Effects of Exogenous Oral Infusion of Volatile Fatty Acids on Ileal Microbiome Profiling and Epithelial Health in Goats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Diets, and Experimental Design
2.3. Sample Collection
2.4. Measurement of the Concentrations of VFAs in Ileal Contents
2.5. ELISA Assay of Inflammatory Cytokines and Tight Junctions of Ileal Epithelium
2.6. Microbial DNA Extraction and 16S rRNA Gene Sequencing
2.7. Bioinformatic Analysis of Ileal Microbiome Data
2.8. Statistical Analysis
3. Results
3.1. Concentrations of VFAs in Ileal Contents
3.2. The Concentrations of Inflammatory Cytokines and Tight Junctions in Ileal Epithelial Tissue
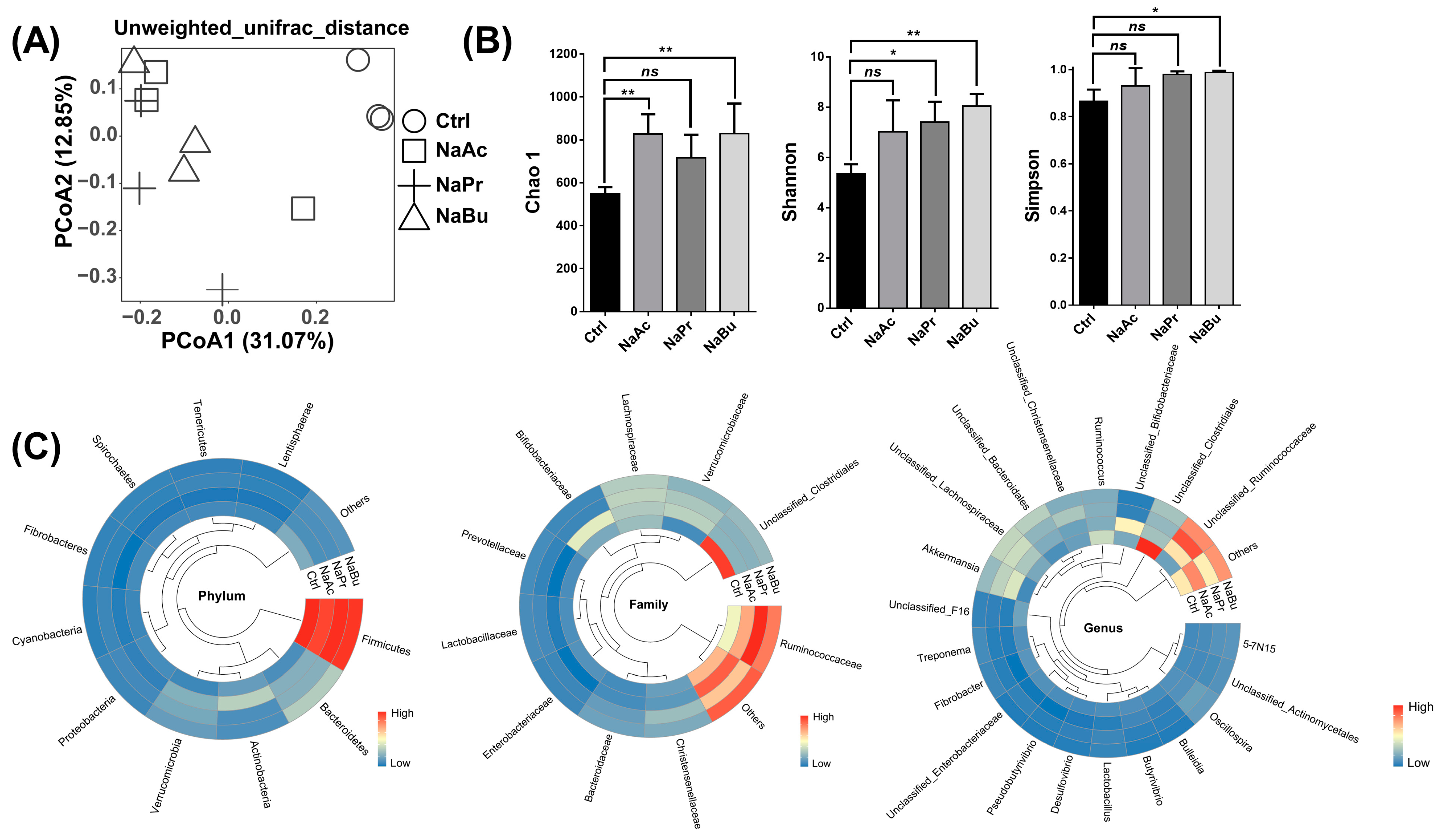
3.3. Distribution of the Microbial Succession Patterns and Microbial Diversities and Abundances in Ileal Contents
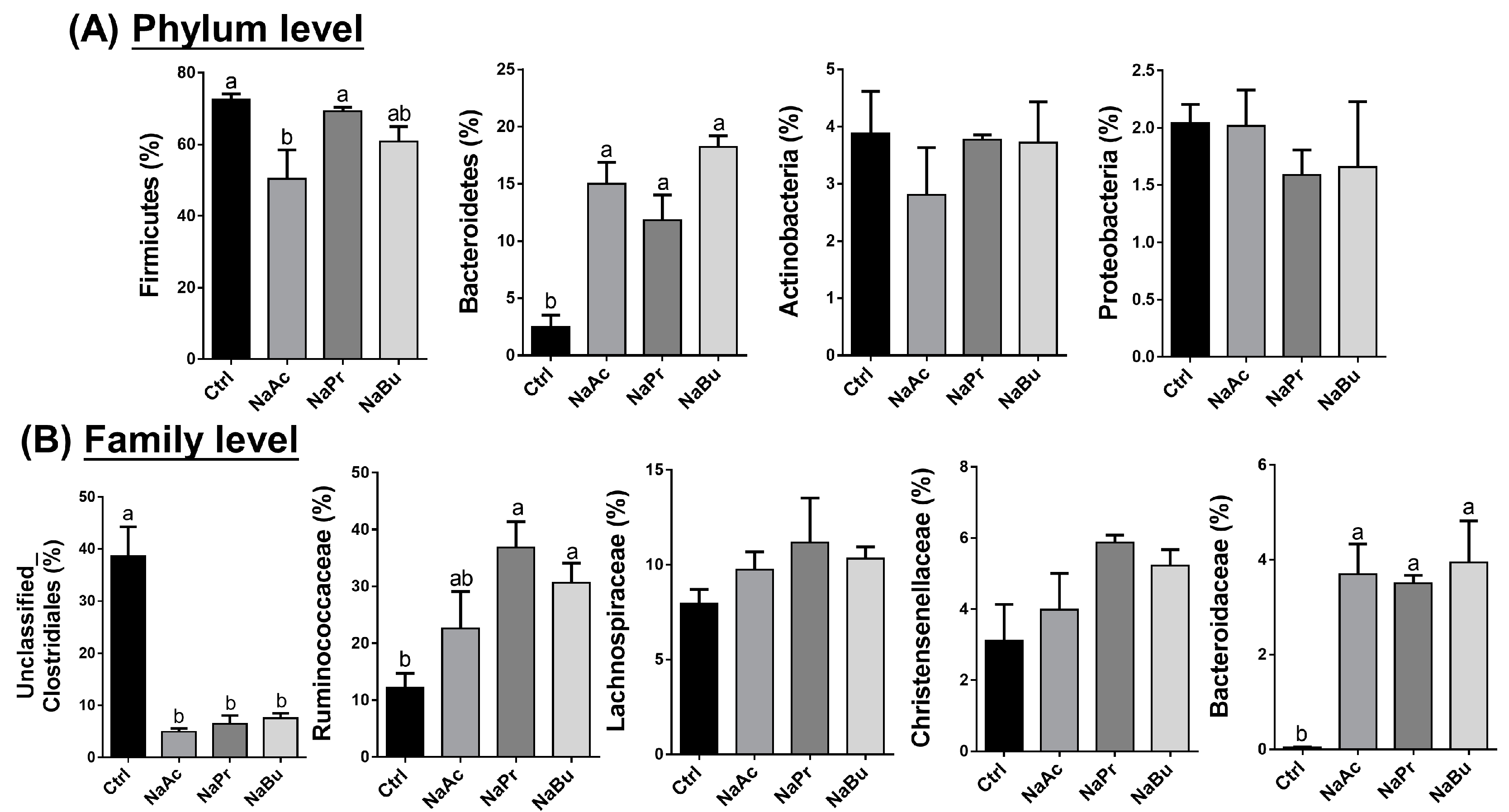
3.4. Selection of Significant Taxonomic Differences under Different Levels in Ileal Contents
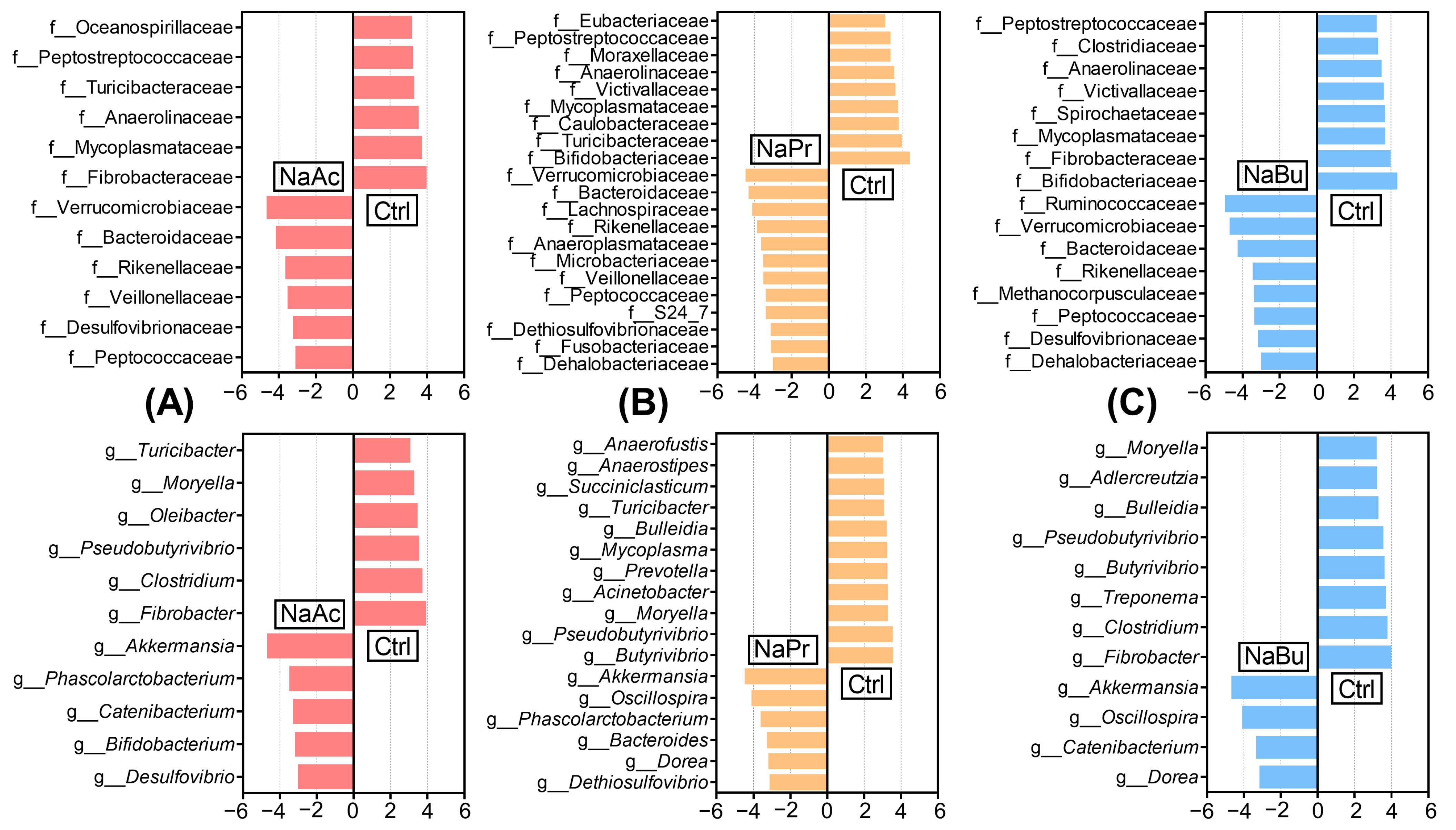
3.5. Core Differential Microbes in Ileal Contents of Four Groups
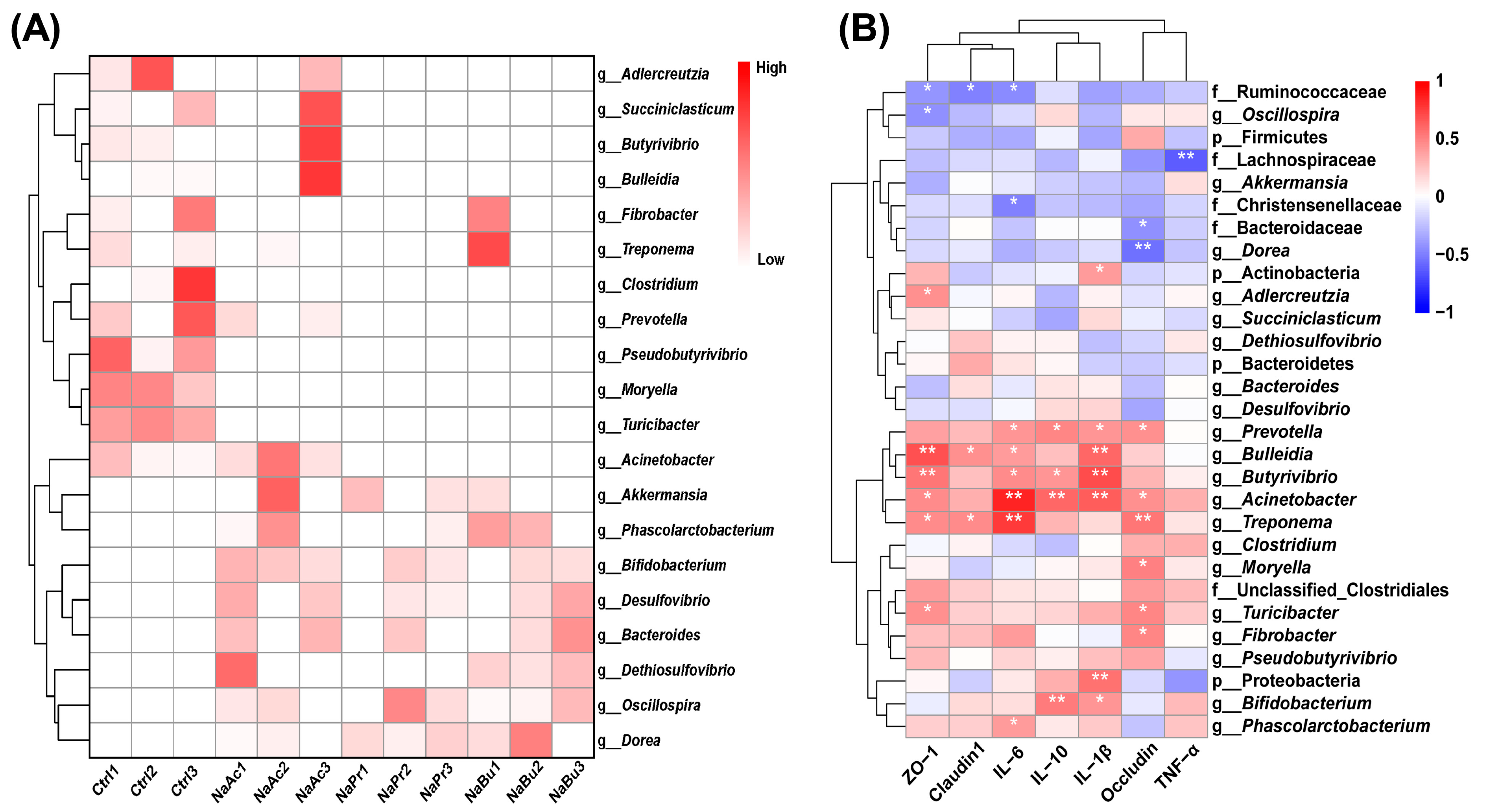
3.6. The Correlations between Ileal Bacteria and Gut Health Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Lai, Z.; Zhang, J.; Zhu, W.; Mao, S. The gastrointestinal microbiome in dairy cattle is constrained by the deterministic driver of the region and the modified effect of diet. Microbiome 2023, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, D.M.; Qin, T.; Mao, S.Y.; Zhu, W.Y.; Yin, Y.Y.; Huang, J.; Heller, R.; Li, Z.P.; Liu, J.H.; et al. Single-cell transcriptomic landscape of the sheep rumen provides insights into physiological programming development and adaptation of digestive strategies. Zool. Res. 2022, 43, 634–647. [Google Scholar] [CrossRef]
- Moraïs, S.; Mizrahi, I. The Road Not Taken: The Rumen Microbiome, Functional Groups, and Community States. Trends Microbiol. 2019, 27, 538–549. [Google Scholar] [CrossRef]
- Na, S.W.; Guan, L.L. Understanding the role of rumen epithelial host-microbe interactions in cattle feed efficiency. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2022, 10, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Górka, P.; Kowalski, Z.M.; Zabielski, R.; Guilloteau, P. Invited review: Use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 2018, 101, 4785–4800. [Google Scholar] [CrossRef]
- Ma, L.; Yang, Y.; Liu, W.; Bu, D. Sodium butyrate supplementation impacts the gastrointestinal bacteria of dairy calves before weaning. Appl. Microbiol. Biotechnol. 2023, 107, 3291–3304. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.C.; Hatungimana, E.; Klanderman, K.D.; Moreland, S.C.; Erickson, P.S. Sodium butyrate and monensin supplementation to postweaning heifer diets: Effects on growth performance, nutrient digestibility, and health. J. Dairy Sci. 2020, 103, 10207–10218. [Google Scholar] [CrossRef]
- Liu, L.; Sun, D.; Mao, S.; Zhu, W.; Liu, J. Infusion of sodium butyrate promotes rumen papillae growth and enhances expression of genes related to rumen epithelial VFA uptake and metabolism in neonatal twin lambs. J. Anim. Sci. 2019, 97, 909–921. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Zhang, R.; He, T.; Huang, G.; Tian, K.; Liu, J.; Chen, J.; Dong, G. Sodium Butyrate More Effectively Mitigates the Negative Effects of High-Concentrate Diet in Dairy Cows than Sodium β-Hydroxybutyrate via Reducing Free Bacterial Cell Wall Components in Rumen Fluid and Plasma. Toxins 2021, 13, 352. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Zebeli, Q.; Patra, A.K.; Greco, G.; Amasheh, S.; Penner, G.B. Symposium review: The importance of the ruminal epithelial barrier for a healthy and productive cow. J. Dairy Sci. 2019, 102, 1866–1882. [Google Scholar] [CrossRef]
- Abuelfatah, K.; Zuki, A.B.; Goh, Y.M.; Sazili, A.Q.; Abubakr, A. Effects of feeding whole linseed on ruminal fatty acid composition and microbial population in goats. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2016, 2, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, B.; Gao, M.; Li, Q.; Gao, Y.; Dong, N.; Liu, G.; Wang, Z.; Gao, W.; Chen, Y.; et al. Dietary Nutritional Level Affects Intestinal Microbiota and Health of Goats. Microorganisms 2022, 10, 2322. [Google Scholar] [CrossRef] [PubMed]
- Hackmann, T.J.; Ngugi, D.K.; Firkins, J.L.; Tao, J. Genomes of rumen bacteria encode atypical pathways for fermenting hexoses to short-chain fatty acids. Environ. Microbiol. 2017, 19, 4670–4683. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Du, H.S.; Wu, Z.Z.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Branched-chain volatile fatty acids and folic acid accelerated the growth of Holstein dairy calves by stimulating nutrient digestion and rumen metabolism. Animal 2020, 14, 1176–1183. [Google Scholar] [CrossRef]
- Brisson, V.; Girard, C.L.; Metcalf, J.A.; Castagnino, D.S.; Dijkstra, J.; Ellis, J.L. Meta-analysis of apparent ruminal synthesis and postruminal flow of B vitamins in dairy cows. J. Dairy Sci. 2022, 105, 7399–7415. [Google Scholar] [CrossRef]
- Zhang, Q.; Koser, S.L.; Bequette, B.J.; Donkin, S.S. Effect of propionate on mRNA expression of key genes for gluconeogenesis in liver of dairy cattle. J. Dairy Sci. 2015, 98, 8698–8709. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W. Gluconeogenesis in cattle: Significance and methodology. J. Dairy Sci. 1977, 60, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Wang, H.; Nan, X.; Guo, Y.; Xiong, B. Calcium Propionate Supplementation Has Minor Effects on Major Ruminal Bacterial Community Composition of Early Lactation Dairy Cows. Front. Microbiol. 2022, 13, 847488. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Yang, W.Z.; Guo, G.; Yang, X.M.; He, D.C.; Dong, K.H.; Huang, Y.X. Effects of calcium propionate supplementation on lactation performance, energy balance and blood metabolites in early lactation dairy cows. J. Anim. Physiol. Anim. Nutr. 2010, 94, 605–614. [Google Scholar] [CrossRef]
- Górka, P.; Sliwinski, B.; Flaga, J.; Olszewski, J.; Nawrocka, P.; Sobkowiak, K.; Miltko, R.; Godlewski, M.M.; Zabielski, R.; Kowalski, Z.M. Effect of exogenous butyrate on the gastrointestinal tract of sheep. II. Hydrolytic activity in the rumen and structure and function of the small intestine. J. Anim. Sci. 2018, 96, 5325–5335. [Google Scholar] [CrossRef]
- Zhan, K.; Yang, T.Y.; Chen, Y.; Jiang, M.C.; Zhao, G.Q. Propionate enhances the expression of key genes involved in the gluconeogenic pathway in bovine intestinal epithelial cells. J. Dairy Sci. 2020, 103, 5514–5524. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.L.; Swanson, K.C. Review: Nutritional regulation of intestinal starch and protein assimilation in ruminants. Animal 2020, 14, s17–s28. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.; Krehbiel, C. Nutrient metabolism by gut tissues. J. Dairy Sci. 1993, 76, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- El-Kadi, S.W.; Baldwin, R.L.t.; McLeod, K.R.; Sunny, N.E.; Bequette, B.J. Glutamate is the major anaplerotic substrate in the tricarboxylic acid cycle of isolated rumen epithelial and duodenal mucosal cells from beef cattle. J. Nutr. 2009, 139, 869–875. [Google Scholar] [CrossRef]
- Koch, C.; Gerbert, C.; Frieten, D.; Dusel, G.; Eder, K.; Zitnan, R.; Hammon, H.M. Effects of ad libitum milk replacer feeding and butyrate supplementation on the epithelial growth and development of the gastrointestinal tract in Holstein calves. J. Dairy Sci. 2019, 102, 8513–8526. [Google Scholar] [CrossRef]
- Zhan, K.; Jiang, M.; Gong, X.; Zhao, G. Effect of short-chain fatty acids on the expression of genes involved in short-chain fatty acid transporters and inflammatory response in goat jejunum epithelial cells. In Vitro Cell Dev. Biol. Anim. 2018, 54, 311–320. [Google Scholar] [CrossRef]
- Zhen, Y.; Xi, Z.; Nasr, S.M.; He, F.; Han, M.; Yin, J.; Ge, L.; Chen, Y.; Wang, Y.; Wei, W.; et al. Multi-Omics Reveals the Impact of Exogenous Short-Chain Fatty Acid Infusion on Rumen Homeostasis: Insights into Crosstalk between the Microbiome and the Epithelium in a Goat Model. Microbiol. Spectr. 2023, 11, e0534322. [Google Scholar] [CrossRef]
- Gualdrón-Duarte, L.B.; Allen, M.S. Effects of acetic acid or sodium acetate infused into the rumen or abomasum on feeding behavior and metabolic response of cows in the postpartum period. J. Dairy Sci. 2018, 101, 2016–2026. [Google Scholar] [CrossRef]
- Bedford, A.; Beckett, L.; Hardin, K.; Dias, N.W.; Davis, T.; Mercadante, V.R.G.; Ealy, A.D.; White, R.R. Propionate Affects Insulin Signaling and Progesterone Profiles in Dairy Heifers. Sci. Rep. 2018, 8, 17629. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, X.; Wang, M.; Zhou, C.; Yan, Q.; Tan, Z. Linkages between Epithelial Microbiota and Host Transcriptome in the Ileum during High-Grain Challenges: Implications for Gut Homeostasis in Goats. J. Agric. Food Chem. 2019, 67, 551–561. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Zha, X.; Ma, Y.; Liu, X.; Elsabagh, M.; Wang, H.; Wang, M. Dietary L-Arginine or N-Carbamylglutamate Alleviates Colonic Barrier Injury, Oxidative Stress, and Inflammation by Modulation of Intestinal Microbiota in Intrauterine Growth-Retarded Suckling Lambs. Antioxidants 2022, 11, 2251. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hu, F.; Guo, C.; Mei, S.; Xie, F.; Zeng, H.; Mao, S. Undernutrition shifted colonic fermentation and digest-associated bacterial communities in pregnant ewes. Appl. Microbiol. Biotechnol. 2020, 104, 5973–5984. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wang, D.; Dong, S.; Cheng, Z.; Na, L.; Sang, M.; Yang, H.; Yang, Z.; Zhang, S.; Yan, Z. Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cells. Int. Immunopharmacol. 2017, 45, 194–200. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Wefer, H.A.; Lundin, S.; Jakobsson, H.E.; Lindberg, M.; Rodin, S.; Engstrand, L.; Andersson, A.F. DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies. Appl. Environ. Microbiol. 2014, 80, 5116–5123. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Borton, M.A.; Sabag-Daigle, A.; Wu, J.; Solden, L.M.; O’Banion, B.S.; Daly, R.A.; Wolfe, R.A.; Gonzalez, J.F.; Wysocki, V.H.; Ahmer, B.M.M.; et al. Chemical and pathogen-induced inflammation disrupt the murine intestinal microbiome. Microbiome 2017, 5, 47. [Google Scholar] [CrossRef]
- Zhang, R.; Ye, H.; Liu, J.; Mao, S. High-grain diets altered rumen fermentation and epithelial bacterial community and resulted in rumen epithelial injuries of goats. Appl. Microbiol. Biotechnol. 2017, 101, 6981–6992. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L.; et al. Dietary Supplementation of Inulin Ameliorates Subclinical Mastitis via Regulation of Rumen Microbial Community and Metabolites in Dairy Cows. Microbiol. Spectr. 2021, 9, e0010521. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Blackmon, T.L.; Sawyer, J.E.; Miller, R.K.; Baber, J.R.; Morrill, J.C.; Cabral, A.R.; Wickersham, T.A. Glucose and acetate metabolism in bovine intramuscular and subcutaneous adipose tissues from steers infused with glucose, propionate, or acetate. J. Anim. Sci. 2018, 96, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lei, X.; Yang, X.; Shen, J.; Zheng, L.; Jin, C.; Cao, Y.; Yao, J. A Metagenomic Insight Into the Hindgut Microbiota and Their Metabolites for Dairy Goats Fed Different Rumen Degradable Starch. Front. Microbiol. 2021, 12, 651631. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, Z.; Wang, S.; Cao, L.; Zhou, L.; Sun, A.; Zhong, Z.; Nabben, M. Microbial-Driven Butyrate Regulates Jejunal Homeostasis in Piglets During the Weaning Stage. Front. Microbiol. 2018, 9, 3335. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.; Peng, A.; Dong, L.; Wang, M.; Yu, L.; Loor, J.J.; Wang, H. Effects of Dietary l-Arginine and N-Carbamylglutamate Supplementation on Intestinal Integrity, Immune Function, and Oxidative Status in Intrauterine-Growth-Retarded Suckling Lambs. J. Agric. Food Chem. 2018, 66, 4145–4154. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Gebeyew, K.; Yang, X.; Tang, S.; Zhou, C.; Khan, N.A.; Tan, Z.; Liu, Y. A low-carbon high inulin diet improves intestinal mucosal barrier function and immunity against infectious diseases in goats. Front. Vet. Sci. 2022, 9, 1098651. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shin, Y.J.; Jang, H.M.; Joo, M.K.; Yoo, J.W.; Kim, D.H. Lactobacillus rhamnosus and Bifidobacterium longum alleviate colitis and cognitive impairment in mice by regulating IFN-γ to IL-10 and TNF-α to IL-10 expression ratios. Sci. Rep. 2021, 11, 20659. [Google Scholar] [CrossRef]


| Ingredients | Percent, % of DM | Nutrients | Level, g/kg of DM |
|---|---|---|---|
| Oat hay | 41.00 | DE (MJ/kg) | 10.25 |
| Corn | 29.50 | CP | 155.60 |
| Soybean meal | 14.50 | NDF | 352.60 |
| Wheat bran | 10.00 | ADF | 196.25 |
| Stone dust | 0.35 | EE | 28.94 |
| CaH2PO4 | 0.15 | Ca | 4.08 |
| NaCl | 0.50 | P | 4.65 |
| Premix | 4.00 | ||
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, Y.; Zhang, C.; Lin, J.; Rahmat, A.; He, F.; Wang, M. Effects of Exogenous Oral Infusion of Volatile Fatty Acids on Ileal Microbiome Profiling and Epithelial Health in Goats. Fermentation 2023, 9, 801. https://doi.org/10.3390/fermentation9090801
Zhen Y, Zhang C, Lin J, Rahmat A, He F, Wang M. Effects of Exogenous Oral Infusion of Volatile Fatty Acids on Ileal Microbiome Profiling and Epithelial Health in Goats. Fermentation. 2023; 9(9):801. https://doi.org/10.3390/fermentation9090801
Chicago/Turabian StyleZhen, Yongkang, Chong Zhang, Jiaqi Lin, Ali Rahmat, Feiyang He, and Mengzhi Wang. 2023. "Effects of Exogenous Oral Infusion of Volatile Fatty Acids on Ileal Microbiome Profiling and Epithelial Health in Goats" Fermentation 9, no. 9: 801. https://doi.org/10.3390/fermentation9090801
APA StyleZhen, Y., Zhang, C., Lin, J., Rahmat, A., He, F., & Wang, M. (2023). Effects of Exogenous Oral Infusion of Volatile Fatty Acids on Ileal Microbiome Profiling and Epithelial Health in Goats. Fermentation, 9(9), 801. https://doi.org/10.3390/fermentation9090801







