Alcohol-Free Beer Produced Using Maltose-Negative Wine Yeast Saccharomyces cerevisiae with Probiotic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Media, and Cultivation
2.3. Wort Fermentation
2.4. Experimental Design Based on Response Surface Methodology
2.5. Analytical Methods
2.6. Sensory Evaluation
2.7. Resistance to the Simulated Gastrointestinal Conditions
2.8. Determining Viability by Flow Cytometry
3. Results
3.1. Growth Characteristics of Cultures in Laboratory Settings
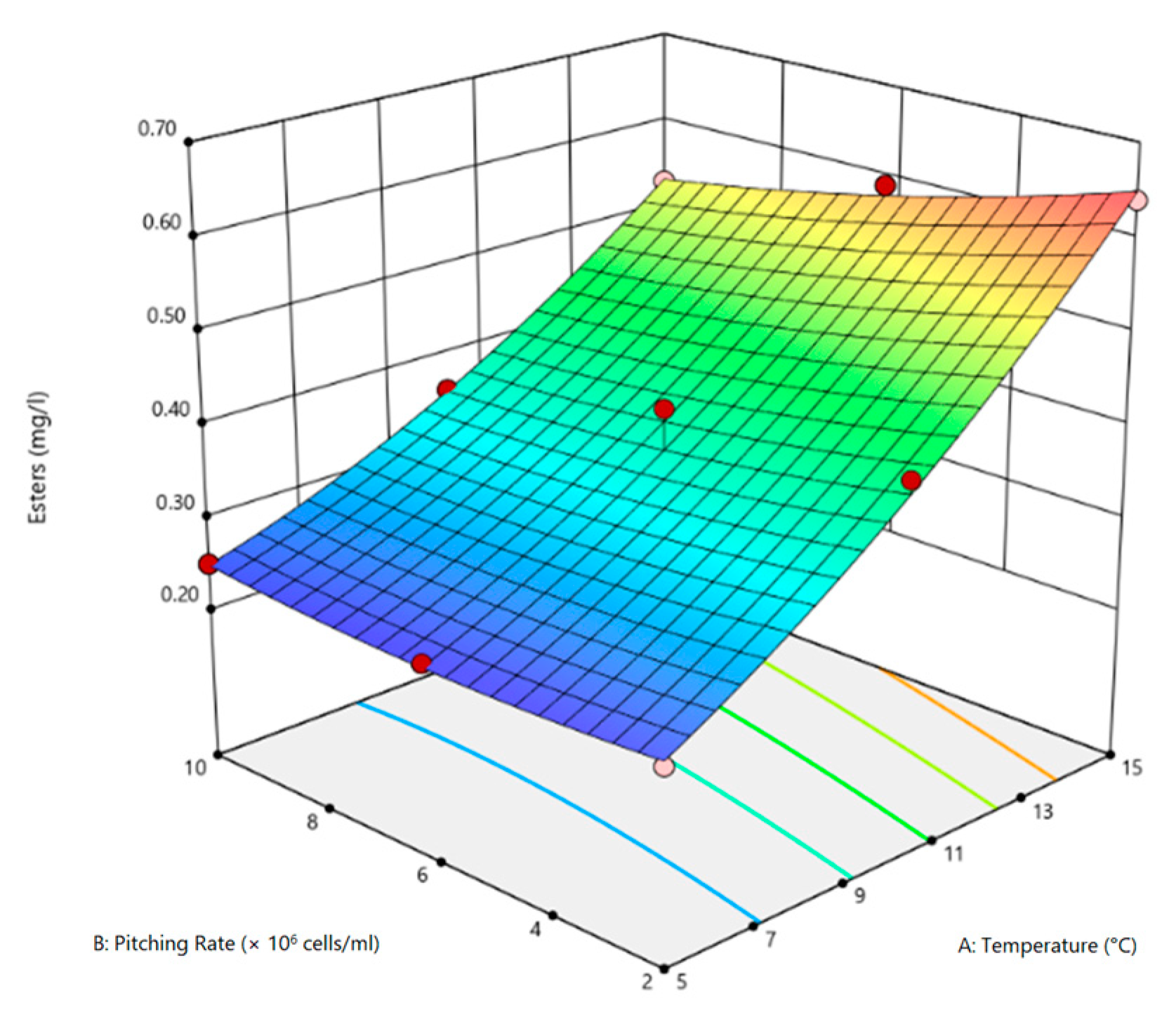
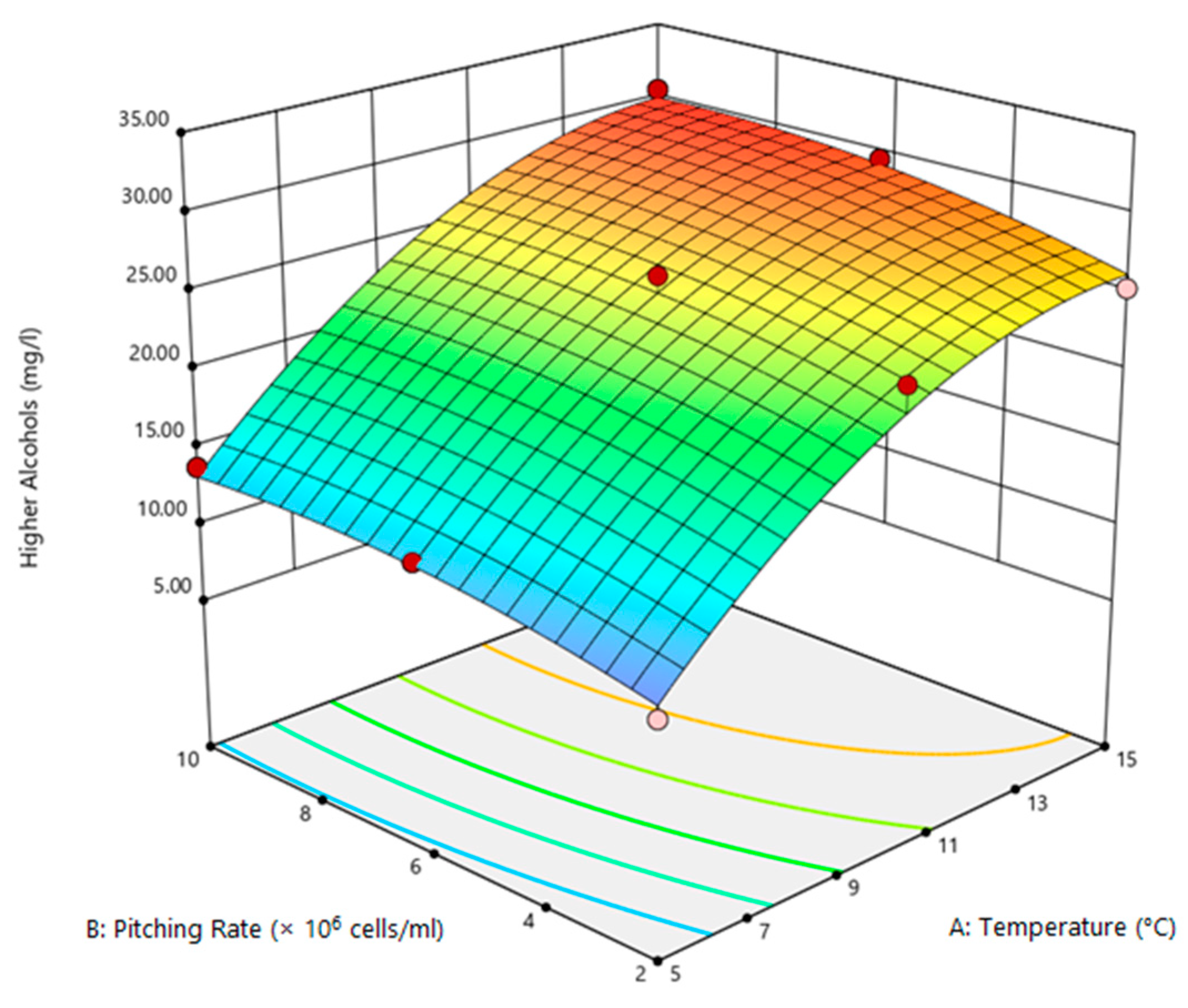
3.2. Optimization of Process Variables
3.3. Statistical Evaluation of Process Variables
3.4. Sensory Evaluation of the Alcohol-Free Beer
3.5. Gastrointestinal Survival Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sancén, M.; Léniz, A.; Macarulla, M.T.; González, M.; Milton-Laskibar, I.; Portillo, M.P. Features of Non-Alcoholic Beer on Cardiovascular Biomarkers. Can It Be a Substitute for Conventional Beer? Nutrients 2023, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Kulieva, V.A.; Hernández-Martínez, E.; Minchán-Velayarce, H.H.; Pasapera-Campos, S.E.; Luque-Vilca, O.M. A Comprehensive Review of the Benefits of Drinking Craft Beer: Role of Phenolic Content in Health and Possible Potential of the Alcoholic Fraction. Curr. Res. Food Sci. 2023, 6, 100477. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Gao, X. Research Progress on the Antioxidant Biological Activity of Beer and Strategy for Applications. Trends Food Sci. Technol. 2021, 110, 754–764. [Google Scholar] [CrossRef]
- European Parliament & Council Regulation (EC) 1924/2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Communities 2006, L404, 1–15.
- Kokole, D.; Jané Llopis, E.; Anderson, P. Non-Alcoholic Beer in the European Union and UK: Availability and Apparent Consumption. Drug Alcohol. Rev. 2022, 41, 550–560. [Google Scholar] [CrossRef]
- Anderson, P.; Jané Llopis, E.; O’donnell, A.; Manthey, J.; Rehm, J. Impact of Low and No Alcohol Beers on Purchases of Alcohol: Interrupted Time Series Analysis of British Household Shopping Data, 2015–2018. BMJ Open 2020, 10, e036371. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; O’donnell, A.; Kokole, D.; Llopis, E.J.; Kaner, E. Is Buying and Drinking Zero and Low Alcohol Beer a Higher Socio-economic Phenomenon? Analysis of British Survey Data, 2015–2018 and Household Purchase Data 2015–2020. Int. J. Environ. Res. Public Health 2021, 18, 10347. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C. Recent Innovations in the Production of Selected Specialty (Non-Traditional) Beers. Folia Microbiol. 2021, 66, 525–541. [Google Scholar] [CrossRef]
- Merinas-Amo, T.; Celestino, M.D.R.; Font, R.; Alonso-Moraga, Á. Safety and Protective Activities of Manufactured Alcohol-Free Beers. Processes 2022, 10, 331. [Google Scholar] [CrossRef]
- Mellor, D.D.; Hanna-Khalil, B.; Carson, R. A Review of the Potential Health Benefits of Low Alcohol and Alcohol-Free Beer: Effects of Ingredients and Craft Brewing Processes on Potentially Bioactive Metabolites. Beverages 2020, 6, 25. [Google Scholar] [CrossRef]
- Staub, C.; Contiero, R.; Bosshart, N.; Siegrist, M. You Are What You Drink: Stereotypes about Consumers of Alcoholic and Non-Alcoholic Beer. Food Qual. Prefer. 2022, 101, 104633. [Google Scholar] [CrossRef]
- Blanco, C.A.; Andrés-Iglesias, C.; Montero, O. Low-Alcohol Beers: Flavor Compounds, Defects, and Improvement Strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 1379–1388. [Google Scholar] [CrossRef]
- Brányik, T.; Silva, D.P.; Baszczyňski, M.; Lehnert, R.; Almeida, E.; Silva, J.B. A Review of Methods of Low Alcohol and Alcohol-Free Beer Production. J. Food Eng. 2012, 108, 493–506. [Google Scholar] [CrossRef]
- Jackowski, M.; Trusek, A. Non-Alcoholic Beer Production—An Overview. Pol. J. Chem. Technol. 2018, 20, 32–38. [Google Scholar] [CrossRef]
- Piornos, J.A.; Koussissi, E.; Balagiannis, D.P.; Brouwer, E.; Parker, J.K. Alcohol-free and low-alcohol beers: Aroma chemistry and sensory characteristics. Compr. Rev. Food Sci. Food Saf. 2023, 22, 233–259. [Google Scholar] [CrossRef]
- Johansson, L.; Nikulin, J.; Juvonen, R.; Krogerus, K.; Magalhães, F.; Mikkelson, A.; Nuppunen-Puputti, M.; Sohlberg, E.; de Francesco, G.; Perretti, G.; et al. Sourdough Cultures as Reservoirs of Maltose-Negative Yeasts for Low-Alcohol Beer Brewing. Food Microbiol. 2021, 94, 103629. [Google Scholar] [CrossRef]
- Yabaci Karaoglan, S.; Jung, R.; Gauthier, M.; Kinčl, T.; Dostálek, P. Maltose-Negative Yeast in Non-Alcoholic and Low-Alcoholic Beer Production. Fermentation 2022, 8, 273. [Google Scholar] [CrossRef]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of Yeasts in the Brewing Process: Tradition and Innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces Cerevisiae Var. Boulardii in Co-Fermentations with S. Cerevisiae for the Production of Craft Beers with Potential Healthy Value-Added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Pereira de Paula, B.; de Souza Lago, H.; Firmino, L.; Fernandes Lemos Júnior, W.J.; Ferreira Dutra Corrêa, M.; Fioravante Guerra, A.; Signori Pereira, K.; Zarur Coelho, M.A. Technological Features of Saccharomyces Cerevisiae Var. Boulardii for Potential Probiotic Wheat Beer Development. LWT 2021, 135, 110233. [Google Scholar] [CrossRef]
- Senkarcinova, B.; Graça Dias, I.A.; Nespor, J.; Branyik, T. Probiotic Alcohol-Free Beer Made with Saccharomyces Cerevisiae Var. Boulardii. LWT 2019, 100, 362–367. [Google Scholar] [CrossRef]
- Davey, H.M. Life, Death, and in-between: Meanings and Methods in Microbiology. Appl. Environ. Microbiol. 2011, 77, 5571–5576. [Google Scholar] [CrossRef]
- Davey, H.; Guyot, S. Estimation of Microbial Viability Using Flow Cytometry. Curr. Protoc. Cytom. 2020, 93, e72. [Google Scholar] [CrossRef]
- Wendel, U. Assessing Viability and Stress Tolerance of Probiotics—A Review. Front. Microbiol. 2022, 12, 818468. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef] [PubMed]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a New Approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Brányik, T.; Kubizniaková, P.; Slaby, M.; Hanzaliková, K.; Ilpars, E.; Matoulková, D.; Vlach, M.; Veverka, L. New Yeast Strain Saccharomyces Cerevisiae CCM 9181 Isolated at End of Spontaneous Fermentation of Grape Pomace, Useful for Producing Low-Alcohol and Non-Alcoholic Beer 2021.
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protocols 1990, 315–322. [Google Scholar] [CrossRef]
- Jurková, M.; Olšovská, J.; Čejka, P. Determination of Sugars and Saccharides in Beer. Kvasny Prumysl 2018, 64, 58–64. [Google Scholar] [CrossRef]
- EBC Analysis Committee EBC Methods of Analysis. Method 9.4.: Original, Real, and Apparent Extract and Original Gravity of Beer. In Analytica EBC; Carl Getränke-Fachverlag: Nürnberg, Germany, 2010. [Google Scholar]
- EBC Analysis Committee EBC Methods of Analysis. Method 9.2.6.: Alcohol in Beer by Near Infrared Spectroscopy. In Analytica EBC; Carl Getränke-Fachverlag: Nürnberg, Germany, 2010. [Google Scholar]
- EBC Analysis Committee EBC Methods of Analysis. Method 9.24.2.: Vicinal Diketones in Beer: Gas Chromatography Method. In Analytica EBC; Carl Getränke-Fachverlag: Nürnberg, Germany, 2010. [Google Scholar]
- EBC Analysis Committee EBC Methods of Analysis. Method 9.39.: Dimethyl Sulphide and Other Lower Boiling Point Volatile Compounds in Beer by Gas Chromatography Method. In Analytica EBC; Carl Getränke-Fachverlag: Nürnberg, Germany, 2009. [Google Scholar]
- Pennacchia, C.; Blaiotta, G.; Pepe, O.; Villani, F. Isolation of Saccharomyces Cerevisiae Strains from Different Food Matrices and Their Preliminary Selection for a Potential Use as Probiotics. J. Appl. Microbiol. 2008, 105, 1919–1928. [Google Scholar] [CrossRef]
- Molecular Probes LIVE/DEAD® FungaLightTM Yeast Viability Kit Quick Facts. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp34952.pdf (accessed on 28 March 2022).
- Gudde, L.R.; Hulce, M.; Largen, A.H.; Franke, J.D. Sterol Synthesis Is Essential for Viability in the Planctomycete Bacterium Gemmata Obscuriglobus. FEMS Microbiol. Lett. 2019, 366, fnz019. [Google Scholar] [CrossRef]
- Decree on Requirements for Beverages, Fermented Vinegar and Yeast. (2018)|FAOLEX. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC192349/ (accessed on 14 July 2023).
- Jackson, J.F.; Linskens, H.F. Analysis of Taste and Aroma. In Molecular Methods of Plant Analysis; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783662048580. [Google Scholar]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef]
- Budroni, M.; Zara, G.; Ciani, M.; Comitini, F. Saccharomyces and Non-Saccharomyces Starter Yeasts; Kanauchi, M., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 4; ISBN 978-953-51-3342-1. [Google Scholar]
- Puerari, C.; Strejc, J.; Souza, A.C.; Karabín, M.; Schwan, R.F.; Brányik, T. Optimization of Alcohol-Free Beer Production by Lager and Cachaça Yeast Strains Using Response Surface Methodology. J. Inst. Brew. 2016, 122, 69–75. [Google Scholar] [CrossRef]
- Landaud, S.; Latrille, E.; Corrieu, G. Top Pressure and Temperature Control the Fusel Alcohol/Ester Ratio through Yeast Growth in Beer Fermentation. J. Inst. Brew. 2001, 107, 107–117. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces Cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Suomalainen, H. Yeast Esterases and Aroma Esters in Alcoholic Beverages. J. Inst. Brew. 1981, 87, 296–300. [Google Scholar] [CrossRef]
- Verbelen, P.J.; Dekoninck, T.M.L.; Saerens, S.M.G.; Van Mulders, S.E.; Thevelein, J.M.; Delvaux, F.R. Impact of Pitching Rate on Yeast Fermentation Performance and Beer Flavour. Appl. Microbiol. Biotechnol. 2009, 82, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, Z.; Wang, Y.; Wei, Z.; Chen, B.; Zhang, H.; Guo, X.; Xiao, D. The Effect of Pitching Rate on the Production of Higher Alcohols by Top-Fermenting Yeast in Wheat Beer Fermentation. Ann. Microbiol. 2019, 69, 713–726. [Google Scholar] [CrossRef]
- Lehnert, R.; Novák, P.; Macieira, F.; Kuřec, M.; Teixeira, J.A.; Brányik, T. Optimisation of Lab-Scale Continuous Alcohol-Free Beer Production. Czech J. Food Sci. 2009, 27, 267–275. [Google Scholar] [CrossRef]
- Pires, E.; Brányik, T. Biochemistry of Beer Fermentation; Springer Briefs in Biochemistry and Molecular Biology; Springer: Cham, Switzerland, 2015; By-products of Beer Fermentation; ISBN 9783319151885. [Google Scholar]
- FAO/WHO Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, April 30 and May 1, 2002. 2002. Available online: https://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000197343.pdf (accessed on 14 July 2023).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Davis, C. Enumeration of Probiotic Strains: Review of Culture-Dependent and Alternative Techniques to Quantify Viable Bacteria. J. Microbiol. Methods 2014, 103, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, F.; Arpentin, G. The Effect of Pesticide Usage on Grape Yeast. BIO Web Conf. 2022, 53, 05001. [Google Scholar] [CrossRef]
- Xie, M.; Xu, L.; Zhang, R.; Zhou, Y.; Xiao, Y.; Su, X.; Shen, C.; Sun, F.; Hashmi, M.Z.; Lin, H.; et al. Viable but Nonculturable State of Yeast Candida Sp. Strain LN1 Induced by High Phenol Concentrations. Appl. Environ. Microbiol. 2021, 87, e01110-21. [Google Scholar] [CrossRef]
- Salma, M.; Rousseaux, S.; Sequeira-Le Grand, A.; Divol, B.; Alexandre, H. Characterization of the Viable but Nonculturable (VBNC) State in Saccharomyces Cerevisiae. PLoS ONE 2013, 8, e77600. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Z.; Sun, W.; Luan, Y.; Piao, M.; Deng, Y. Characterization and Formation Mechanisms of Viable, but Putatively Non-Culturable Brewer’s Yeast Induced by Isomerized Hop Extract. LWT 2022, 155, 112974. [Google Scholar] [CrossRef]
- Decent Number—0.5%—Ferdinand Brewery—Real Beer. Available online: https://www.pivovarferdinand.cz/decent-number/decent-number-0-5-1 (accessed on 14 July 2023).
| Symbol | Independent Variable (Units) | Coded Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| T | Temperature (°C) | 5 | 10 | 15 |
| P | Pitching rate (×106 cells/mL) | 2 | 6 | 10 |
| Factors | Responses | |||
|---|---|---|---|---|
| T | P | HA (mg/L) | ES (mg/L) | Ethanol (% v/v) |
| +1 | −1 | 25.30 | 0.64 | 0.44 |
| −1 | 0 | 13.20 | 0.24 | 0.16 |
| −1 | +1 | 13.80 | 0.25 | 0.17 |
| 0 | −1 | 24.01 | 0.43 | 0.38 |
| 0 | +1 | 24.30 | 0.36 | 0.41 |
| +1 | 0 | 29.50 | 0.59 | 0.46 |
| +1 | +1 | 30.50 | 0.53 | 0.46 |
| −1 | −1 | 09.70 | 0.24 | 0.09 |
| 0 | 0 | 24.10 | 0.33 | 0.41 |
| 0 | 0 | 24.30 | 0.36 | 0.42 |
| 0 | 0 | 26.10 | 0.42 | 0.42 |
| Temperature (°C) | Iso-α-Bitter Acids (IBU) | Ethanol (% w/v) | µ (h−1) |
|---|---|---|---|
| 30 | 0 | 0 | 0.38 ± 0.01 |
| 25 | 0 | 0 | 0.41 ± 0.01 |
| 20 | 0 | 0 | 0.34 ± 0.02 |
| 15 | 0 | 0 | 0.18 ± 0.06 |
| 8 | 0 | 0 | 0.08 ± 0.01 |
| 3 | 0 | 0 | - |
| 20 | 15 | 0 | 0.34 ± 0.01 |
| 20 | 30 | 0 | 0.34 ± 0.02 |
| 20 | 50 | 0 | 0.28 ± 0.03 |
| 20 | 0 | 0.5 | 0.34 ± 0.01 |
| 20 | 0 | 2.5 | 0.31 ± 0.02 |
| 20 | 0 | 5.0 | 0.26 ± 0.01 |
| Source | SSQ | df | MSQ | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.19 | 5 | 0.04 | 39.68 | 0.0005 |
| T | 0.18 | 1 | 0.18 | 185.68 | <0.0001 |
| P | 4.80 × 10−3 | 1 | 4.80 × 10−3 | 5.06 | 0.0744 |
| TP | 3.60 × 10−3 | 1 | 3.60 × 10−3 | 3.78 | 0.1095 |
| T2 | 2.60 × 10−3 | 1 | 2.60 × 10−3 | 2.70 | 0.1614 |
| P2 | 4.00 × 10−4 | 1 | 4.00 × 10−4 | 0.37 | 0.5680 |
| LoF | 6.00 × 10−4 | 3 | 2.00 × 10−4 | ||
| PE | 4.20 × 10−3 | 2 | 2.10 × 10−3 | ||
| R2 | 0.9754 | ||||
| Adj.R2 | 0.9508 |
| Source | SSQ | df | MSQ | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 461.26 | 5 | 92.25 | 50.01 | 0.0003 |
| T | 393.66 | 1 | 393.66 | 213.40 | <0.0001 |
| P | 15.33 | 1 | 15.33 | 8.31 | 0.0345 |
| TP | 0.30 | 1 | 0.30 | 0.16 | 0.7023 |
| T2 | 39.11 | 1 | 39.11 | 21.20 | 0.0058 |
| P2 | 3.20 | 1 | 3.20 | 1.73 | 0.2449 |
| LoF | 6.80 | 3 | 2.27 | ||
| PE | 2.43 | 2 | 1.21 | ||
| R2 | 0.9804 | ||||
| Adj.R2 | 0.9608 |
| Compounds | Analyte | Concentration (mg/L) |
|---|---|---|
| Higher alcohols | isobutanol | 1.80 |
| n-propanol | 4.00 | |
| 2-phenylethanol | 1.20 | |
| 2- and 3-methylbutanol | 18.30 | |
| Esters | ethyl acetate | 0.48 |
| isoamyl acetate | 0.14 | |
| 2-phenylethyl acetate | 0.02 | |
| Aldehydes | acetaldehyde | 1.40 |
| benzaldehyde | 1.5 × 10−3 | |
| Vicinal diketones | diacetyl | 0.167 |
| Sulphur substances | pentanedione | 0.012 |
| methional | 4.96 × 10−3 |
| AGJ (Cells/mL) | Survival % in AGJ | AGJ + AIF (Cells/mL) | Survival % in AGJ + AIF | ||
|---|---|---|---|---|---|
| Strain | 0 h | 2.5 h | 5 h | ||
| S. boulardii | 4.6 × 108 | 4.6 × 108 | 100.0 a ± 2.1 | 1.9 × 107 | 4.1 a ± 0.3 |
| S. pastorianus | 8.5 × 108 | 6.0 × 108 | 63.6 b ± 3.4 | 8.9 × 106 | 1.1 b± 0.2 |
| S. cerevisiae | 1.6 × 109 | 5.4 × 108 | 37.4 c ± 2.1 | 6.4 × 107 | 4.0 a ± 0.01 |
| Strain | Survival % | |
|---|---|---|
| AGJ (2.5 h) | AGJ + AIF (5 h) | |
| S. boulardii | 99.8 a ± 0.00 | 99.2 a ± 0.26 |
| S. pastorianus | 97.4 b ± 0.67 | 5.7 b ± 3.35 |
| S. cerevisiae | 99.7 a ± 0.06 | 99.0 a ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İlpars, E.; Titlová, Š.; Hanzalíková, K.; Křížová, I.; Brányik, T. Alcohol-Free Beer Produced Using Maltose-Negative Wine Yeast Saccharomyces cerevisiae with Probiotic Potential. Fermentation 2023, 9, 805. https://doi.org/10.3390/fermentation9090805
İlpars E, Titlová Š, Hanzalíková K, Křížová I, Brányik T. Alcohol-Free Beer Produced Using Maltose-Negative Wine Yeast Saccharomyces cerevisiae with Probiotic Potential. Fermentation. 2023; 9(9):805. https://doi.org/10.3390/fermentation9090805
Chicago/Turabian Styleİlpars, Emre, Štěpánka Titlová, Katarína Hanzalíková, Ivana Křížová, and Tomáš Brányik. 2023. "Alcohol-Free Beer Produced Using Maltose-Negative Wine Yeast Saccharomyces cerevisiae with Probiotic Potential" Fermentation 9, no. 9: 805. https://doi.org/10.3390/fermentation9090805
APA Styleİlpars, E., Titlová, Š., Hanzalíková, K., Křížová, I., & Brányik, T. (2023). Alcohol-Free Beer Produced Using Maltose-Negative Wine Yeast Saccharomyces cerevisiae with Probiotic Potential. Fermentation, 9(9), 805. https://doi.org/10.3390/fermentation9090805







