Single-Cell Oil Production by Engineered Ashbya gossypii from Non-Detoxified Lignocellulosic Biomass Hydrolysate
Abstract
1. Introduction
2. Materials and Methods
2.1. A. gossypii Strains and Culture Conditions
2.2. Media Composition and Preparation
2.3. Lipid Production
2.4. HPLC Analyses
2.5. Lipid Extraction and GC-MS Quantification
2.6. Nile Red Lipid Staining
3. Results and Discussion
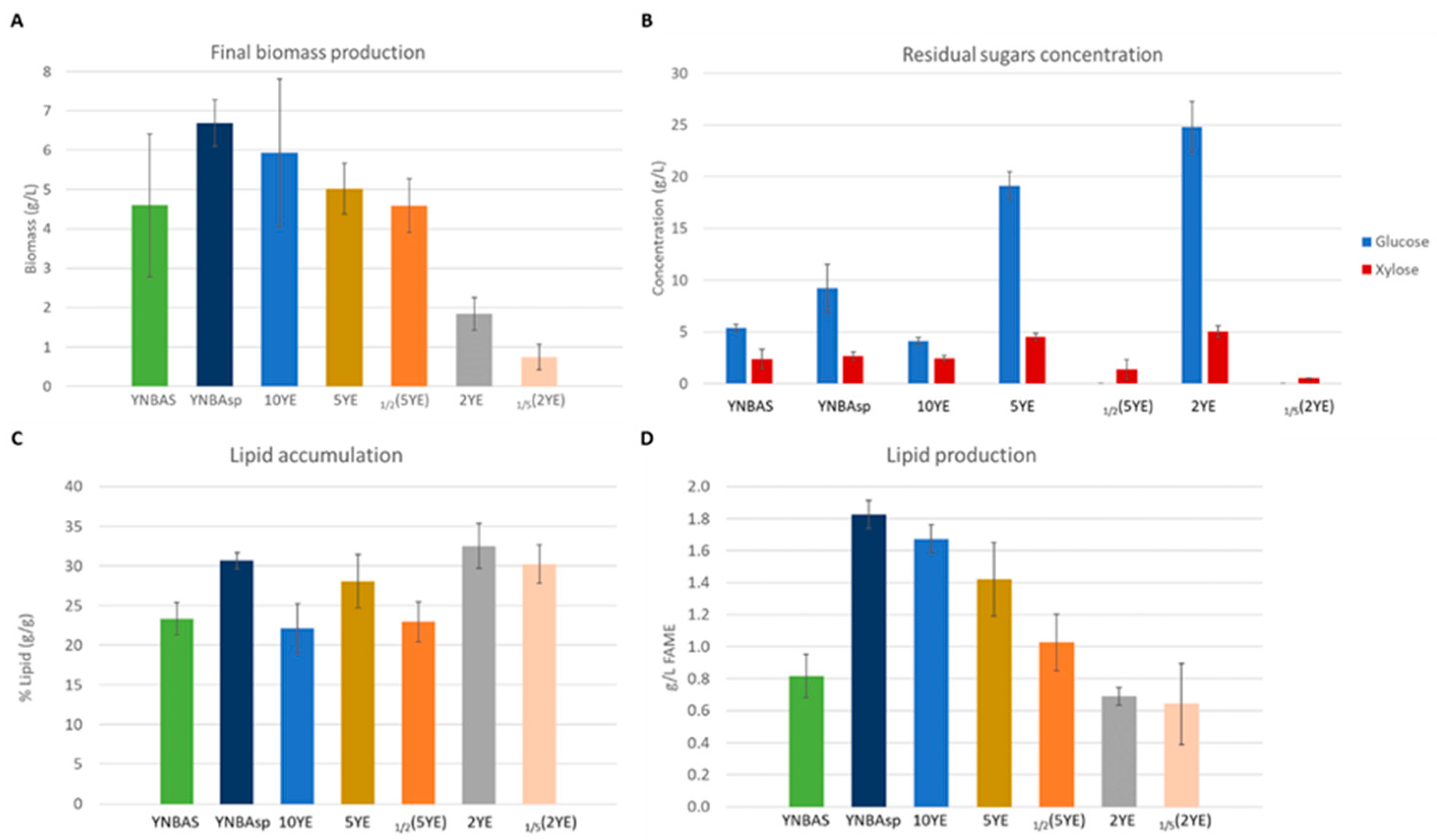
3.1. Performance of Xylose-Utilizing Oleaginous A. gossypii Strains in Non-Detoxified EBH-Mimicking Media
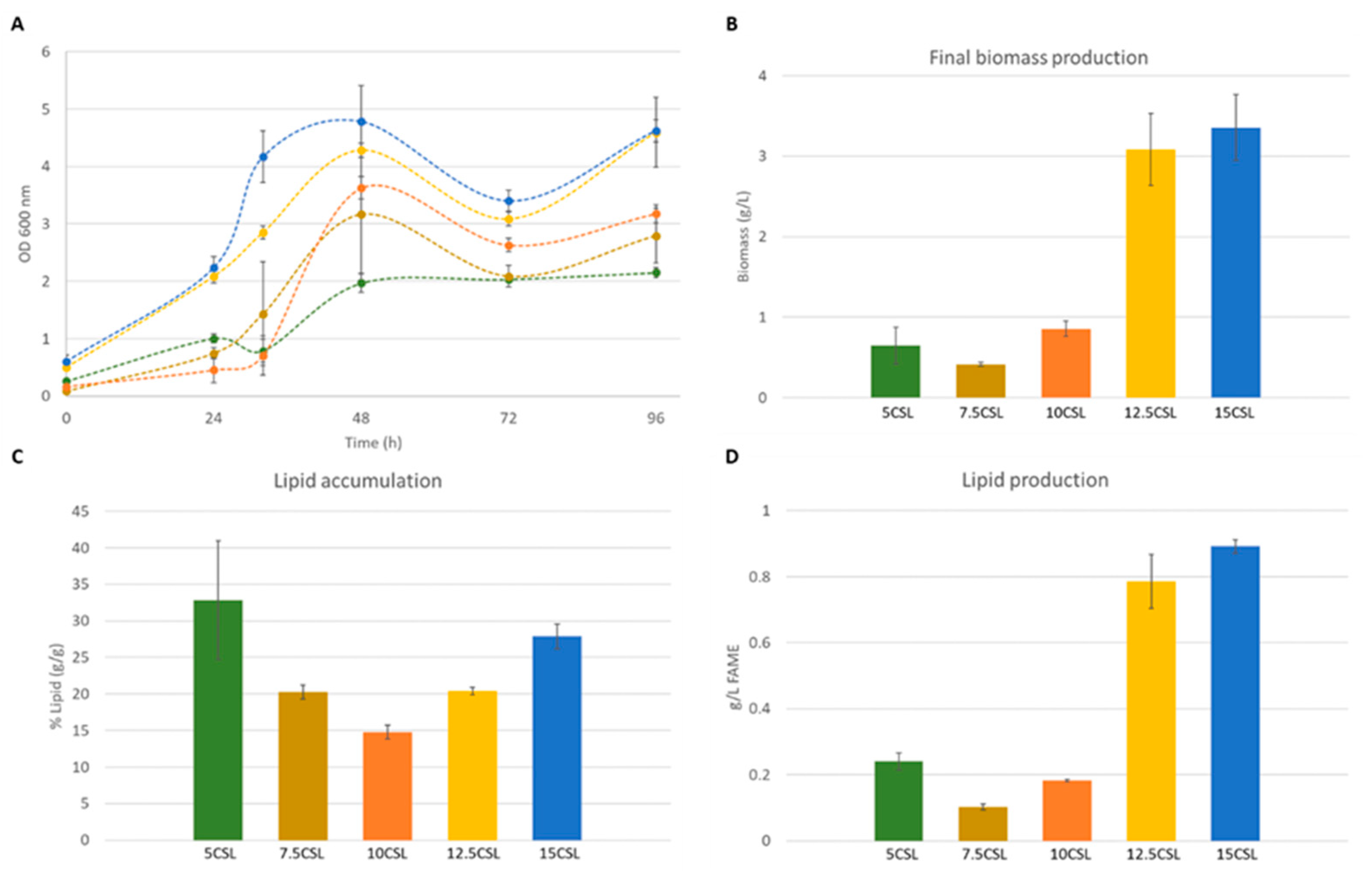
3.2. A. gossypii A877 Performance Using Different Media Supplementations
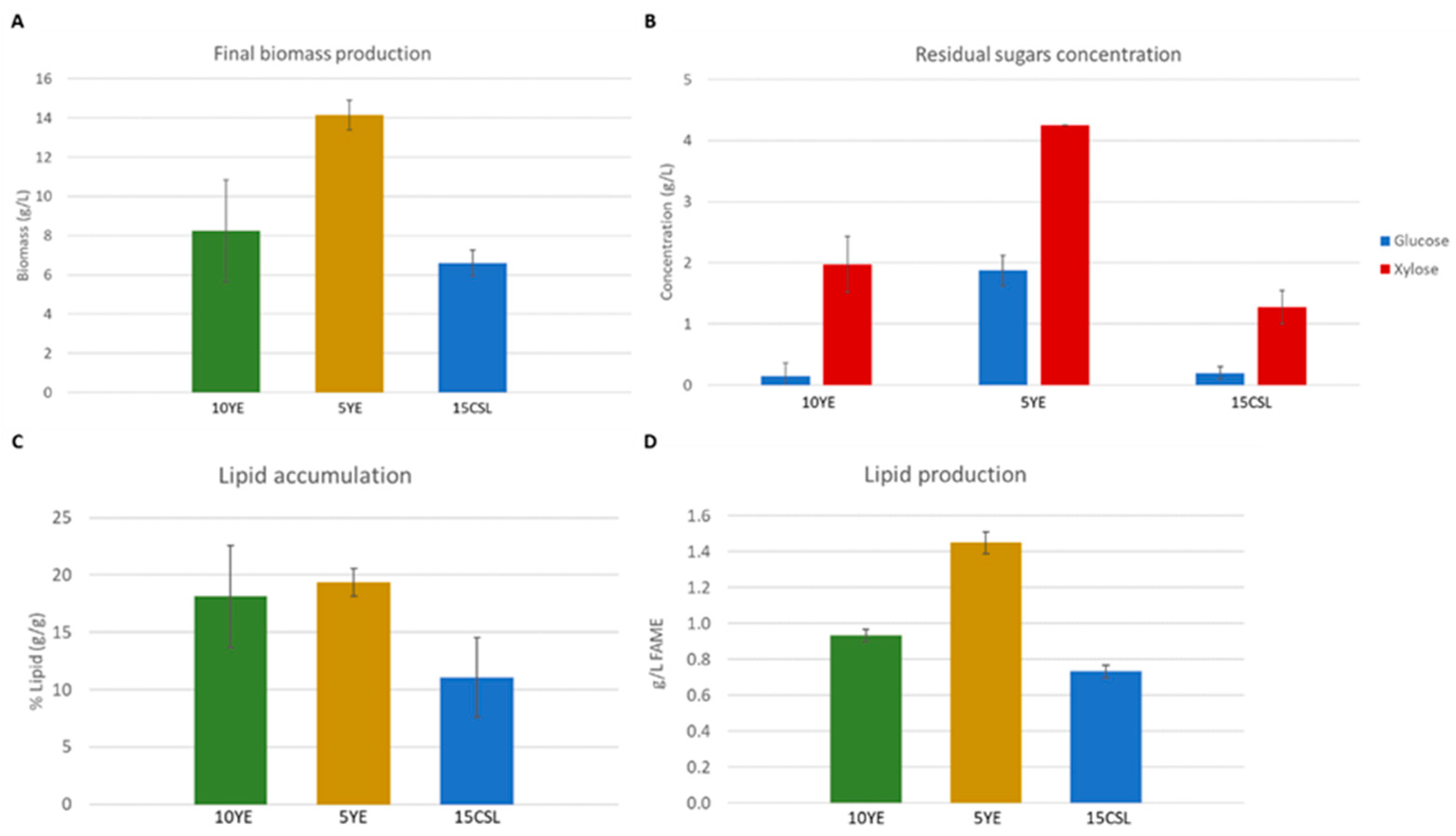
3.3. Lipid Accumulation and Production by Strain A877 in Non-Detoxified EBH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meijaard, E.; Abrams, J.F.; Slavin, J.L.; Sheil, D. Dietary Fats, Human Nutrition and the Environment: Balance and Sustainability. Front. Nutr. 2022, 9, 878644. [Google Scholar] [CrossRef]
- Mhlongo, S.I.; Ezeokoli, O.T.; Roopnarain, A.; Ndaba, B.; Sekoai, P.T.; Habimana, O.; Pohl, C.H. The Potential of Single-Cell Oils Derived From Filamentous Fungi as Alternative Feedstock Sources for Biodiesel Production. Front. Microbiol. 2021, 12, 637381. [Google Scholar] [CrossRef]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production Strategies and Applications of Microbial Single Cell Oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Santos, M.A.; Jiménez, A.; Revuelta, J.L. Tuning single-cell oil production in Ashbya gossypii by engineering the elongation and desaturation systems. Biotechnol. Bioeng. 2014, 9, 1782–1791. [Google Scholar] [CrossRef]
- Cunha, J.T.; Soares, P.O.; Baptista, S.L.; Costa, C.E.; Domingues, L. Engineered Saccharomyces cerevisiae for lignocellulosic valorization: A review and perspectives on bioethanol production. Bioengineered 2020, 11, 883–903. [Google Scholar] [CrossRef]
- Baptista, S.L.; Costa, C.E.; Cunha, J.T.; Soares, P.O.; Domingues, L. Metabolic engineering of Saccharomyces cerevisiae for the production of top value chemicals from biorefinery carbohydrates. Biotechnol. Adv. 2021, 47, 107697. [Google Scholar] [CrossRef] [PubMed]
- Romaní, A.; Larramendi, A.; Yáñez, R.; Cancela, Á.; Sánchez, Á.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus nitens bark by organosolv pretreatment for the production of advanced biofuels. Ind. Crop. Prod. 2019, 132, 327–335. [Google Scholar] [CrossRef]
- Zanuso, E.; Ruiz, H.A.; Domingues, L.; Teixeira, J.A. Magnetic Nanoparticles as Support for Cellulase Immobilization Strategy for Enzymatic Hydrolysis Using Hydrothermally Pretreated Corn Cob Biomass. Bioenerg. Res. 2022, 15, 1946–1957. [Google Scholar] [CrossRef]
- Zanuso, E.; Gomes, D.G.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Enzyme immobilization as a strategy towards efficient and sustainable lignocellulosic biomass conversion into chemicals and biofuels: Current status and perspectives. Sustain. Energy Fuels 2021, 5, 4233–4247. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Costa, C.E.; Sá-Correia, I.; Domingues, L. Molecular and physiological basis of Saccharomyces cerevisiae tolerance to adverse lignocellulose-based process conditions. Appl. Microbiol. Biotechnol. 2019, 103, 159–175. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering Toward White Biotechnology. Bioenergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Zuccaro, G.; Kumar, M.; Kumar, S.P.J.; Garlapati, V.K.; Postemsky, P.D.; Kumar, N.S.S.; Chandel, A.K.; Simal-Gandara, J. Biodiesel Production from Lignocellulosic Biomass Using Oleaginous Microbes: Prospects for Integrated Biofuel Production. Front. Microbiol. 2021, 12, 658284. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar] [CrossRef]
- Domingos, M.; de Souza-Cruz, P.B.; Ferraz, A.; Prata, A.M.R. A new bioreactor design for culturing basidiomycetes: Mycelial biomass production in submerged cultures of Ceriporiopsis subvermispora. Chem. Eng. Sci. 2017, 170, 670–676. [Google Scholar] [CrossRef]
- Pereira, F.B.; Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Optimization of low-cost medium for very high gravity ethanol fermentations by Saccharomyces cerevisiae using statistical experimental designs. Bioresour. Technol. 2010, 101, 7856–7863. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, T.Q.; Silva, R.; Domingues, L. Ashbya gossypii beyond industrial riboflavin production: A historical perspective and emerging biotechnological applications. Biotechnol. Adv. 2015, 33, 1774–1786. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Buey, R.M.; Revuelta, J.L. The filamentous fungus Ashbya gossypii as a competitive industrial inosine producer. Biotechnol. Bioeng. 2016, 113, 2060–2063. [Google Scholar] [CrossRef]
- Serrano-Amatriain, C.; Ledesma-Amaro, R.; López-Nicolás, R.; Ros, G.; Jiménez, A.; Revuelta, J.L. Folic Acid Production by Engineered Ashbya gossypii. Metab. Eng. 2016, 38, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Jiménez, A.; Revuelta, J.L. Pathway Grafting for Polyunsaturated Fatty Acids Production in Ashbya gossypii through Golden Gate Rapid Assembly. ACS Synth. Biol. 2018, 7, 2340–2347. [Google Scholar] [CrossRef]
- Díaz-Fernández, D.; Aguiar, T.Q.; Martín, V.I.; Romaní, A.; Silva, R.; Domingues, L.; Revuelta, J.L.; Jiménez, A. Microbial lipids from industrial wastes using xylose-utilizing Ashbya gossypii strains. Bioresour. Technol. 2019, 293, 122054. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Aguiar, T.Q.; Coelho, E.; Jiménez, A.; Revuelta, J.L.; Domingues, L. Metabolic engineering of Ashbya gossypii for deciphering the de novo biosynthesis of γ-lactones. Microb. Cell Factories 2019, 18, 62. [Google Scholar] [CrossRef]
- Silva, R.; Aguiar, T.Q.; Domingues, L. Orotic acid production from crude glycerol by engineered Ashbya gossypii. Bioresour. Technol. Rep. 2022, 17, 100992. [Google Scholar] [CrossRef]
- Muñoz-Fernández, G.; Martínez-Buey, R.; Revuelta, J.L.; Jiménez, A. Metabolic engineering of Ashbya gossypii for limonene production from xylose. Biotechnol. Biofuels Bioprod. 2022, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Santos, M.A.; Jiménez, A.; Revuelta, J.L. Strain design of Ashbya gossypii for single-cell oil production. Appl. Environ. Microbiol. 2014, 80, 1237–1244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomes, D.G.; Michelin, M.; Romaní, A.; Domingues, L.; Teixeira, J.A. Co-production of biofuels and value-added compounds from industrial Eucalyptus globulus bark residues using hydrothermal treatment. Fuel 2021, 285, 119265. [Google Scholar] [CrossRef]
- Díaz-Fernández, D.; Lozano-Martínez, P.; Buey, R.M.; Revuelta, J.L.; Jiménez, A. Utilization of xylose by engineered strains of Ashbya gossypii for the production of microbial oils. Biotechnol. Biofuels 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.L.; Romaní, A.; Cunha, J.T.; Domingues, L. Multi-feedstock biorefinery concept: Valorization of winery wastes by engineered yeast. J. Environ. Manag. 2023, 326, 116623. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Xiong, L.; Lin, X.; Huang, C.; Li, H.; Chen, X.; Chen, X. CaCO3 supplementation alleviates the inhibition of formic acid on acetone/butanol/ethanol fermentation by Clostridium acetobutylicum. Biotechnol. Lett. 2017, 39, 97–104. [Google Scholar] [CrossRef]
- Aguiar, T.Q.; Silva, R.; Domingues, L. New biotechnological applications for Ashbya gossypii: Challenges and perspectives. Bioengineered 2017, 8, 309–315. [Google Scholar] [CrossRef]
- Caspeta, L.; Nielsen, J. Economic and environmental impacts of microbial biodiesel. Nat. Biotechnol. 2013, 31, 789–793. [Google Scholar] [CrossRef]
- Dirmeier, R.; O’Brien, K.M.; Engle, M.; Dodd, A.; Spears, E.; Poyton, R.O. Exposure of yeast cells to anoxia induces transient oxidative stress: Implications for the induction of hypoxic genes. J. Biol. Chem. 2002, 277, 34773–34784. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Nicaud, J.M.; Gaillardin, C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, W.; Shen, H.; Zhao, Z.K.; Yang, Z.; Yan, J.; Zhao, M. Co-utilization of corn stover hydrolysates and biodiesel-derived glycerol by Cryptococcus curvatus for lipid production. Bioresour. Technol. 2016, 219, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Do Kim, J.; Gong, G.; Ko, J.K.; Um, Y.; Han, S.O.; Lee, S.M. High-yield lipid production from lignocellulosic biomass using engineered xylose-utilizing Yarrowia lipolytica. Glob. Chang. Biol. Bioenergy 2020, 12, 670–679. [Google Scholar] [CrossRef]


| Strain | Parental Strain | Genotype | Relevant Phenotype | Reference |
|---|---|---|---|---|
| A729 | ATCC 10895 | ACL107Cp::loxP-AgGPDp, AGR324Cp::loxP-AgGPDp, ABR229Cp::loxP-AgGPDp, ADR304W::loxP-AgGPDp-Bspta-AgPGK1t, AGR034W::loxP-AgGPDp-AnxpkA-AgENO2t | Ino−, Xyl+ | [26] |
| A842 | A729 | A729, ACR165W850−1139::loxP, AAR071W::loxP-AgGPDp-aar071wT1975G, T3463G | Ino−, Xyl+, oleaginous | [20] |
| A877 | A842 | A842, AFR171W::loxP-KanMX-loxP-AgGPDp-AgACR140C-AgPGK1t | Ino−, Xyl+, oleaginous, G418R | [20] |
| Media | Concentration (g/L) | C/N Ratio | |||
|---|---|---|---|---|---|
| YE | 1x YNB + AS | 1x YNB + Asp | CSL | ||
| SM-2 to 10 YE | 2 to 10 | - | - | - | ~150 to ~30 |
| SM1/2(5YE) | 5 | - | - | - | ~30 |
| SM1/5(2YE) | 2 | - | - | - | ~30 |
| SM-YNBAS | - | 5 | - | - | 29 |
| SM-YNBAsp | - | - | 2 | - | 74 |
| SM-5 to 15 CSL | - | - | - | 5 to 15 | ~65 to ~22 |
| EBH-2 to 10 YE | 2 to 10 | - | - | - | ~150 to ~30 |
| EBH-15/17CSL | - | - | - | 15 or 17 | ~22 or ~19 |
| Microorganism | Medium Composition (g/L) | Lipid Titer (g/L) | Lipid Content (%) | Reference |
|---|---|---|---|---|
| A. gossypii A877 | Non-detoxified EBH | 1.42 | 11.0 | This study |
| G: 84.0 | ||||
| X: 8.65 | ||||
| A. gossypii A877 | 50% detoxified corn cob hydrolysate + 4% molasses supplemented with YE and tryptone | 3.90 | 38.1 | [20] |
| G: 2.60 | ||||
| X: 15.1 | ||||
| S: 20.3 | ||||
| Y. lipolytica YSXID | Miscanthus hydrolysates (Fed-batch) supplemented with YNB containing amino acids and ammonium sulfate | 12.01 | 42.4 | [34] |
| G: 35.2 + 32.7 | ||||
| X: 32.8 + 22.8 | ||||
| C. curvatus | Corn stover enzymatic hydrolysates supplemented with YE, ammonium sulfate, and trace elements | 4.6 | 39.4 | [33] |
| G: 18.8 | ||||
| X: 14.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francisco, M.; Aguiar, T.Q.; Abreu, G.; Marques, S.; Gírio, F.; Domingues, L. Single-Cell Oil Production by Engineered Ashbya gossypii from Non-Detoxified Lignocellulosic Biomass Hydrolysate. Fermentation 2023, 9, 791. https://doi.org/10.3390/fermentation9090791
Francisco M, Aguiar TQ, Abreu G, Marques S, Gírio F, Domingues L. Single-Cell Oil Production by Engineered Ashbya gossypii from Non-Detoxified Lignocellulosic Biomass Hydrolysate. Fermentation. 2023; 9(9):791. https://doi.org/10.3390/fermentation9090791
Chicago/Turabian StyleFrancisco, Miguel, Tatiana Q. Aguiar, Gabriel Abreu, Susana Marques, Francisco Gírio, and Lucília Domingues. 2023. "Single-Cell Oil Production by Engineered Ashbya gossypii from Non-Detoxified Lignocellulosic Biomass Hydrolysate" Fermentation 9, no. 9: 791. https://doi.org/10.3390/fermentation9090791
APA StyleFrancisco, M., Aguiar, T. Q., Abreu, G., Marques, S., Gírio, F., & Domingues, L. (2023). Single-Cell Oil Production by Engineered Ashbya gossypii from Non-Detoxified Lignocellulosic Biomass Hydrolysate. Fermentation, 9(9), 791. https://doi.org/10.3390/fermentation9090791






