Effect of Alkaline Mineral Complex Buffer Supplementation on Milk Performance, Serum Variables, Rumen Fermentation and Rumen Microbiota of Transition Dairy Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Treatments
2.2. Experimental Feed
2.3. Feed and Milk Collection, Preservation, and Analysis and DMI Recording
2.4. Collection, Preservation and Pretreatment of Cow Blood Samples
2.5. Collection, Preservation and Pretreatment of Rumen Fluid Samples
2.6. Data Analysis
3. Results
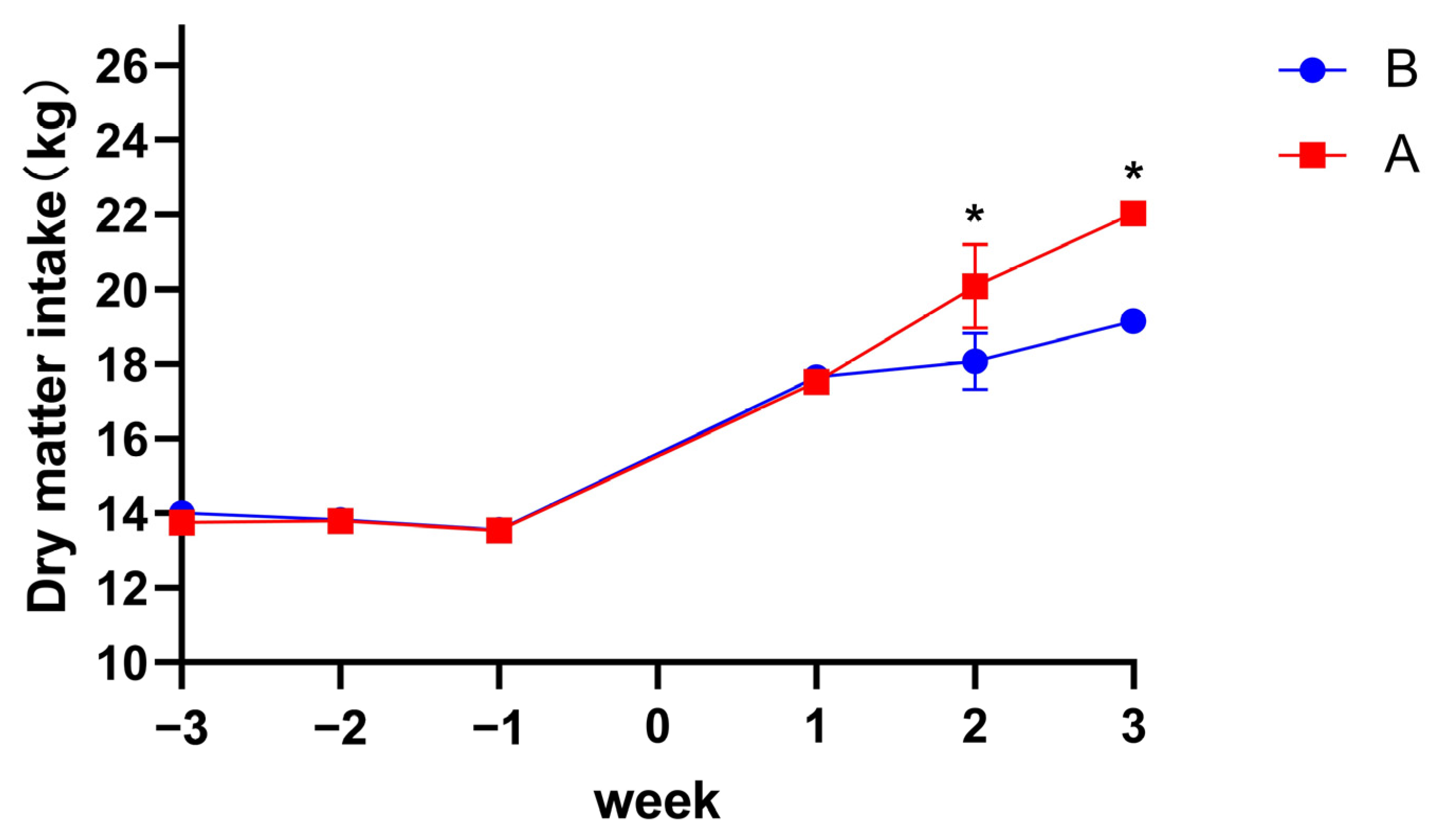
3.1. Milk Performance and DMI
3.2. Serum Variables
3.3. Rumen Fermentation
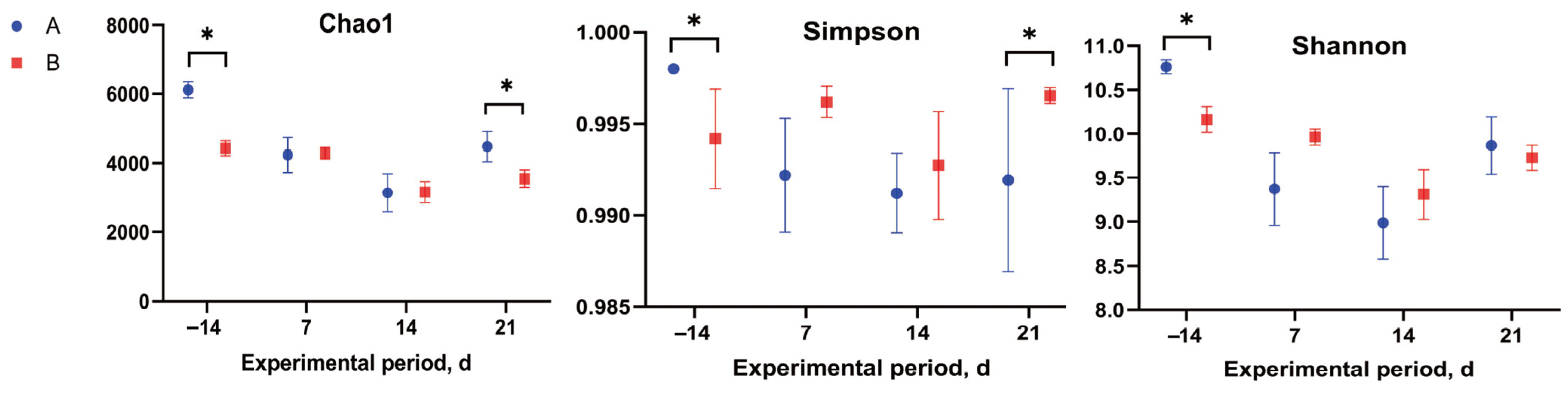
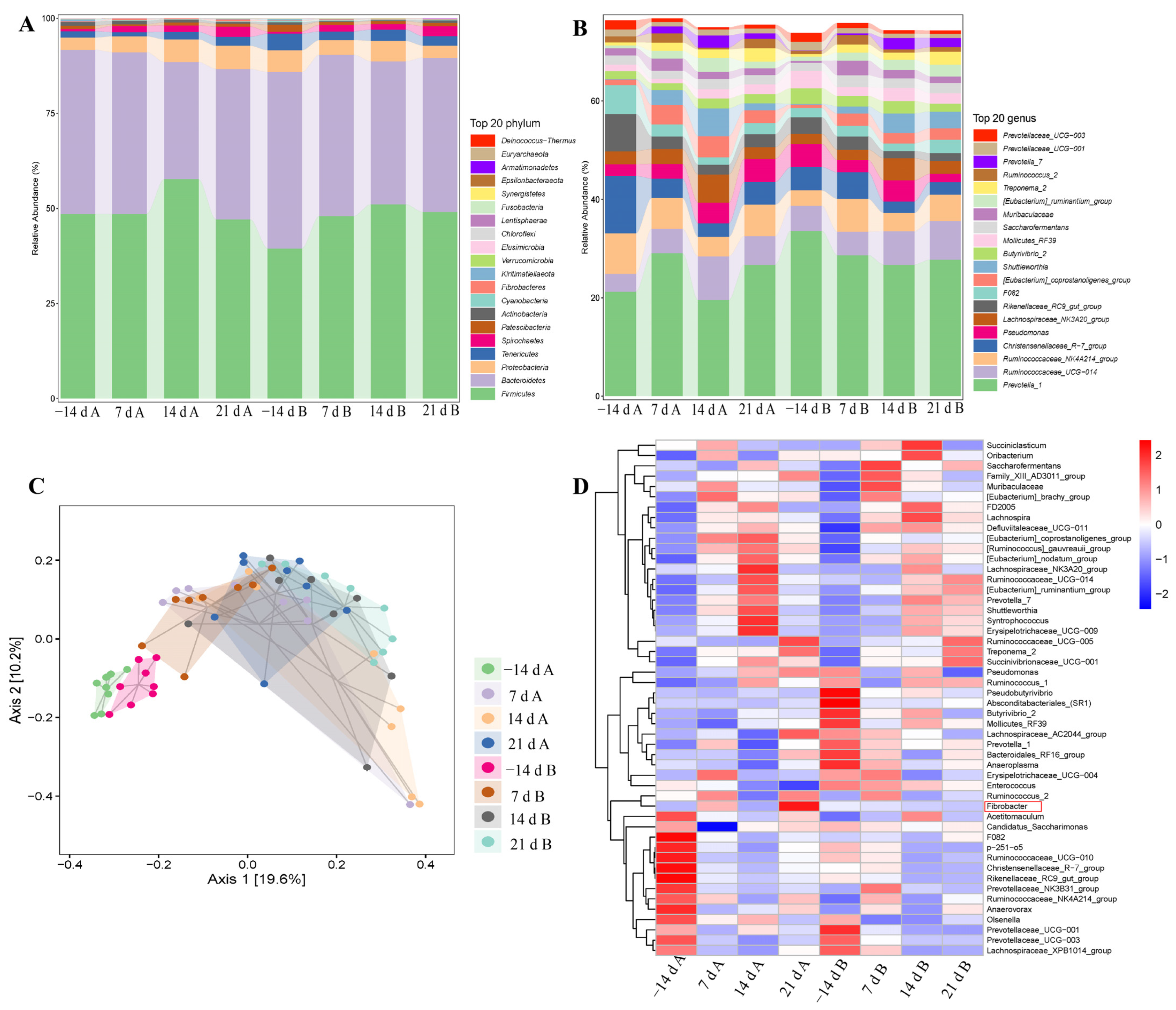
3.4. Rumen Microbiota Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Gaowa, N.; Wang, Y.; Li, H.; Cao, Z.; Yang, H.; Zhang, X.; Li, S. Complementary hepatic metabolomics and proteomics reveal the adaptive mechanisms of dairy cows to the transition period. J. Dairy Sci. 2022, 106, 2071–2088. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Harper, M.T.; Melgar, A.; Räisänen, S.; Chen, X.; Nedelkov, K.; Fetter, M.; Ott, T.; Wall, E.H.; Hristov, A.N. Dietary supplementation with rumen-protected capsicum during the transition period improves the metabolic status of dairy cows. J. Dairy Sci. 2021, 104, 11609–11620. [Google Scholar] [CrossRef] [PubMed]
- Tröscher-Mußotter, J.; Deusch, S.; Borda-Molina, D.; Frahm, J.; Dänicke, S.; Camarinha-Silva, A.; Huber, K.; Seifert, J. Cow’s microbiome from antepartum to postpartum: A long-term study covering two physiological challenges. Front. Microbiol. 2022, 13, 1000750. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Lombardelli, R. Some new aspects of nutrition, health conditions and fertility of intensively reared dairy cows. Ital. J. Anim. Sci. 2009, 8, 491–518. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Andersen, J.B. Integration of metabolism and intake regulation: A review focusing on periparturient animals. J. Dairy Sci. 2000, 83, 1573–1597. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.W. Review: Lipid biology in the periparturient dairy cow: Contemporary perspectives. Animal 2020, 14, s165–s175. [Google Scholar] [CrossRef] [PubMed]
- El-Kasrawy, N.I.; Swelum, A.A.; Abdel-Latif, M.A.; Alsenosy, A.; Beder, N.A.; Alkahtani, S.; Abdel-Daim, M.M.; Abd, E.A. Efficacy of different drenching regimens of gluconeogenic precursors during transition period on body condition score, production, reproductive performance, subclinical ketosis and economics of dairy cows. Animals 2020, 10, 937. [Google Scholar] [CrossRef]
- Ha, S.; Kang, S.; Jeong, M.; Han, M.; Lee, J.; Chung, H.; Park, J. Characteristics of Holstein cows predisposed to ketosis during the post-partum transition period. Vet. Med. Sci. 2023, 9, 307–314. [Google Scholar] [CrossRef]
- Li, S.; Hao, Y.; Wang, W.; Wang, Y. Research progress on glucose-lipid metabolism and health feeding in transition dairy cows. Chin. J. Anim. Nutr. 2020, 32, 4708–4715. (In Chinese) [Google Scholar]
- Huang, S.; Ji, S.; Yan, H.; Hao, Y.; Zhang, J.; Wang, Y.; Cao, Z.; Li, S. The day-to-day stability of the ruminal and fecal microbiota in lactating dairy cows. Microbiologyopen 2020, 9, e990. [Google Scholar] [CrossRef]
- Bach, A.; Lopez-Garcia, A.; Gonzalez-Recio, O.; Elcoso, G.; Fabregas, F.; Chaucheyras-Durand, F.; Castex, M. Changes in the rumen and colon microbiota and effects of live yeast dietary supplementation during the transition from the dry period to lactation of dairy cows. J. Dairy Sci. 2019, 102, 6180–6198. [Google Scholar] [CrossRef]
- Lima, F.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.; Ganda, E.K.; Filho, J.C.; Lorenzo, G.; Trojacanec, P.; Bicalhoa, R.C. Prepartum and postpartum rumen fluid microbiomes: Characterization and correlation with production traits in dairy cows. Appl. Environ. Microb. 2015, 81, 1327–1337. [Google Scholar] [CrossRef]
- Zhu, Z.; Kristensen, L.; Difford, G.F.; Poulsen, M.; Noel, S.J.; Abu Al-Soud, W.; Sørensen, S.J.; Lassen, J.; Løvendahl, P.; Højberg, O. Changes in rumen bacterial and archaeal communities over the transition period in primiparous Holstein dairy cows. J. Dairy Sci. 2018, 101, 9847–9862. [Google Scholar] [CrossRef]
- Pitta, D.W.; Kumar, S.; Vecchiarelli, B.; Shirley, D.J.; Bittinger, K.; Baker, L.D.; Ferguson, J.D.; Thomsen, N. Temporal dynamics in the ruminal microbiome of dairy cows during the transition period. J. Anim Sci. 2014, 92, 4014–4022. [Google Scholar] [CrossRef] [PubMed]
- Penner, G.B.; Steele, M.A.; Aschenbach, J.R.; McBride, B.W. Ruminant Nutrition Symposium: Molecular adaptation of ruminal epithelia to highly fermentable diets. J. Anim Sci. 2011, 89, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.A.; Penner, G.B.; Chaucheyras-Durand, F.; Guan, L.L. Development and physiology of the rumen and the lower gut: Targets for improving gut health. J. Dairy Sci. 2016, 99, 4955–4966. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Kmicikewycz, A.D.; Harvatine, K.J.; Heinrichs, A.J. Effects of corn silage particle size, supplemental hay, and forage-to-concentrate ratio on rumen pH, feed preference, and milk fat profile of dairy cattle. J. Dairy Sci. 2015, 98, 4850–4868. [Google Scholar] [CrossRef]
- Li, S.; Khafipour, E.; Krause, D.O.; Kroeker, A.; Rodriguez-Lecompte, J.C.; Gozho, G.N.; Plaizier, J.C. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J. Dairy Sci. 2012, 95, 294–303. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Zebeli, Q. Diet-induced inflammation: From gut to metabolic organs and the consequences for the health and longevity of ruminants. Res. Vet. Sci. 2018, 120, 17–27. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Danesh, M.M.; Derakhshani, H.; Golder, H.; Khafipour, E.; Kleen, J.L.; Lean, I.; Loor, J.; Penner, G.; Zebeli, Q. Review: Enhancing gastrointestinal health in dairy cows. Animal 2018, 12, s399–s418. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Benedito, J.L.; Abuelo, A.; Castillo, C. Ruminal acidosis in feedlot: From aetiology to prevention. Sci. World J. 2014, 2014, 702572. [Google Scholar] [CrossRef] [PubMed]
- Elmhadi, M.E.; Ali, D.K.; Khogali, M.K.; Wang, H. Subacute ruminal acidosis in dairy herds: Microbiological and nutritional causes, consequences, and prevention strategies. Anim. Nutr. 2022, 10, 148–155. [Google Scholar] [CrossRef]
- Hu, W.; Murphy, M.R. Statistical evaluation of early- and mid-lactation dairy cow responses to dietary sodium bicarbonate addition. Anim. Feed Sci. Technol. 2005, 119, 43–54. [Google Scholar] [CrossRef]
- Enemark, J.M. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): A review. Vet. J. 2008, 176, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Golder, H.M.; Celi, P.; Rabiee, A.R.; Lean, I.J. Effects of feed additives on rumen and blood profiles during a starch and fructose challenge. J. Dairy Sci. 2014, 97, 985–1004. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, B.; Dai, X.; Xu, Y.; Kang, J.; Li, J. Drinking alkaline mineral water confers diarrhea resistance in maternally separated piglets by maintaining intestinal epithelial regeneration via the brain-microbe-gut axis. J. Adv. Res. 2022; in press. [Google Scholar] [CrossRef]
- Bao, Y.M.; Choct, M. Trace mineral nutrition for broiler chickens and prospects of application of organically complexed trace minerals: A review. Anim. Prod. Sci. 2009, 49, 269. [Google Scholar] [CrossRef]
- Jurkic, L.M.; Cepanec, I.; Pavelic, S.K.; Pavelic, K. Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutr. Metab. 2013, 10, 2. [Google Scholar] [CrossRef]
- Pavelic, K.; Hadzija, M.; Bedrica, L.; Pavelic, J.; Dikic, I.; Katic, M.; Kralj, M.; Bosnar, M.H.; Kapitanovic, S.; Poljak-Blazi, M.; et al. Natural zeolite clinoptilolite: New adjuvant in anticancer therapy. J. Mol. Med. 2001, 78, 708–720. [Google Scholar] [CrossRef]
- Shin, D.W.; Yoon, H.; Kim, H.S.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H. Effects of alkaline-reduced drinking water on irritable bowel syndrome with diarrhea: A randomized double-blind, placebo-controlled pilot study. Evid.-Based Complement. Altern. Med. 2018, 2018, 9147914. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.; Conti, V.; Corbi, G.; Filippelli, A. Hydropinotherapy with sulphurous mineral water as complementary treatment to improve glucose metabolism, oxidative status, and quality of life. Antioxidants 2021, 10, 1773. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.R.; Kang, J.X.; Zhao, B.C.; Dai, X.Y.; Qiu, B.H.; Li, J.L. Effects of alkaline mineral complex water supplementation on growth performance, inflammatory response, and intestinal barrier function in weaned piglets. J. Anim. Sci. 2022, 100, skac251. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, X.W.; Kang, J.X.; Zhao, B.C.; Xu, Y.R.; Li, J.L. Metasilicate-based alkaline mineral water confers diarrhea resistance in maternally separated piglets via the microbiota-gut interaction. Pharmacol. Res. 2023, 187, 106580. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Wang, J. Effects of Compound Buffer on Performance and Blood Biochemical Indices of Lactating Dairy Cows; China Agricultural University: Beijing, China, 2022. (In Chinese) [Google Scholar]
- Greenland, M.S.; Waldron, B.L.; Isom, S.C.; Fonnesbeck, S.D.; Peel, M.D.; Rood, K.A.; Thornton, K.J.; Miller, R.L.; Hadfield, J.A.; Henderson, B.; et al. Dry matter intake and feed efficiency of heifers from 4 dairy breed types grazing organic grass and grass-birdsfoot trefoil mixed pastures. J. Dairy Sci. 2023, 106, 3918–3931. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Huang, S.; Liu, G.; Zhang, J.; Liu, G.; Cao, Z.; Wang, Y.; Wang, W.; Li, S. Effects of different parts on the chemical composition, silage fermentation profile, in vitro and in situ digestibility of paper mulberry. Animals 2021, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Wang, Y.; Xie, T.; Yang, Z.T.; Wang, J.D.; Zheng, Y.H.; Guo, C.; Zhang, Y.; Wang, Q.Q.; Wang, Z.H.; et al. Effects of high-forage diets containing raw flaxseeds or soybean on in vitro ruminal fermentation, gas emission, and microbial profile. Microorganisms 2021, 9, 2304. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Guo, C.; Wu, Y.; Li, S.; Cao, Z.; Wang, Y.; Mao, J.; Shi, H.; Shi, R.; Sun, X.; Zheng, Y.; et al. Effects of different forage types on rumen fermentation, microflora, and production performance in peak-lactation dairy cows. Fermentation 2022, 8, 507. [Google Scholar] [CrossRef]
- Liu, E.; VandeHaar, M.J. Relationship of residual feed intake and protein efficiency in lactating cows fed high- or low-protein diets. J. Dairy Sci. 2020, 103, 3177–3190. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Yang, H.J. In vitro ruminal methanogenesis of a hay-rich substrate in response to different combination supplements of nitrocompounds; pyromellitic diimide and 2-bromoethanesulphonate. Anim. Feed Sci. Technol. 2011, 163, 20–32. [Google Scholar] [CrossRef]
- Claesson, M.J.; O’Sullivan, O.; Wang, Q.; Nikkilä, J.; Marchesi, J.R.; Smidt, H.; de Vos, W.M.; Ross, R.P.; O’Toole, P.W. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Jaccard, P. The distribution of the flora in the alpine zone.1. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microb. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Ferguson, J.D. Nutrition and reproduction in dairy herds. Vet. Clin. N. Am.-Food A 2005, 21, 325–347. [Google Scholar] [CrossRef]
- Bertics, S.J.; Grummer, R.R.; Cadorniga-Valino, C.; Stoddard, E.E. Effect of prepartum dry matter intake on liver triglyceride concentration and early lactation. J. Dairy Sci. 1992, 75, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Haisan, J.; Inabu, Y.; Sugino, T.; Oba, M. Effects of starch concentration of close-up diets on rumen pH and plasma metabolite responses of dairy cows to grain challenges after calving. J. Dairy Sci. 2020, 103, 11461–11471. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ji, S.; Suen, G.; Wang, F.; Li, S. The rumen bacterial community in dairy cows is correlated to production traits during freshening period. Front. Microbiol. 2021, 12, 630605. [Google Scholar] [CrossRef]
- Harlow, B.E.; Flythe, M.D.; Klotz, J.L.; Harmon, D.L.; Aiken, G.E. Effect of biochanin A on the rumen microbial community of Holstein steers consuming a high fiber diet and subjected to a subacute acidosis challenge. PLoS ONE 2021, 16, e253754. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M.I.; Booth, A.N.; Bohstedt, G.; Hart, E.B. The “In vitro” conversion of inorganic nitrogen to protein by microorganisms from the cow’s rumen. J. Dairy Sci. 1940, 23, 1123–1129. [Google Scholar] [CrossRef]
- Slyter, L.L.; Bryant, M.P.; Wolin, M.J. Effect of pH on population and fermentation in a continuously cultured rumen ecosystem1. Appl. Microbiol. 1966, 14, 573–578. [Google Scholar] [CrossRef]
- Johansen, M.; Lund, P.; Weisbjerg, M.R. Feed intake and milk production in dairy cows fed different grass and legume species: A meta-analysis. Animal 2018, 12, 66–75. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; El-Tarabany, M.S. Impact of three THI levels on somatic cell count, milk yield and composition of multiparous Holstein cows in a subtropical region. J. Therm. Biol. 2017, 64, 73–77. [Google Scholar] [CrossRef]
- Matamoros, C.; Cai, J.; Patterson, A.D.; Harvatine, K.J. Comparison of the effects of short-term feeding of sodium acetate and sodium bicarbonate on milk fat production. J. Dairy Sci. 2021, 104, 7572–7582. [Google Scholar] [CrossRef]
- Khalesi, M.; FitzGerald, R.J. Insolubility in milk protein concentrates: Potential causes and strategies to minimize its occurrence. Crit. Rev. Food Sci. 2022, 62, 6973–6989. [Google Scholar] [CrossRef]
- Haque, M.N.; Rulquin, H.; Andrade, A.; Faverdin, P.; Peyraud, J.L.; Lemosquet, S. Milk protein synthesis in response to the provision of an “ideal” amino acid profile at 2 levels of metabolizable protein supply in dairy cows. J. Dairy Sci. 2012, 95, 5876–5887. [Google Scholar] [CrossRef] [PubMed]
- Spek, J.W.; Dijkstra, J.; van den Borne, J.J.G.C.; Bannink, A. Short communication: Assessing urea transport from milk to blood in dairy cows. J. Dairy Sci. 2012, 95, 6536–6541. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.P.; Smith, R.H. Concentrations of amino acids and urea in the plasma of the ruminating calf and estimation of the amino acid requirements. Brit. J. Nutr. 1974, 32, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.C.; Williams, C.C.; Jenny, B.F.; Fernandez, J.M.; Bateman, H.G.; Nipper, W.A.; Lovejoy, J.C.; Gantt, D.T.; Goodier, G.E. Effects of Feeding Milk Replacer Once Versus Twice Daily on Glucose Metabolism in Holstein and Jersey Calves1. J. Dairy Sci. 2002, 85, 2335–2343. [Google Scholar] [CrossRef]
- Coma, J.; Carrion, D.; Zimmerman, D.R. Use of plasma urea nitrogen as a rapid response criterion to determine the lysine requirement of pigs. J. Anim. Sci. 1995, 73, 472–481. [Google Scholar] [CrossRef]
- Sun, X.; Guo, C.; Zhang, Y.; Wang, Q.; Yang, Z.; Wang, Z.; Wang, W.; Cao, Z.; Niu, M.; Li, S. Effect of diets enriched in n-6 or n-3 fatty acids on dry matter intake, energy balance, oxidative stress, and milk fat profile of transition cows. J. Dairy Sci. 2023, 106, 5416–5432. [Google Scholar] [CrossRef]
- Weber, C.; Hametner, C.; Tuchscherer, A.; Losand, B.; Kanitz, E.; Otten, W.; Sauerwein, H.; Bruckmaier, R.M.; Becker, F.; Kanitz, W.; et al. Hepatic gene expression involved in glucose and lipid metabolism in transition cows: Effects of fat mobilization during early lactation in relation to milk performance and metabolic changes. J. Dairy Sci. 2013, 96, 5670–5681. [Google Scholar] [CrossRef]
- Du, X.; Liu, G.; Loor, J.J.; Fang, Z.; Bucktrout, R.; Yang, Y.; Ye, Q.; Shi, Z.; Shen, T.; Wang, X.; et al. Impaired hepatic autophagic activity in dairy cows with severe fatty liver is associated with inflammation and reduced liver function. J. Dairy Sci. 2018, 101, 11175–11185. [Google Scholar] [CrossRef]
- Andjelic, B.; Djokovic, R.; Cincovic, M.; Bogosavljevic-Boskovic, S.; Petrovic, M.; Mladenovic, J.; Cukic, A. Relationships between milk and blood biochemical parameters and metabolic status in dairy cows during lactation. Metabolites 2022, 12, 733. [Google Scholar] [CrossRef]
- Cozzi, G.; Ravarotto, L.; Gottardo, F.; Stefani, A.L.; Contiero, B.; Moro, L.; Brscic, M.; Dalvit, P. Short communication: Reference values for blood parameters in Holstein dairy cows: Effects of parity, stage of lactation, and season of production. J. Dairy Sci. 2011, 94, 3895–3901. [Google Scholar] [CrossRef]
- Kozeniecki, M.; Ludke, R.; Kerner, J.; Patterson, B. Micronutrients in liver disease: Roles, risk factors for deficiency, and recommendations for supplementation. Nutr. Clin. Pract. 2020, 35, 50–62. [Google Scholar] [CrossRef]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef]
- Kim, E.J.; Bu, S.Y.; Sung, M.K.; Kang, M.H.; Choi, M.K. Analysis of antioxidant and anti-inflammatory activity of silicon in murine macrophages. Biol. Trace Elem. Res. 2013, 156, 329–337. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Dai, D.; Cao, Z.; Wang, Y.; Li, S.; Wang, W. Preliminary investigation of the effects of rosemary extract supplementation on milk production and rumen fermentation in high-producing dairy cows. Antioxidants 2022, 11, 1715. [Google Scholar] [CrossRef]
- Ma, Y.F.; Wu, Z.H.; Gao, M.; Loor, J.J. Nuclear factor erythroid 2-related factor 2 antioxidant response element pathways protect bovine mammary epithelial cells against H2O2-induced oxidative damage in vitro. J. Dairy Sci. 2018, 101, 5329–5344. [Google Scholar] [CrossRef]
- Drikic, M.; Windeyer, C.; Olsen, S.; Fu, Y.; Doepel, L.; De Buck, J. Determining the IgG concentrations in bovine colostrum and calf sera with a novel enzymatic assay. J. Anim. Sci. Biotechnol. 2018, 9, 69. [Google Scholar] [CrossRef]
- Behrouz, S.; Saadat, S.; Memarzia, A.; Sarir, H.; Folkerts, G.; Boskabady, M.H. The Antioxidant, Anti-Inflammatory and Immunomodulatory Effects of Camel Milk. Front. Immunol. 2022, 13, 855342. [Google Scholar] [CrossRef]
- Cammack, K.M.; Austin, K.J.; Lamberson, W.R.; Conant, G.C.; Cunningham, H.C. Ruminnat Nutrition Symposium: Tiny but mighty: The role of the rumen microbes in livestock production. J. Anim. Sci. 2018, 96, 752–770. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Rajion, M.A.; Adeyemi, K.D.; Jafari, S.; Jahromi, M.F.; Oskoueian, E.; Meng, G.Y.; Ghaffari, M.H. Dietary n-6: N-3 Fatty Acid Ratios Alter Rumen Fermentation Parameters and Microbial Populations in Goats. J. Agric. Food Chem. 2017, 65, 737–744. [Google Scholar] [CrossRef]
- Lin, L.; Lai, Z.; Zhang, J.; Zhu, W.; Mao, S. The gastrointestinal microbiome in dairy cattle is constrained by the deterministic driver of the region and the modified effect of diet. Microbiome 2023, 11, 10. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xi, Y.M.; Wang, Z.D.; Zeng, H.F.; Han, Z. Combined signature of rumen microbiome and metabolome in dairy cows with different feed intake levels. J. Anim. Sci. 2020, 98, skaa070. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut microbiota and BMI throughout childhood: The role of Firmicutes, Bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Han, L.; Xue, W.; Cao, H.; Chen, X.; Qi, F.; Ma, T.; Tu, Y.; Diao, Q.; Zhang, C.; Cui, K. Comparison of rumen fermentation parameters and microbiota of yaks from different altitude regions in Tibet, China. Front. Microbiol. 2021, 12, 807512. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, X.; Yang, X.; Wang, J.; Li, T.; Hua, G.; Han, D.; Da, L.; Li, R.; Rong, W.; et al. Characteristics of bacterial microbiota in different intestinal segments of Aohan Fine-Wool sheep. Front. Microbiol. 2022, 13, 874536. [Google Scholar] [CrossRef]
- Soukup, S.T.; Stoll, D.A.; Danylec, N.; Schoepf, A.; Kulling, S.E.; Huch, M. Metabolism of daidzein and genistein by gut bacteria of the class Coriobacteriia. Foods 2021, 10, 2741. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Xie, X.; Yang, C.; Guan, L.L.; Wang, J.; Xue, M.; Liu, J.X. Persistence of cellulolytic bacteria Fibrobacter and Treponema after short-term corn stover-based dietary intervention reveals the potential to improve rumen fibrolytic function. Front. Microbiol. 2018, 9, 1363. [Google Scholar] [CrossRef]
- Yeoman, C.J.; Fields, C.J.; Lepercq, P.; Ruiz, P.; Forano, E.; White, B.A.; Mosoni, P. In vivo competitions between Fibrobacter succinogenes, Ruminococcus flavefaciens, and Ruminoccus albus in a gnotobiotic sheep model revealed by multi-omic analyses. Mbio 2021, 12, 16. [Google Scholar] [CrossRef]

| Dietary Composition (Fresh Weight) | Content | Nutrient Compositions (% of Dry Matter Unless Noted) | Content |
|---|---|---|---|
| Corn silage, kg/d | 10.20 | Dry matter (% of fresh weight), % | 43.59 ± 0.19 |
| Oat hay, kg/d | 7.50 | Total digestible nutrients, % | 63.10 |
| Corn starch, kg/d | 0.52 | NEL, Mcal/kg 1 | 1.43 |
| Cottonseed meal, kg/d | 0.52 | Crude protein, % | 12.00 ± 0.27 |
| Corn distillers dried grains with solubles, kg/d 1 | 1.29 | Neutral detergent fiber, % | 46.85 ± 4.21 |
| Rapeseed meal, kg/d | 1.29 | Acid detergent fiber, % | 33.50 ± 0.17 |
| Commercial Premix, kg/d 2 | 0.41 | Lignin, % | 4.46 ± 0.32 |
| Total, kg/d | 21.73 | Starch, % | 12.12 ± 0.26 |
| Ether extract, % | 4.16 ± 0.24 | ||
| Ash, % | 10.48 ± 0.47 |
| Dietary Composition (Fresh Weight) | Content | Nutrient Compositions (% of Dry Matter Unless Noted) | Content |
|---|---|---|---|
| Corn silage, kg/d | 26.50 | Dry matter (% of fresh weight), % | 48.57 ± 2.21 |
| Alfalfa hay, kg/d | 3.40 | NEL, Mcal/kg | 1.63 |
| Cotton seed, kg/d | 1.35 | Total digestible nutrients, % | 72.15 |
| steam-flaked corn, kg/d | 0.80 | Crude protein, % | 16.29 ± 0.12 |
| Bran, kg/d | 1.15 | Neutral detergent fiber, % | 30.16 ± 1.75 |
| Corn starch, kg/d | 4.69 | Acid detergent fiber, % | 23.05 ± 1.25 |
| Soybean meal, kg/d | 2.84 | Lignin, % | 3.73 ± 0.36 |
| Cottonseed meal, kg/d | 0.86 | Starch, % | 26.21 ± 0.34 |
| Corn distillers dried grains with solubles, kg/d | 2.59 | Ether extract, % | 5.87 ± 0.23 |
| Expanded soybean, kg/d | 0.31 | Ash, % | 9.45 ± 0.32 |
| Calcium bicarbonate, kg/d | 0.25 | ||
| Sodium bicarbonate, kg/d | 0.19 | ||
| Commercial premix 1, kg/d 1 | 0.05 | ||
| Commercial premix 2, kg/d 2 | 0.62 | ||
| Total, kg/d | 45.60 |
| Ingredient | Content | Chemical Formula |
|---|---|---|
| Sodium metasilicate pentahydrate | 200 g/L | 5H2O·Na2SiO3 |
| Potassium bicarbonate | 100 g/L | KHCO3 |
| Zinc oxide | 10 mg/L | ZnO |
| Bis-(carboxyethyl germanium) sesquioxide | 1 mg/L | Ge-132 |
| Item | Group | SEM 5 | p-Value | |||
|---|---|---|---|---|---|---|
| A 1 | B 2 | Group | Time | Group × Time | ||
| Dry matter intake (before calving), kg | 13.69 | 13.79 | 0.14 | 0.47 | 0.12 | 0.72 |
| Dry matter intake (after calving), kg | 20.16 | 18.29 | 0.70 | 0.01 | 0.01 | 0.34 |
| Milk production, kg/d | 38.25 | 36.10 | 1.03 | 0.03 | <0.01 | 0.01 |
| FCM yield, kg 3 | 35.02 | 34.14 | 0.95 | 0.36 | <0.01 | 0.02 |
| ECM yield, kg 4 | 39.14 | 37.32 | 1.05 | 0.09 | <0.01 | 0.01 |
| Milk lactose, % | 5.03 | 5.10 | 0.06 | 0.06 | <0.01 | <0.01 |
| Milk fat, % | 3.43 | 3.64 | 0.09 | 0.03 | 0.29 | 0.73 |
| Milk protein, % | 3.45 | 3.22 | <0.01 | <0.01 | <0.01 | <0.01 |
| Milk somatic cell count, ×1000/mL | 58.65 | 58.83 | 0.19 | 0.90 | <0.01 | 0.90 |
| Milk urea nitrogen, mg/dL | 12.30 | 13.64 | 0.26 | <0.01 | <0.01 | 0.01 |
| Milk lactose yield, kg/d | 1.92 | 1.83 | 0.05 | 0.08 | <0.01 | 0.01 |
| Milk fat yield, kg/d | 1.31 | 1.31 | <0.01 | 0.95 | <0.01 | 0.02 |
| Milk protein yield, kg/d | 1.33 | 1.18 | 0.04 | <0.01 | <0.01 | <0.01 |
| Item | Group | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| A 1 | B 2 | Group | Time | Group × Time | ||
| INS, mIU/mL | 16.31 | 16.58 | 1.07 | 0.80 | 0.47 | 0.63 |
| GLU, mmol/L | 3.62 | 3.64 | 0.02 | 0.84 | 0.15 | 0.38 |
| TC, mmol/L | 3.24 | 2.73 | 0.13 | <0.01 | <0.01 | 0.57 |
| TG, mmol/L | 0.19 | 0.20 | <0.01 | 0.31 | 0.12 | 0.10 |
| ALT, U/L | 22.76 | 25.79 | 1.04 | <0.01 | 0.47 | 0.38 |
| AST, U/L | 95.14 | 120.05 | 8.24 | <0.01 | <0.01 | 0.06 |
| TP, g/L | 69.92 | 70.38 | 0.46 | 0.70 | <0.01 | 0.23 |
| ALB, g/L | 31.82 | 32.43 | 0.61 | 0.33 | 0.02 | 0.27 |
| GLB, g/L | 38.10 | 37.95 | 1.20 | 0.90 | <0.01 | 0.42 |
| T-BIL, umol/L | 4.45 | 4.46 | 0.44 | 0.99 | <0.01 | 0.70 |
| ALP, U/L | 42.39 | 38.12 | 4.77 | 0.37 | 0.90 | 0.29 |
| BUN, mmol/L | 5.27 | 5.74 | 0.21 | 0.03 | <0.01 | 0.09 |
| Cr, umol/L | 84.79 | 86.64 | 2.02 | 0.36 | <0.01 | 0.09 |
| SOD, U/mL | 44.17 | 43.17 | 0.94 | 0.29 | 0.45 | 0.12 |
| MDA, nmol/mL | 1.47 | 1.52 | 0.03 | 0.04 | 0.29 | 0.11 |
| H2O2, mmol/L | 25.13 | 49.46 | 5.34 | <0.01 | <0.01 | <0.01 |
| CAT, U/ML | 17.33 | 18.28 | 0.58 | 0.11 | 0.23 | 0.10 |
| BHBA, mmol/L | 0.46 | 0.45 | 0.01 | 0.48 | 0.74 | 0.48 |
| NEFA, umol/L | 38.03 | 37.91 | 1.37 | 0.93 | <0.01 | 0.52 |
| IgG, mg/mL | 17.57 | 16.90 | 0.39 | 0.09 | 0.06 | 0.17 |
| IgA, ug/mL | 588.52 | 561.34 | 14.53 | 0.07 | 0.54 | 0.60 |
| IgM, mg/mL | 4.14 | 3.86 | 0.17 | 0.10 | 0.44 | 0.29 |
| IL-6, ng/L | 401.35 | 398.48 | 12.71 | 0.82 | 0.59 | 0.27 |
| GM-CSF, pg/mL | 54.90 | 55.13 | 1.98 | 0.91 | <0.01 | 0.80 |
| Item | Physiological Stage | Group | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| A 1 | B 2 | Group | Time | Group × Time | |||
| pH | Prenatal 4 | 6.79 | 7.30 | 0.25 | 0.10 | - | - |
| Postpartum 5 | 6.75 | 6.38 | 0.12 | 0.01 | 0.36 | 0.04 | |
| Ammonia nitrogen, mg/dL | Prenatal | 1.61 | 1.49 | 0.10 | 0.24 | - | - |
| Postpartum | 1.29 | 1.31 | 0.02 | 0.77 | <0.01 | 0.40 | |
| Acetic acid, mmol/L | Prenatal | 53.90 | 46.76 | 7.04 | 0.34 | - | - |
| Postpartum | 53.07 | 57.59 | 4.10 | 0.28 | 0.28 | 0.67 | |
| Propionic acid, mmol/L | Prenatal | 17.16 | 15.45 | 1.85 | 0.38 | - | - |
| Postpartum | 23.34 | 25.94 | 2.44 | 0.30 | 0.20 | 0.31 | |
| Butyric acid, mmol/L | Prenatal | 10.21 | 10.44 | 1.53 | 0.89 | - | - |
| Postpartum | 9.18 | 11.32 | 1.09 | 0.06 | 0.77 | 0.39 | |
| Acetic acid/propionic acid | Prenatal | 3.12 | 3.03 | 0.15 | 0.60 | - | - |
| Postpartum | 2.32 | 2.29 | 0.13 | 0.86 | 0.77 | 0.27 | |
| Total VFA, mmol/L 3 | Prenatal | 81.27 | 72.65 | 10.00 | 0.41 | - | - |
| Postpartum | 85.59 | 94.85 | 6.48 | 0.17 | 0.21 | 0.51 | |
| Plylum, % | −14d A | 7d A | 14d A | 21d A | −14d B | 7d B | 14d B | 21d B |
|---|---|---|---|---|---|---|---|---|
| Firmicutes | 48.53 | 48.51 | 57.67 | 47.08 | 39.45 | 47.93 | 51.09 | 49.02 |
| Bacteroidetes | 43.15 | 42.54 | 30.83 | 39.61 | 46.39 | 42.46 | 37.58 | 40.61 |
| Proteobacteria | 3.23 | 4.20 | 5.95 | 6.06 | 5.73 | 3.84 | 5.39 | 3.17 |
| Tenericutes | 1.69 | 1.08 | 1.95 | 2.35 | 4.38 | 2.31 | 2.95 | 2.50 |
| Spirochaetes | 0.62 | 1.68 | 1.72 | 2.67 | 0.56 | 1.66 | 1.46 | 2.61 |
| Patescibacteria | 0.84 | 0.41 | 0.78 | 0.82 | 1.74 | 0.82 | 0.78 | 0.90 |
| Actinobacteria | 0.98 | 0.92 | 0.70 | 0.45 | 0.73 | 0.22 | 0.26 | 0.67 |
| Cyanobacteria | 0.03 | 0.20 | 0.15 | 0.18 | 0.34 | 0.29 | 0.20 | 0.25 |
| Fibrobacteres | 0.13 | 0.28 | 0.11 | 0.46 | 0.18 | 0.17 | 0.15 | 0.14 |
| Kiritimatiellaeota | 0.50 | 0.07 | 0.04 | 0.19 | 0.29 | 0.15 | 0.03 | 0.03 |
| Genus, % | −14d A | 7d A | 14d A | 21d A | −14d B | 7d B | 14d B | 21d B |
|---|---|---|---|---|---|---|---|---|
| Prevotella_1 | 21.25 | 29.07 | 19.54 | 26.75 | 33.58 | 28.66 | 26.74 | 27.75 |
| Ruminococcaceae_UCG-014 | 3.61 | 4.92 | 8.90 | 5.75 | 5.14 | 4.76 | 6.76 | 7.84 |
| Ruminococcaceae_NK4A214_group | 8.23 | 6.28 | 3.92 | 6.46 | 3.10 | 6.68 | 3.76 | 5.35 |
| Christensenellaceae_R-7_group | 11.66 | 3.98 | 2.75 | 4.60 | 4.78 | 5.41 | 2.32 | 2.56 |
| Pseudomonas | 2.39 | 2.95 | 4.20 | 4.71 | 4.67 | 2.52 | 4.29 | 1.67 |
| Lachnospiraceae_NK3A20_group | 2.64 | 3.02 | 5.75 | 2.35 | 2.05 | 2.07 | 4.52 | 2.60 |
| Rikenellaceae_RC9_gut_group | 7.59 | 2.57 | 1.97 | 2.62 | 3.40 | 2.70 | 1.43 | 1.62 |
| F082 | 5.88 | 2.49 | 1.51 | 2.30 | 1.91 | 2.17 | 1.57 | 2.74 |
| [Eubacterium]_coprostanoligenes_group | 1.12 | 3.84 | 4.28 | 2.60 | 0.62 | 2.46 | 2.08 | 2.32 |
| Shuttleworthia | 0.09 | 3.07 | 5.69 | 1.38 | 0.15 | 1.47 | 3.96 | 3.43 |
| Butyrivibrio_2 | 1.61 | 1.44 | 1.96 | 1.86 | 3.18 | 2.10 | 2.53 | 1.58 |
| Mollicutes_RF39 | 1.34 | 0.85 | 1.85 | 2.03 | 3.56 | 1.82 | 2.66 | 2.12 |
| Saccharofermentans | 1.83 | 1.71 | 2.11 | 1.87 | 1.64 | 2.41 | 1.96 | 2.13 |
| Muribaculaceae | 1.45 | 2.49 | 1.50 | 1.41 | 0.36 | 2.99 | 1.74 | 1.31 |
| [Eubacterium]_ruminantium_group | 0.60 | 1.54 | 2.81 | 1.35 | 0.90 | 1.59 | 2.06 | 2.37 |
| Treponema_2 | 0.61 | 1.67 | 1.71 | 2.67 | 0.52 | 1.66 | 1.46 | 2.60 |
| unclassified_Lachnospiraceae | 0.83 | 1.83 | 2.57 | 1.18 | 1.23 | 0.97 | 2.12 | 1.94 |
| Ruminococcus_2 | 1.23 | 1.89 | 0.38 | 1.94 | 0.67 | 1.91 | 0.63 | 0.96 |
| Prevotella_7 | 0.01 | 1.40 | 2.44 | 1.10 | 0.01 | 0.40 | 2.34 | 1.86 |
| Prevotellaceae_UCG-001 | 1.41 | 0.89 | 1.24 | 1.03 | 1.81 | 1.06 | 0.85 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Kong, F.; Li, S.; Wang, X.; Sun, X.; Du, W.; Dai, D.; Wang, S.; Xie, B.; Xu, X. Effect of Alkaline Mineral Complex Buffer Supplementation on Milk Performance, Serum Variables, Rumen Fermentation and Rumen Microbiota of Transition Dairy Cows. Fermentation 2023, 9, 792. https://doi.org/10.3390/fermentation9090792
Guo C, Kong F, Li S, Wang X, Sun X, Du W, Dai D, Wang S, Xie B, Xu X. Effect of Alkaline Mineral Complex Buffer Supplementation on Milk Performance, Serum Variables, Rumen Fermentation and Rumen Microbiota of Transition Dairy Cows. Fermentation. 2023; 9(9):792. https://doi.org/10.3390/fermentation9090792
Chicago/Turabian StyleGuo, Cheng, Fanlin Kong, Shengli Li, Xiaowei Wang, Xiaoge Sun, Wen Du, Dongwen Dai, Shuo Wang, Biao Xie, and Xiaofeng Xu. 2023. "Effect of Alkaline Mineral Complex Buffer Supplementation on Milk Performance, Serum Variables, Rumen Fermentation and Rumen Microbiota of Transition Dairy Cows" Fermentation 9, no. 9: 792. https://doi.org/10.3390/fermentation9090792
APA StyleGuo, C., Kong, F., Li, S., Wang, X., Sun, X., Du, W., Dai, D., Wang, S., Xie, B., & Xu, X. (2023). Effect of Alkaline Mineral Complex Buffer Supplementation on Milk Performance, Serum Variables, Rumen Fermentation and Rumen Microbiota of Transition Dairy Cows. Fermentation, 9(9), 792. https://doi.org/10.3390/fermentation9090792






