Changes in the Concentration of Amino Acids and Bacterial Community in the Rumen When Feeding Artemisia absinthium and Cobalt Chloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Informed Consent
2.2. Feeding and Keeping of Animals
2.3. Amino Acid Analysis
2.4. Extraction of Total DNA
2.5. Library Preparation and Sequencing
2.6. Bioinformatic Processing of Sequencing Data
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durmic, Z.; Moate, P.J.; Eckard, R.; Revell, D.K.; Williams, R.; E Vercoe, P. In vitro screening of selected feed additives, plant essential oils and plant extracts for rumen methane mitigation. J. Sci. Food Agric. 2013, 94, 1191–1196. [Google Scholar] [CrossRef]
- Lira-Casas, R.; Ramirez-Bribiesca, J.E.; A Zavaleta-Mancera, H.; Hidalgo-Moreno, C.; Cruz-Monterrosa, R.G.; Crosby-Galvan, M.M.; A Mendez-Rojas, M.; A Domínguez-Vara, I. Designing and evaluation of urea microcapsules in vitro to improve nitrogen slow release availability in rumen. J. Sci. Food Agric. 2018, 99, 2541–2547. [Google Scholar] [CrossRef]
- Michalak, M.; Wojnarowski, K.; Cholewińska, P.; Szeligowska, N.; Bawej, M.; Pacoń, J. Selected Alternative Feed Additives Used to Manipulate the Rumen Microbiome. Animals 2021, 11, 1542. [Google Scholar] [CrossRef]
- Rabee, A.E.; Younan, B.R.; Kewan, K.Z.; Sabra, E.A.; Lamara, M. Modulation of rumen bacterial community and feed utilization in camel and sheep using combined supplementation of live yeast and microalgae. Sci. Rep. 2022, 12, 12990. [Google Scholar] [CrossRef] [PubMed]
- Valldecabres, A.; Gilmore, S.P.; Embree, J.J.; Zhelev, I.Z.; Gaffney, J.R.; A Marotz, C.; Yang, F.; Izzo, A.S.; Embree, M.M.; Lago, A. Effects of rumen-native microbial feed supplementation on milk yield, composition, and feed efficiency in lactating dairy cows. J. Anim. Sci. 2022, 100, skac275. [Google Scholar] [CrossRef] [PubMed]
- Bennato, F.; Martino, C.; Di Domenico, M.; Ianni, A.; Chai, B.; Di Marcantonio, L.; Cammà, C.; Martino, G. Metagenomic Characterization and Volatile Compounds Determination in Rumen from Saanen Goat Kids Fed Olive Leaves. Veter. Sci. 2022, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler chicks challenged with Salmonella typhimurium. Environ. Sci. Pollut. Res. 2016, 23, 24151–24157. [Google Scholar] [CrossRef]
- Thomas, M.; Webb, M.; Ghimire, S.; Blair, A.; Olson, K.; Fenske, G.J.; Fonder, A.T.; Christopher-Hennings, J.; Brake, D.; Scaria, J. Metagenomic characterization of the effect of feed additives on the gut microbiome and antibiotic resistome of feedlot cattle. Sci. Rep. 2017, 7, 12257. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2017, 4, 137–150. [Google Scholar] [CrossRef]
- Teobaldo, R.W.; Granja-Salcedo, Y.T.; Cardoso, A.d.S.; Constancio, M.T.L.; Brito, T.R.; Romanzini, E.P.; Reis, R.A. The Impact of Mineral and Energy Supplementation and Phytogenic Compounds on Rumen Microbial Diversity and Nitrogen Utilization in Grazing Beef Cattle. Microorganisms 2023, 11, 810. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.; Castillejos, L.; Ferret, A. Invited Review: Essential Oils as Modifiers of Rumen Microbial Fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, L.; Ke, S.; Chen, X.; Kenéz, Á.; Xu, W.; Wang, D.; Zhang, F.; Li, Y.; Cui, Z.; et al. Yak rumen microbiome elevates fiber degradation ability and alters rumen fermentation pattern to increase feed efficiency. Anim. Nutr. 2022, 11, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef] [PubMed]
- Daugaliyeva, A.; Daugaliyeva, S.; Ashanin, A.; Beltramo, C.; Mamyrova, L.; Yessembekova, Z.; Peletto, S. Prokaryotic Diversity of Ruminal Content and Its Relationship with Methane Emissions in Cattle from Kazakhstan. Life 2022, 12, 1911. [Google Scholar] [CrossRef]
- Dixit, S.; Kumar, S.; Sharma, R.; Banakar, P.S.; Singh, M.; Keshri, A.; Tyagi, A.K. Rumen multi-omics addressing diet–host–microbiome interplay in farm animals: A review. Anim. Biotechnol. 2022, 17, 1–19. [Google Scholar] [CrossRef]
- Li, F.; Hitch, T.C.A.; Chen, Y.; Creevey, C.J.; Guan, L.L. Comparative metagenomic and metatranscriptomic analyses reveal the breed effect on the rumen microbiome and its associations with feed efficiency in beef cattle. Microbiome 2019, 7, 6. [Google Scholar] [CrossRef]
- Clemmons, B.A.; Voy, B.H.; Myer, P.R. Altering the Gut Microbiome of Cattle: Considerations of Host-Microbiome Interactions for Persistent Microbiome Manipulation. Microb. Ecol. 2018, 77, 523–536. [Google Scholar] [CrossRef]
- Redoy, M.R.A.; Shuvo, A.A.S.; Cheng, L.; Al-Mamun, M. Effect of herbal supplementation on growth, immunity, rumen histology, serum antioxidants and meat quality of sheep. Animal 2020, 14, 2433–2441. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Beshbishy, A.M.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Rashidi, R.; Akaberi, M.; Gholoobi, A.; Ghazavi, H. Artemisia absinthium extract attenuates the quinolinic acid-induced cell injury in OLN-93 cells. Curr. Cancer Drug Targets 2023, 20, 42–47. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.; Chen, L.; Zhang, Y.; Pei, C.; Zhang, S. Effects of folic acid and cobalt sulphate supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Holstein calves. Br. J. Nutr. 2021, 127, 1313–1319. [Google Scholar] [CrossRef]
- González-Montaña, J.-R.; Escalera-Valente, F.; Alonso, A.J.; Lomillos, J.M.; Robles, R.; Alonso, M.E. Relationship between Vitamin B12 and Cobalt Metabolism in Domestic Ruminant: An Update. Animals 2020, 10, 1855. [Google Scholar] [CrossRef]
- Kalashnikov, A.; Fisinin, V.; Shcheglova, V.; Kleimenova, N. Feeding Norms and Rations—Reference Manual, 3rd ed.; Russia Russian Agricultural Academy: Moscow, Russia, 2003; 456p. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18–20 September 2017; ACM: New York, NY, USA; pp. 28:1–28:5. Available online: https://www.rawgraphs.io/ (accessed on 6 June 2023).
- Lachenmeier, D.W. Wormwood (Artemisia absinthium L.)—A curious plant with both neurotoxic and neuroprotective properties? J. Ethnopharmacol. 2010, 131, 224–227. [Google Scholar] [CrossRef]
- Koulu, M.; Örmä, S.; Liljeblad, A.; Niemelä, P. Artemisaiae as medicinal and herbal medicinal plants from ancient times to the present day. Duodecim Laaketieteellinen Aikakauskirja 2016, 132, 1763–1770. [Google Scholar]
- Artegoitia, V.M.; Foote, A.P.; Lewis, R.M.; Freetly, H.C. Rumen Fluid Metabolomics Analysis Associated with Feed Efficiency on Crossbred Steers. Sci. Rep. 2017, 7, 14. [Google Scholar] [CrossRef]
- Li, L.; Ning, N.; Wei, J.-A.; Huang, Q.-L.; Lu, Y.; Pang, X.-F.; Wu, J.-J.; Zhou, J.-W.; Luo, G.-A.; Han, L. Metabonomics Study on the Infertility Treated with Zishen Yutai Pills Combined with In Vitro Fertilization-embryo Transfer. Front. Pharmacol. 2021, 12, 686133. [Google Scholar] [CrossRef]
- Petrič, D.; Mravčáková, D.; Kucková, K.; Kišidayová, S.; Cieslak, A.; Szumacher-Strabel, M.; Huang, H.; Kolodziejski, P.; Lukomska, A.; Slusarczyk, S.; et al. Impact of Zinc and/or Herbal Mixture on Ruminal Fermentation, Microbiota, and Histopathology in Lambs. Front. Veter. Sci. 2021, 8, 630971. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Seo, M.; Park, H.; Lee, W.K.; Guan, L.L.; Yoon, J.; Caetano-Anolles, K.; Ahn, H.; Kim, S.-Y.; Kang, Y.-M.; et al. Gut microbiota Modulated by Probiotics and Garcinia cambogia Extract Correlate with Weight Gain and Adipocyte Sizes in High Fat-Fed Mice. Sci. Rep. 2016, 6, 33566. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Liu, H.; Zhao, J.; Gu, X.; Wei, X.; Zhang, X.; Ma, N.; Johnston, L.J.; Bai, Y.; Zhang, W.; et al. Amino acids metabolism by rumen microorganisms: Nutrition and ecology strategies to reduce nitrogen emissions from the inside to the outside. Sci. Total. Environ. 2021, 800, 149596. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Brover, V.; Tolstoy, I.; Yutin, N. Phylogenomic analysis of the family Peptostreptococcaceae (Clostridium cluster XI) and proposal for reclassification of Clostridium litorale (Fendrich et al. 1991) and Eubacterium acidaminophilum (Zindel et al. 1989) as Peptoclostridium litorale gen. nov. comb. nov. and Peptoclostridium acidaminophilum comb. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5506–5513. [Google Scholar] [CrossRef]
- Duan, Y.H.; Zeng, L.M.; Li, F.N.; Kong, X.F.; Xu, K.; Guo, Q.P.; Wang, W.L.; Zhang, L.Y. β-hydroxy-β-methyl butyrate promotes leucine metabolism and improves muscle fibre composition in growing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1328–1339. [Google Scholar] [CrossRef]
- Tsekouras, N.; Meletis, E.; Kostoulas, P.; Labronikou, G.; Athanasakopoulou, Z.; Christodoulopoulos, G.; Billinis, C.; Papatsiros, V.G. Detection of Enterotoxigenic Escherichia coli and Clostridia in the Aetiology of Neonatal Piglet Diarrhoea: Important Factors for Their Prevention. Life 2023, 13, 1092. [Google Scholar] [CrossRef]
- Ryazanov, V.; Duskaev, G.; Sheida, E.; Nurzhanov, B.; Kurilkina, M. Rumen fermentation, methane concentration, and blood metabolites of cattle receiving dietetical phytobiotic and cobalt (II) chloride. Vet. World 2022, 15, 2551–2557. [Google Scholar] [CrossRef]
- Gharechahi, J.; Vahidi, M.F.; Bahram, M.; Han, J.-L.; Ding, X.-Z.; Salekdeh, G.H. Metagenomic analysis reveals a dynamic microbiome with diversified adaptive functions to utilize high lignocellulosic forages in the cattle rumen. ISME J. 2020, 15, 1108–1120. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, H.; Yan, H.; Azarfar, A.; Shi, H.; Alugongo, G.; Li, S.; Cao, Z.; Wang, Y. Comparison of rumen bacteria distribution in original rumen digesta, rumen liquid and solid fractions in lactating Holstein cows. J. Anim. Sci. Biotechnol. 2017, 8, 636–642. [Google Scholar] [CrossRef]
- Nathani, N.M.; Patel, A.K.; Mootapally, C.S.; Reddy, B.; Shah, S.V.; Lunagaria, P.M.; Joshi, C.G. Effect of roughage on rumen microbiota composition in the efficient feed converter and sturdy Indian Jaffrabadi buffalo (Bubalus bubalis). BMC Genom. 2015, 16, 1116. [Google Scholar] [CrossRef]
- Kim, J.N.; Cann, I.K.; Mackie, R.I. Purification, characterization, and expression of multiple glutamine synthetases from Prevotella ruminicola 23. J. Bacteriol. 2012, 194, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, J.S.; Rivera, C.N.; Vaughan, R.A. Branched-Chain Amino Acids and Mitochondrial Biogenesis: An Overview and Mechanistic Summary. Mol. Nutr. Food Res. 2022, 66, e2200109. [Google Scholar] [CrossRef] [PubMed]
- Zolnikova, O.Y.; Potskhverashvili, N.D.; Kudryavtseva, A.V.; Krasnov, G.S.; Guvatova, Z.G.; Truhmanov, A.S.; Kokina, N.I.; Ivashkin, V.T. Changes in gut microbiota with bronchial asthma. Ter. Arkh. 2020, 92, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Ueki, A.; Ohtaki, Y.; Kaku, N.; Watanabe, K.; Ueki, K. Anaerocella delicata gen. nov., sp. nov., a strictly anaerobic bacterium in the phylum Bacteroidetes isolated from a methanogenic reactor of cattle farms. J. Gen. Appl. Microbiol. 2012, 58, 405–412. [Google Scholar] [CrossRef]
- Vargas-Rodriguez, C.; Yuan, K.; Titgemeyer, E.; Mamedova, L.; Griswold, K.; Bradford, B. Effects of supplemental chromium propionate and rumen-protected amino acids on productivity, diet digestibility, and energy balance of peak-lactation dairy cattle. J. Dairy Sci. 2014, 97, 3815–3821. [Google Scholar] [CrossRef]
- Higgs, R.; Chase, L.; Schwab, C.; Sloan, B.; Luchini, D.; LaPierre, P.; Van Amburgh, M. Balancing dairy cattle diets for rumen nitrogen and methionine or all essential amino acids relative to metabolizable energy. J. Dairy Sci. 2023, 106, 1826–1836. [Google Scholar] [CrossRef]
- Kim, M.H.; Seo, J.Y.; Liu, K.H.; Kim, J.-S. Protective Effect of Artemisia annua L. Extract against Galactose-Induced Oxidative Stress in Mice. PLoS ONE 2014, 9, e101486. [Google Scholar] [CrossRef]
- Ivanov, M.; Gašić, U.; Stojković, D.; Kostić, M.; Mišić, D.; Soković, M. New Evidence for Artemisia absinthium L. Application in Gastrointestinal Ailments: Ethnopharmacology, Antimicrobial Capacity, Cytotoxicity, and Phenolic Profile. Evidence-Based Complement. Altern. Med. 2021, 14, 9961089. [Google Scholar] [CrossRef]
- Kim, S.; Adesogan, A.; Kim, J.; Ko, Y. Influence of replacing rice straw with wormwood (Artemisia montana) silage on feed intake, digestibility and ruminal fermentation characteristics of sheep. Anim. Feed. Sci. Technol. 2006, 128, 1–13. [Google Scholar] [CrossRef]

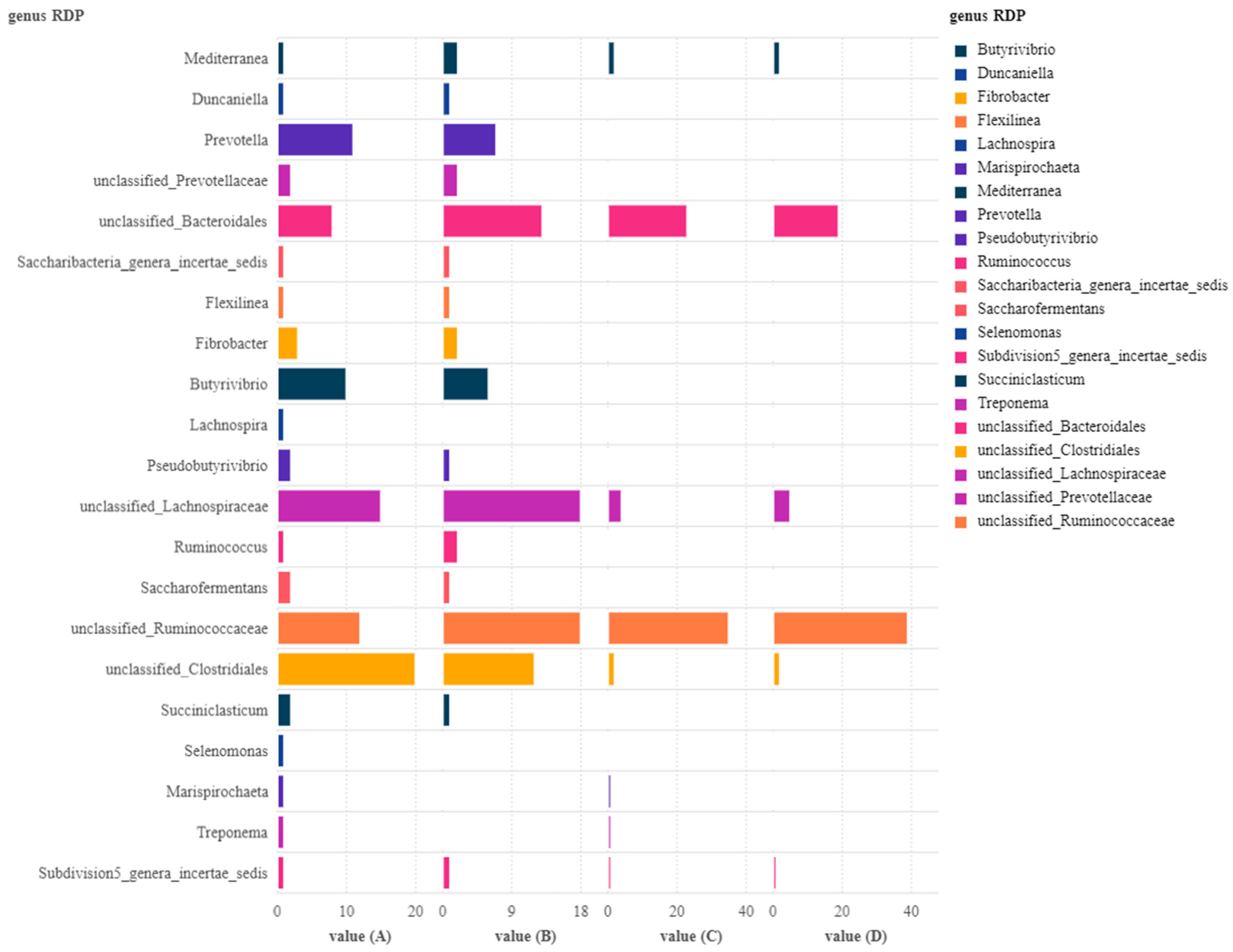


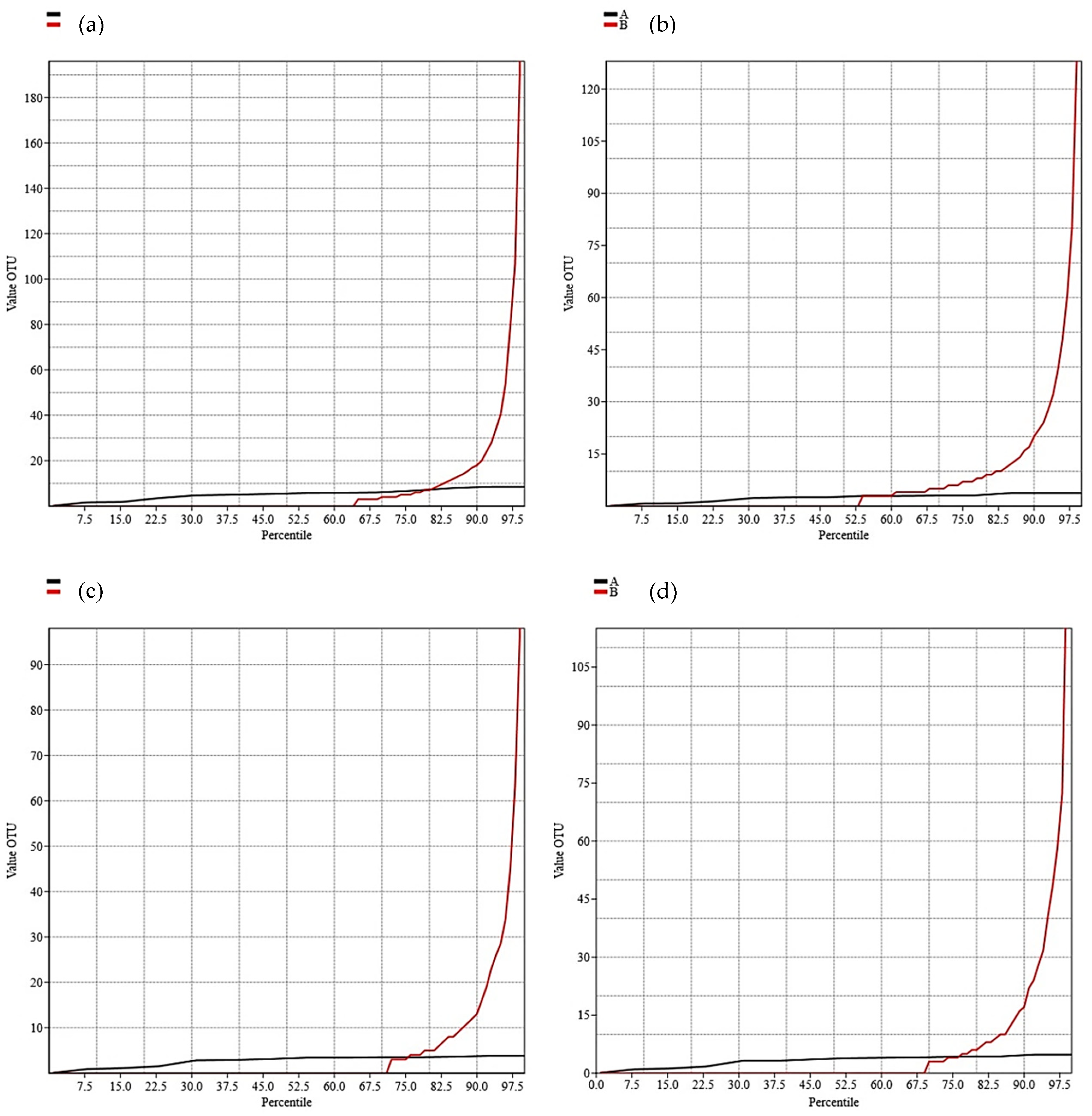
| Indices | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Taxa S | 465.1 ± 39.6 | 657.3 ± 43.0 | 408.6 ± 15.5 | 454.1 ± 36.0 * |
| Individuals | 17,640.6 ± 547.4 | 15,727.7 ± 24.6 * | 8129.7 ± 580.6 | 10,762.0 ± 1417.1 * |
| Dominance D | 0.04 ± 0.0081 | 0.02 ± 0.0044 * | 0.02 ± 0.0008 | 0.02 ± 0.0011 |
| Shannon H | 4.86 ± 0.17 | 5.16 ± 0.16 ** | 5.14 ± 0.02 | 5.15 ± 0.07 * |
| Pielou’s evenness | 0.24 ± 0.015 | 0.28 ± 0.024 | 0.42 ± 0.011 * | 0.39 ± 0.008 * |
| Amino Acids | Groups | ||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Glycogenic | arginine | 5.7 ± 0.06 | 3.7 ± 0.04 * | 3.5 ± 0.07 | 4.3 ± 0.02 |
| histidine | 1.8 ± 0.02 | 0.8 ± 0.01 | 1.1 ± 0.03 | 1.2 ± 0.04 | |
| valine | 6.8 ± 0.03 | 3.0 ± 0.01 * | 3.4 ± 0.06 * | 4.1 ± 0.01 * | |
| alanine | 7.9 ± 0.02 | 3.0 ± 0.01 ** | 3.5 ± 0.05 * | 4.3 ± 0.02 ** | |
| glycine | 5.8 ± 0.07 | 2.9 ± 0.04 | 3.4 ± 0.02 | 4.0 ± 0.01 | |
| threonine | 6.0 ± 0.05 | 2.9 ± 0.07 | 3.6 ± 0.06 | 3.9 ± 0.05 | |
| serine | 5.3 ± 0.02 | 2.5 ± 0.01 | 3.1 ± 0.03 | 3.6 ± 0.08 | |
| methionine | 3.5 ± 0.04 | 1.4 ± 0.02 * | 1.5 ± 0.02 * | 1.7 ± 0.03 * | |
| Ketogenic | lysine | 8.5 ± 0.05 | 3.7 ± 0.04 * | 3.8 ± 0.03 | 4.8 ± 0.05 * |
| Gluco-ketogenic | tyrosine | 4.7 ± 0.04 | 2.5 ± 0.02 | 2.9 ± 0.05 | 3.2 ± 0.07 |
| phenylalanine | 5.0 ± 0.03 | 2.3 ± 0.07 | 2.8 ± 0.04 | 3.2 ± 0.05 | |
| leucine + isoleucine | 1.6 ± 0.01 | 0.74 ± 0.02 ** | 0.87 ± 0.02 * | 0.97 ± 0.04 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryazanov, V.; Tarasova, E.; Duskaev, G.; Kolpakov, V.; Miroshnikov, I. Changes in the Concentration of Amino Acids and Bacterial Community in the Rumen When Feeding Artemisia absinthium and Cobalt Chloride. Fermentation 2023, 9, 751. https://doi.org/10.3390/fermentation9080751
Ryazanov V, Tarasova E, Duskaev G, Kolpakov V, Miroshnikov I. Changes in the Concentration of Amino Acids and Bacterial Community in the Rumen When Feeding Artemisia absinthium and Cobalt Chloride. Fermentation. 2023; 9(8):751. https://doi.org/10.3390/fermentation9080751
Chicago/Turabian StyleRyazanov, Vitaly, Ekaterina Tarasova, Galimzhan Duskaev, Vladimir Kolpakov, and Ivan Miroshnikov. 2023. "Changes in the Concentration of Amino Acids and Bacterial Community in the Rumen When Feeding Artemisia absinthium and Cobalt Chloride" Fermentation 9, no. 8: 751. https://doi.org/10.3390/fermentation9080751
APA StyleRyazanov, V., Tarasova, E., Duskaev, G., Kolpakov, V., & Miroshnikov, I. (2023). Changes in the Concentration of Amino Acids and Bacterial Community in the Rumen When Feeding Artemisia absinthium and Cobalt Chloride. Fermentation, 9(8), 751. https://doi.org/10.3390/fermentation9080751







