultivation methods showed that film mulching resulted in significantly higher sweetness than the other methods, whereas no significant difference in sweetness occurred among the other samples; however, the sourness, bitterness, and astringency of the samples from film mulching and tunnel farming were lower than those from open-field and greenhouse farming.
3.3. Metabolomics Analysis
The flesh metabolite profiles of C. unshiu were analyzed using GC-MS (Table S1), UPLC-Q-TOF MS (Table S2), and HPLC (Figures S2–S5), and the sample discrimination was visualized using a PLS-DA score plot (Figure 2). The statistical parameters, including the goodness of fit (R2X = 0.341; R2Y = 0.523), predictability (Q2 = 0.507), p-values, and cross-validation determined via the permutation test of the PLS-DA model, indicated that the PLS-DA model used in this study was statistically acceptable. The score plot showed that the C. unshiu samples were separated from each other by t(1) and t(2).
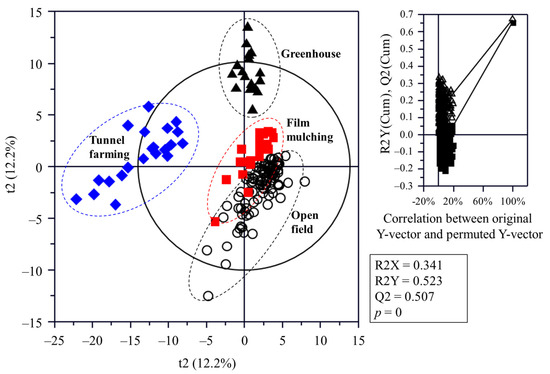
Figure 2.
Partial least squares discriminant analysis score plots of Citrus unshiu metabolites cultivated using different methods and the respective quality parameters. The statistical acceptability of the partial least squares discriminant analysis model was evaluated via the R2X, R2Y, Q2, and p-value, and the differences were tested through cross-validation with a permutation test (n = 200).
To identify the metabolites contributing to the separation between the samples in the score plot, the variable importance in projection (VIP) and p-values of all the metabolites (including fragments: 23 metabolites by GC-MS, 14 metabolites by UPLC-Q-TOF MS, and 1 metabolite by HPLC) were analyzed (Table 2, Tables S1, and S2). Among them, 41 metabolites with a VIP ≥ 0.76 and a p-value ≤ 0.05 were identified as major metabolites: four sugars (sucrose, myo-inositol, mannose, and xylofuranose), four acidic compounds (citric acid, malic acid, ascorbic acid, and oxalic acid), eight amino acids (arginine, acetylvaline, stachydrine, phenylalanine, tryptophan, serine, aspartic acid, and asparagine), seven lipids (palmitic acid, stearic acid, phytosphingosine, oleamide, lysophosphatidylcholines (LPCs; 16:0 and 18:1), and lysophosphatidylethanolamine (LPE; 16:0)), two flavonoids (hesperidin and didymin), three limonoids (limonin, nomilin, and zapoterin), and ten volatile terpenes (β-myrcene, limonene, β-phellandrene, γ-terpinene, m-cymene, α-terpinolene, linalool, α-terpineol, valencene, and α-farnesene).

Table 2.
Identification of the major metabolites in C. unshiu contributing to the difference in the samples.
3.4. Metabolic Pathway of Identified Metabolites
A citrus metabolomic pathway is proposed based on the identified metabolites, and the relative abundances of the identified metabolites were compared according to the different cultivation methods (Figure 3). The cultivation methods significantly affected the citrus primary metabolites (sugars, lipids, amino acids, and acidic compounds) and secondary metabolites (flavonoids, limonoids, and volatile terpenes). With regard to sugars, the levels of sucrose and myo-inositol were slightly lower in greenhouses, film mulching, and tunnel farming than in open-field farming. In contrast, the minor sugars mannose and xylofuranose were not detected in fruits from open-field farming. Among the acidic compounds, the ascorbic acid levels were lower in greenhouse and tunnel farming than in the other methods, whereas the levels of oxalic acid, malic acid, and citric acid were lower in film munching and tunnel farming. In particular, the malic acid levels in film munching and tunnel farming, and the oxalic acid levels in tunnel farming, were 2–3-fold and 5.4–6.5-fold lower, respectively. In terms of amino acids, tunnel farming resulted in higher levels of acetylvaline, phenylalanine, tryptophan, arginine, and stachydrine than the other methods; however, no significant differences were observed between the other methods. Greenhouse farming produced the highest levels of aspartic acid and asparagine, whereas the serine levels in open-field farming and film mulching were 20.9–30.7-fold higher than those in greenhouse and tunnel farming. With regard to lipids, the levels of fatty acids (palmitic acid, stearic acid, and oleamide) were 2.1–8.8-fold lower with tunnel farming than with the other methods, whereas the LPCs, LPEs, and phytosphingosine levels were the lowest in greenhouse and the highest in tunnel farming.
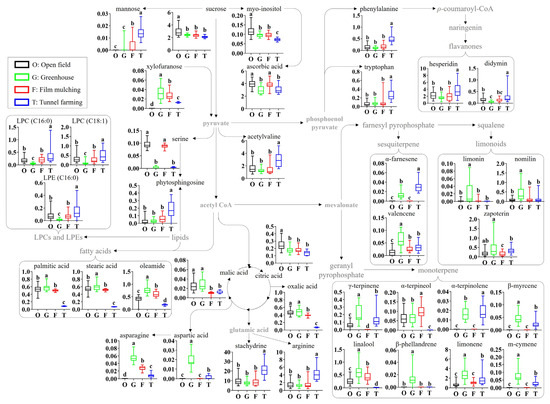
Figure 3.
Schematic diagram of the metabolomic pathway and relative abundances of the citrus metabolites. The metabolomic pathway was retrieved from the KEGG database (https://www.kegg.jp/ accessed on 9 June 2023) and was visualized with some modifications. Different letters in the bar indicate significant differences according to Duncan’s test (p < 0.05). O, open field; G, greenhouse; F, film mulching; T, tunnel farming; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine.
The secondary metabolite profiles, including flavonoids and terpenes, also showed the significant effects of the cultivation method. The levels of flavonoids, including hesperidin and didymin, were 1.5–4.7-fold higher with tunnel farming than with the other methods, which produced largely similar results. Open-field farming and film mulching produced relatively low levels of most identified terpenes compared to greenhouse farming, which showed the highest levels, except for linalool, α-terpinolene, and β-phellandrene. The levels of limonoids, such as limonin, nomilin, and zapoterin, were 3.1–19.5, 3.1–4.5, and 1.5–2.8-fold higher, respectively, with greenhouse farming than with the other methods. The compound α-farnesene was not detected in open-field farming and film mulching, whereas the valencene level with greenhouse farming was 2.0–4.3-fold higher than that produced by the other methods. The levels of monoterpenes, including γ-terpinene, α-terpinolene, β-myrcene, limonene, and m-cymene, were 1.1–14.3- and 1.2–2.9-fold higher, respectively, with greenhouse and tunnel farming than with of the other methods. However, linalool and α-terpinolene were not detected in tunnel farming, while β-phellandrene was detected only in greenhouse farming.
3.5. Correlation Analysis
The correlation between the soil nutrients, general citrus characteristics, and metabolite profiles was analyzed and visualized using heat maps (Figure 4). Regarding the correlation between the soil nutrients and general citrus characteristics (Figure 4A), the soil water content showed a strong positive correlation with the TA (r = 0.79), resulting in a strong negative correlation with the SSC/TA ratio (r = −0.83) and with the flavor quality (sourness, r = 0.79; astringency, r = 0.76; bitterness, r = 0.71), except for sweetness (r = −0.64). The SSC was negatively correlated with the pH and N+ (r < −0.56) and positively correlated with P+ and K+ (r > 0.51).
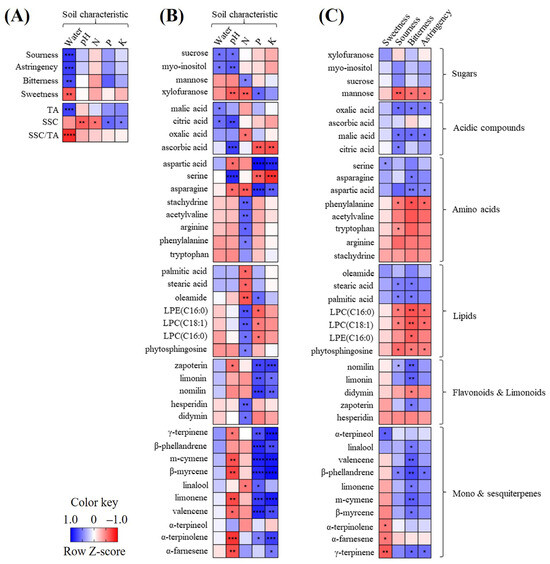
Figure 4.
Heat map indicating the relationships of sensory quality, general characteristics, and soil characteristics (A); heat map of metabolites and soil characteristics (B); heat map of metabolites and sensory quality (C). The heat map colors represent the z-score-transformed raw data of the metabolites with significant differences between the groups. Blue and red indicate the increase and decrease in the metabolite levels, respectively. Asterisks (*, **, ***, and ****) indicate the level of significance (p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively). LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine.
The correlation analyses of the soil nutrients and citrus metabolites (Figure 4B) showed that the soil P+ and K+ were positively correlated with xylofuranose, aspartic acid, asparagine, limonoids, and most terpenes, and negatively with serine. The soil pH was positively correlated with citric acid and ascorbic acid and negatively with most terpenes.
Correlation tests of the sensory quality and citrus metabolites (Figure 4C) showed that bitterness and sourness had a strong negative or strong correlation with metabolites, whereas sweetness was not correlated with sugars. In particular, bitterness was positively correlated with terpenes, limonoids, acidic compounds, aspartic acid, asparagine, and fatty acids and negatively with LPCs and LPE(C16:0). Sourness was positively correlated with acidic compounds, but negatively with mannose.
4. Discussion
The quality of citrus fruits generally depends on the environmental growing conditions, including the soil properties, sunlight, and temperature [28]. Cultivation methods that regulate environmental conditions play an important role in determining the quality of citrus fruits [2]. The effects of cultivation methods such as film mulching and greenhouse and tunnel farming on the quality of various fruits such as ponkan and grapes have been previously reported [29,30,31], whereas such effects of cultivation methods on the quality of C. unshiu have not been examined thus far. In the present study, we investigated the quality differences in C. unshiu produced using four different cultivation methods with regard to the soil properties, general citrus qualities, sensory qualities, and metabolite profiles.
Various cultivation methods, including film mulching and greenhouse and tunnel farming, are typically used to improve citrus quality by regulating growth factors such as the soil characteristics, irradiation, water supply, and temperature compared to the open-field method [28]. Previous studies have demonstrated that greenhouse and tunnel farming reduced sunlight exposure approximately 1.5-fold compared to open fields [32] and helped control the water supply [33]. Furthermore, film mulching increases sunlight exposure approximately two-fold compared with open fields [12] and prevents rainwater uptake by the soil [34]. Our results showed significant differences in the soil water content between the cultivation methods, i.e., open field > greenhouse > tunnel farming > film mulching (Table 1); sunlight exposure, however, was not assessed in the present study. The soil water content, which is a predominant factor affecting fruit quality under drought stress [35], showed a strong positive correlation with sourness, bitterness, and astringency and a strong negative correlation with sweetness, resulting in a positive correlation with the SSC/TA ratio (Figure 4). The SSC/TA ratio is generally accepted in the citrus industry as an important parameter for evaluating citrus flavor, and a ratio of 12 is considered the minimum for the good eating quality of mandarins [36]. The SSC/TA of C. unshiu from film mulching, which had the lowest soil water content and was considered to experience high sunlight exposure, was the highest, along with pronounced sweetness and low sourness (Figure 1). A similar result was previously reported for light irradiation levels of 60–70% and moderate soil water stress through irrigation of 50–60% during the fruit growing of apples, which improved the fruit quality by increasing the SSC while reducing the TA [37].
The observed differences in these sensory parameters depending on the cultivation method were caused by differences in the primary and secondary metabolite profiles of C. unshiu. Previous studies showed that the soil water content and sunlight exposure affected the secondary metabolite profile and volatile compound levels of white grapes [38], quince [18], and grape berry [39]. A low soil water content causes drought and salt stress in citrus plants, resulting in reduced plant growth and leaf area, which ultimately affects photosynthesis, respiration, and metabolite profiles [40,41,42]. Moderate drought stress decreases the content of citric and malic acids in fruits such as nectarines [43] and grapes [44], which is in line with our results (Figure 3), whereas the opposite was reported for ponkan fruits grown in soil with 40% maximum water capacity under water stress [45]. In bitter oranges, drought and salinity stress increase the content of terpenes and flavonoids in the leaves [46] but decrease the fatty acid content in the roots [47]. However, in the current study, the primary and secondary metabolites of C. unshiu were not associated with soil water content but with soil mineral content, including N+, P+, and K+ (Figure 3 and Figure 4). Previous studies reported that N supplementation caused a decrease in phenylpropanoid metabolism in tomato fruits [48], while moderate nitrogen supplementation and N+-K+ balancing (N+:K+ ratio 3.6–4.3) produced the highest concentrations of phenolic compounds in grape berries [49]. However, in the present study, the soil P+ and K+ contents were positively correlated with the levels of most secondary metabolites, except for those of flavones and α-terpineol (Figure 4). Phosphorus can contribute to terpenoid production by supporting the production of terpenoid precursors and suppressing the emission of isoprene to outer cell membranes [50]. Furthermore, potassium fertilizer can increase the yield of essential oils in some aromatic crops, including oregano [51] and Artemisia annua [52]. In addition to the soil water and mineral contents, the soil pH plays a pivotal role in plant health by influencing the availability of essential nutrients, including minerals. Notably, the correlation analysis between the soil pH and metabolites revealed trends contrary to those observed for P+ and K+ (Figure 4). Despite the established optimal soil pH range of 5.5 to 6.5 for citrus production [53], and previous studies indicating a positive correlation between the soil pH and fruit quality [10,54], in this study, the scientific evidence has been insufficient to comprehensively elucidate the relationship with citrus quality.
Citrus flavor is a combination of various metabolites involved in the basic taste (sugars, acidic compounds, phenolic compounds, and limonoids) and volatile compounds [36]. In particular, acidic compounds play a dominant role in citrus taste by stimulating bitterness and suppressing or partially masking sweetness [55]. The bitterness and astringency of secondary metabolites, including flavonoids and limonoids, which have various health benefits [1], contribute to the taste of citrus [56,57,58]. In the present study, no significant difference occurred in the relative abundance of sugars between the cultivation methods (Figure 3); however, the sweetness of the fruits produced through film mulching was higher than that of the fruits from the other cultivation methods (Figure 1D). This may be due to the suppression of sweetness by the bitter compounds, including γ-terpinene, β-phellandrene, m-cymene, β-myrcene, valencene, limonene, linalool, nomilin, limonin, and zapoterin, which were increased by the other cultivation methods, and previous studies have reported that sweetness can be suppressed by bitterness [59]. In addition, cultivation-dependent changes in these metabolite profiles, which have various health benefits [1,4], may affect the quality and extent of the health benefits of citrus fruits; however, these functional properties were not investigated in the present study.
5. Conclusions
This study comprehensively investigated the soil characteristics, general characteristics, sensory evaluation, and metabolite profiles of the flesh of C. unshiu cultivated using four different methods (open field, greenhouse, film mulching, and tunnel farming). Notably, the soil water content, which varied with the cultivation methods, strongly influenced the taste perceptions, correlating with sweetness, sourness, bitterness, and astringency. However, the metabolite profiles associated with the citrus quality parameters were more closely tied to the soil mineral content, especially P+, and K+, than the soil water content. While primary metabolites like sugars remained consistent, secondary metabolites showed significant differences among the cultivation methods. In particular, sweetness was higher under film mulching than under the other cultivation methods because of the suppression of sweetness by bitter compounds. Although we did not investigate the relationship between the citrus quality and other cultivation parameters, these findings significantly contribute to our understanding of how cultivation methods impact C. unshiu quality and its correlation with metabolite profiles. These results provide valuable insights for optimizing various cultivation methods for C. unshiu in the citrus industry, paving the way for enhanced citrus fruit quality and production practices.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10010054/s1, Table S1: Identification of major metabolites via UPLC-Q-TOF MS and HPLC; Table S2: Identification of major metabolites via GC-MS; Figure S1: Average temperature (A), average rainfall (B), average duration of sunshine and solar radiation quantity (C) of Jeju, Korea; Figure S2: Representative chromatograms of C. unshiu metabolites analyzed via UPLC-Q-TOF MS; Figure S3: Representative chromatograms of C. unshiu non-volatile compounds analyzed via GC-MS; Figure S4: Representative chromatograms of C. unshiu volatile terpenes analyzed via GC-MS; Figure S5: Representative chromatograms of C. unshiu ascorbic acid analyzed via HPLC.
Author Contributions
Conceptualization, D.-S.K. and H.-J.K.; methodology, D.-S.K. and S.S.K.; software, S.-M.J.; validation, D.-S.K. and H.-J.K.; formal analysis, S.-M.J. investigation, S.-M.J., D.-S.K. and S.S.K.; writing—original draft preparation, S.-M.J., D.-S.K. and H.-J.K.; writing—review and editing, D.-S.K. and H.-J.K.; visualization, S.-M.J. and D.-S.K.; supervision, H.-J.K.; project administration, H.-J.K.; funding acquisition, S.S.K. and H.-J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01496903)”, Rural Development Administration, Republic of Korea. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A3072463).
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of phytochemical and nutritional characteristics and food applications of Citrus L. fruits. Front. Nutr. 2022, 9, 968604. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive compounds of citrus Fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus waste as source of bioactive compounds: Extraction and utilization in health and food industry. Molecules 2023, 27, 1636. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, H.J.; Park, K.J.; Kang, S.B.; Park, Y.; Han, S.G.; Kim, M.; Song, Y.H.; Kim, D.S. Metabolomic profiling of Citrus unshiu during different stages of fruit development. Plants 2022, 11, 967. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, S.; Park, S.M.; Yun, S.H.; Gab, H.S.; Kim, S.S.; Kim, H.J. Comparative metabolomics analysis of citrus varieties. Foods 2021, 10, 2826. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jin, R.; Yang, Z.; Wang, X.; You, G.; Guo, J.; Zhang, Y.; Liu, F.; Pan, S. Comparative study on physicochemical, nutritional and enzymatic properties of two Satsuma mandarin (Citrus unshiu Marc.) varieties from different regions. J. Food Compos. Anal. 2021, 95, 103614. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Lesce, M.R.; Spinelli, M.; Fontanarosa, C.; Paradiso, R.; Amoresano, A.; Arena, C. Modulation of antioxidant compounds in fruits of Citrus reticulata Blanco using postharvest LED irradiation. Biology 2023, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- Carmona, L.; Sulli, M.; Diretto, G.; Alquézar, B.; Alves, M.; Peña, L. Improvement of antioxidant properties in fruit from two blood and blond orange cultivars by postharvest storage at low temperature. Antioxidants 2022, 11, 547. [Google Scholar] [CrossRef]
- Treeby, M.T.; Henriod, R.E.; Bevington, K.B.; Milne, D.J.; Storey, R. Irrigation management and rootstock effects on navel orange [Citrus sinensis (L.) Osbeck] fruit quality. Agric. Water Manag. 2007, 91, 24–32. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Tan, Q.; Sun, X.; Wei, W.; Hu, C. Biochar is superior to lime in improving acidic soil properties and fruit quality of Satsuma mandarin. Sci. Total Environ. 2020, 714, 136722. [Google Scholar] [CrossRef]
- Wang, S.; Xie, W.; Yan, X. Effects of future climate change on citrus quality and yield in China. Sustainability 2022, 14, 9366. [Google Scholar] [CrossRef]
- Xuemei, J.; Qiong, Y.; Ya, W.; Yanmei, L.; Yongqiang, Z.; Rangjin, X.; Shaolan, H.; Lie, D.; Shilai, Y.; Qiang, L.; et al. Effects of DuPont Tyvek® non-woven material mulching on fruit quality and chlorophyll fluorescence in Wanzhou Rose Orange. Sci. Hortic. 2017, 219, 31–36. [Google Scholar] [CrossRef]
- Cozzolino, E.; Mola, I.D.; Ottaiano, L.; Bilotto, M.; Petriccione, M.; Ferrara, E.; Mori, M.; Morra, L. Assessing yield and quality of melon (Cucumis melo L.) improved by biodegradable mulching film. Plants 2023, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Gecer, M.K.; Orman, E.; Gundogdu, M.; Ercisli, S.; Karunakaran, R. Identification of metabolites changes and quality in strawberry fruit: Effect of cultivation in high tunnel and open field. Plants 2022, 11, 1368. [Google Scholar] [CrossRef]
- Wu, B.P.; Zhang, C.; Gao, Y.B.; Zheng, W.W.; Xu, K. Changes in sugar accumulation and related enzyme activities of red bayberry (Myrica rubra) in greenhouse cultivation. Horticulturae 2021, 7, 429. [Google Scholar] [CrossRef]
- Helaly, A.A.; Goda, Y.; Abd El-Rehim, A.S.; Mohamed, A.A.; El-Zeiny, O.A.H. Effect of polyethylene mulching type on the growth, yield and fruits quality of Physalis pubescens. Adv. Plants Agric. Res. 2017, 6, 154–160. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Pereira-Caro, G.; Cardeñosa, V.; Muriel, J.L.; Moreno-Rojas, J.M. Study of the quality attributes of selected blueberry (Vaccinium corymbosum L.) varieties grown under different irrigation regimes and cultivation systems. Appl. Sci. 2020, 10, 8459. [Google Scholar] [CrossRef]
- Griñán, I.; Galindo, A.; Rodríguez, P.; Morales, D.; Corell, M.; Centeno, A.; Collado-González, J.; Torrecillas, A.; Carbonell-Barrachina, A.A.; Hernández, F. Volatile composition and sensory and quality attributes of quince (Cydonia oblonga Mill.) fruits as affected by water stress. Sci. Hortic. 2019, 244, 68–74. [Google Scholar] [CrossRef]
- Nam, H.A.; Ramakrishnan, S.R.; Kwon, J.H. Effects of electron-beam irradiation on the quality characteristics of mandarin oranges (Citrus unshiu (Swingle) Marcov) during storage. Food Chem. 2019, 286, 338–345. [Google Scholar] [CrossRef]
- Huang, S.; Lim, S.Y.; Lau, H.; Ni, W.; Li, S.F.Y. Effect of glycinebetaine on metabolite profiles of cold-stored strawberry revealed by 1H NMR-based metabolomics. Food Chem. 2022, 393, 133452. [Google Scholar] [CrossRef]
- Perotti, V.E.; Moreno, A.S.; Trípodi, K.E.; Meier, G.; Bello, F.; Cocco, M.; Vázquez, D.; Anderson, C.; Podestá, F.E. Proteomic and metabolomic profiling of Valencia orange fruit after natural frost exposure. Physiol. Plant. 2015, 153, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Moing, A.; Allwood, J.W.; Aharoni, A.; Baker, J.; Beale, M.H.; Ben-Dor, S.; Biais, B.; Brigante, F.; Burger, Y.; Deborde, C.; et al. Comparative metabolomics and molecular phylogenetics of melon (Cucumis melo, Cucurbitaceae) biodiversity. Metabolites 2020, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Madden, R.T.; Sung, J.; Chambers, A.H.; Crane, J.; Wang, Y. Pathway-based metabolomics analysis reveals biosynthesis of key flavor compounds in mango. J. Agric. Food Chem. 2021, 70, 10389–10399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, X.; Li, Y.; Fan, Y.; Li, Y.; Cao, Y.; An, W.; Shi, Z.; Zhao, J.; Guo, S. Changes in metabolome and nutritional quality of Lycium barbarum fruits from three typical growing areas of China as revealed by widely targeted metabolomics. Metabolites 2020, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, Y.; Zhao, Z.; Qu, G.; Cao, J. Transcriptomics integrated with metabolomics reveals underlying mechanisms of cold-induced flesh bleeding in plum (cv. Friar) fruit during storage. Postharvest Biol. Technol. 2022, 192, 112032. [Google Scholar] [CrossRef]
- Obreza, T.A.; Zekri, M.; Hanlon, E.A.; Morgan, K.; Schumann, A.; Rouse, R. Soil and Leaf Tissue Testing for Commercial Citrus Production; SL253.04 Fla.; Cooperative Extension Service, University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2010; pp. 1–11. [Google Scholar]
- Kim, D.S.; Jeong, S.M.; Jo, S.H.; Chanmuang, S.; Kim, S.S.; Park, S.M.; Yun, S.H.; Han, S.G.; Cho, J.Y.; Kang, I.; et al. Comparative analysis of physicochemical properties and storability of a new citrus variety, Yellowball, and its parent. Plants 2023, 12, 2863. [Google Scholar] [CrossRef]
- Jin, L.F.; Guo, D.Y.; Ning, D.Y.; Hussain, S.B.; Liu, Y.Z. Covering the trees of Kinokuni tangerine with plastic film during fruit ripening improves sweetness and alters the metabolism of cell wall components. Acta Physiol. Plant. 2018, 40, 182. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Lin, Q.; Qian, J.; Zhao, C.; Wang, D.; Liu, C.; Wang, Z.; Sun, C.; Chen, K. Low temperature induced changes in citrate metabolism in ponkan (Citrus reticulata Blanco cv. Ponkan) fruit during maturation. PLoS ONE 2016, 11, e0156703. [Google Scholar] [CrossRef]
- Yue, X.; Wei, S.; Liu, W.; Lu, J.; Fang, Y.; Zhang, Z.; Ju, Y. Effect of rain-shelter cultivation on the monoterpenes profile of Muscat Hamburg grapes and wines. Sci. Hortic. 2021, 285, 110136. [Google Scholar] [CrossRef]
- Bülent, K. Effect of light intensity and temperature on growth and quality parameters of grafted vines. Not. Bot. Horti Agrobot. 2014, 42, 507–515. [Google Scholar] [CrossRef][Green Version]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. High tunnel cultivation of sweet cherry (Prunus avium L.): Physiological and production variables. Sci. Hortic. 2019, 251, 108–117. [Google Scholar] [CrossRef]
- Shimazaki, M.; Nesumi, H. A method for high-quality citrus production using drip fertigation and plastic sheet mulching. Jpn. Agric. Res. Q. 2016, 50, 301–306. [Google Scholar] [CrossRef][Green Version]
- Alam, A.; Hariyanto, B.; Ullah, H.; Salin, K.R.; Datta, A. Effects of silicon on growth, yield and fruit quality of cantaloupe under drought stress. Silicon 2021, 13, 3153–3162. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 10, 1–6. Available online: https://access.portico.org/stable?au=phx64r3rfpq (accessed on 13 September 2023).
- Wang, Y.; Liu, L.; Wang, Y.; Tao, H.; Fan, J.; Zhao, Z.; Guo, Y. Effects of soil water stress on fruit yield, quality and their relationship with sugar metabolism in ‘Gala’ apple. Sci. Hortic. 2019, 258, 108753. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario–a review. Front. Plant Sci. 2021, 12, 262. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Morianou, G.; Kourgialas, N. Drought and salinity in citriculture: Optimal practices to alleviate salinity and water stress. Agronomy 2021, 11, 1283. [Google Scholar] [CrossRef]
- Rafie-Rad, Z.; Moradkhani, M.; Golchin, A.; Razam, T.; Eash, N.S. Abiotic Stresses Management in Citrus. In Citrus Research—Horticultural and Human Health Aspects; Gonzatto, M.P., Santos, J.S., Eds.; IntechOpen: London, UK, 2022; pp. 1–19. [Google Scholar] [CrossRef]
- Rao, M.J.; Feng, B.; Ahmad, M.H.; Tahir ul Qamar, M.; Aslam, M.Z.; Khalid, M.F.; Hussain, S.; Zhong, R.; Ali, Q.; Xu, Q.; et al. LC-MS/MS-based metabolomics approach identified novel antioxidant flavonoids associated with drought tolerance in citrus species. Front. Plant Sci. 2023, 14, 1150854. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, Z. Responses of ‘Spring Bright’ and ‘Summer Bright’ nectarines to deficit irrigation: Fruit growth and concentration of sugars and organic acids. Sci. Hortic. 2012, 135, 112–119. [Google Scholar] [CrossRef]
- Geng, K.; Zhang, Y.; Lv, D.; Li, D.; Wang, Z. Effects of water stress on the sugar accumulation and organic acid changes in Cabernet Sauvignon grape berries. Hort. Sci. 2022, 49, 164–178. [Google Scholar] [CrossRef]
- Zhang, G.; Xie, S. Influence of water stress on the citric acid metabolism related gene expression in the ponkan fruits. Agric. Sci. 2014, 5, 1513–1521. [Google Scholar] [CrossRef]
- Eirini, S.; Paschalina, C.; Ioannis, T.; Kortessa, D.T. Effect of drought and salinity on volatile organic compounds and other secondary metabolites of Citrus aurantium leaves. Nat. Prod. Commun. 2017, 12, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Lamine, M.; Gargouri, M.; Mliki, A. Identification of the NaCl-responsive metabolites in Citrus roots: A lipidomic and volatomic signature. Plant Signal. Behav. 2020, 15, 1777376. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.X.; Son, S.; Lee, S.; Jung, E.; Lee, Y.; Sung, J.; Lee, C. Combined effects of nutrients × water × light on metabolite composition in tomato fruits (Solanum lycopersicum L.). Plants 2021, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Martín, P.; Álamo, M.; González, M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Ormeño, E.; Fernandez, C. Effect of soil nutrient on production and diversity of volatile terpenoids from plants. Curr. Bioact. Compd. 2012, 8, 71–79. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Ayad, H.S.; Hendawy, S.F. Effect of potassium humate and nitrogen fertilizer on herb and essential oil of oregano under different irrigation intervals. Ozean J. Appl. Sci. 2009, 2, 319–323. [Google Scholar]
- Davies, M.J.; Atkinson, C.J.; Burns, C.; Woolly, J.G.; Hipps, N.A.; Arroo, R.R.J.; Dungey, N.; Robinson, T.; Brown, P.; Flockart, I.; et al. Enhancement of artemisinin concentration and yield in response to optimization of nitrogen and potassium supply to Artemisia annua. Ann. Bot. 2009, 104, 315–323. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, F.; Wu, Y.; Xhou, T.; Chang, Y.; Lian, X.; Yin, T.; Ye, L.; Li, Y.; Lu, X. Profiles of citrus orchard nutrition and fruit quality in Hunan Province, China. Int. J. Fruit Sci. 2022, 22, 779–793. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Wei, J.; Jiang, J.; Li, Y.; Chen, J.; Yu, H. Growth, fruit Yield, photosynthetic characteristics, and leaf microelement concentration of two blueberry cultivars under different long-term soil pH treatments. Agronomy 2019, 9, 357. [Google Scholar] [CrossRef]
- Feng, S.; Gmitter, F.G., Jr.; Grosser, J.W.; Wang, Y. Identification of key flavor compounds in citrus fruits: A flavoromics approach. ACS Food Sci. Technol. 2021, 1, 2076–2085. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Ding, F.; Sun, D.; Ma, Z.; Cheng, Y.; Xu, J. Content changes of bitter compounds in ‘Guoqing No. 1’Satsuma mandarin (Citrus unshiu Marc.) during fruit development of consecutive 3 seasons. Food Chem. 2014, 145, 963–969. [Google Scholar] [CrossRef]
- Glabasnia, A.; Dunkel, A.; Frank, O.; Hofmann, T. Decoding the nonvolatile sensometabolome of orange juice (Citrus sinensis). J. Agric. Food Chem. 2018, 66, 2354–2369. [Google Scholar] [CrossRef]
- Jin, H.; Fishman, Z.H.; Ye, M.; Wang, L.; Zuker, C.S. Top-down control of sweet and bitter taste in the mammalian brain. Cell 2021, 184, 257–271.e16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).