Algae: The Reservoir of Bioethanol
Abstract
1. Introduction
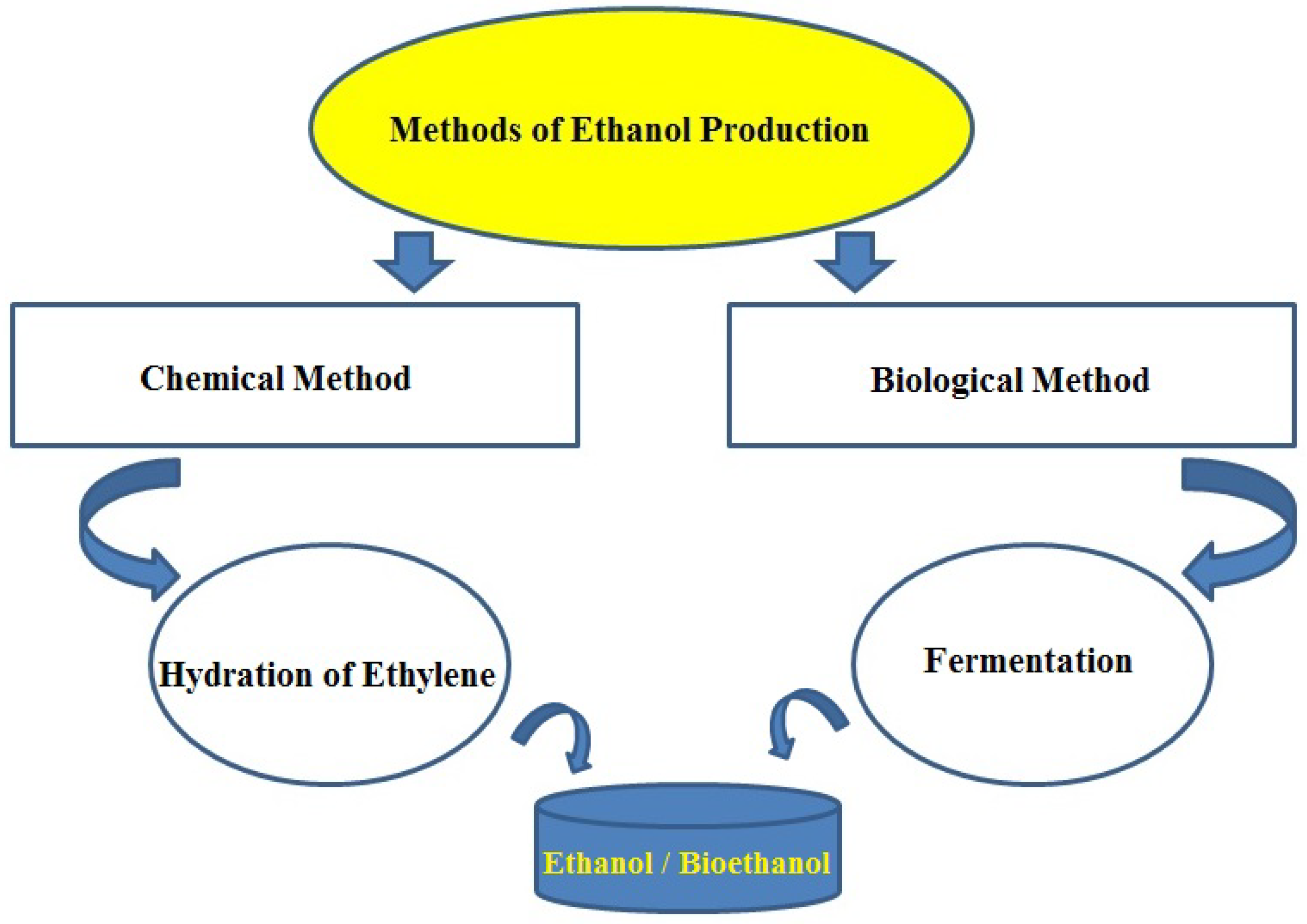
2. Production of Ethanol
3. Biological Sources Used for Ethanol Production
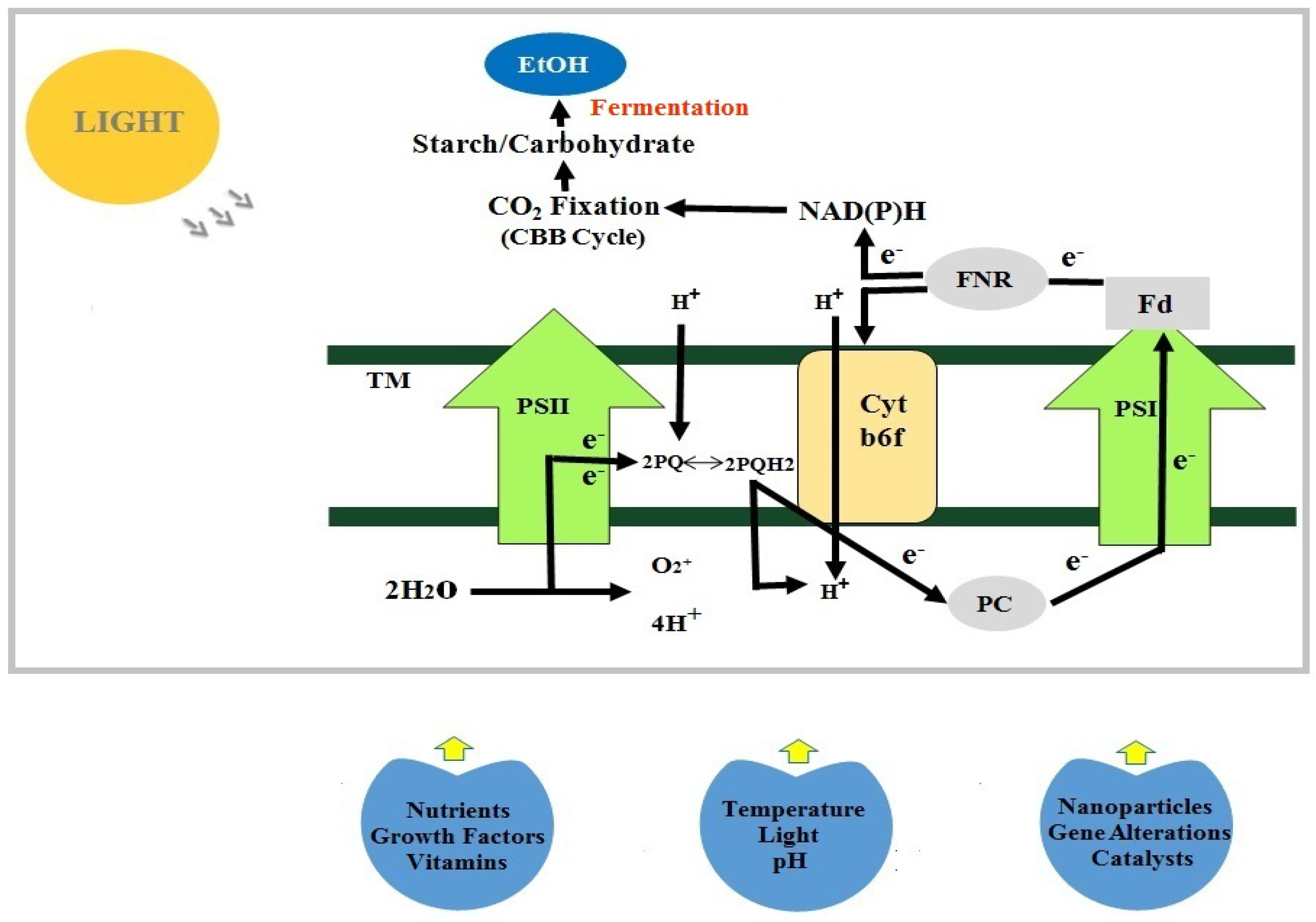
4. Effects of Various Factors on Growth and Ethanol Production from Algae
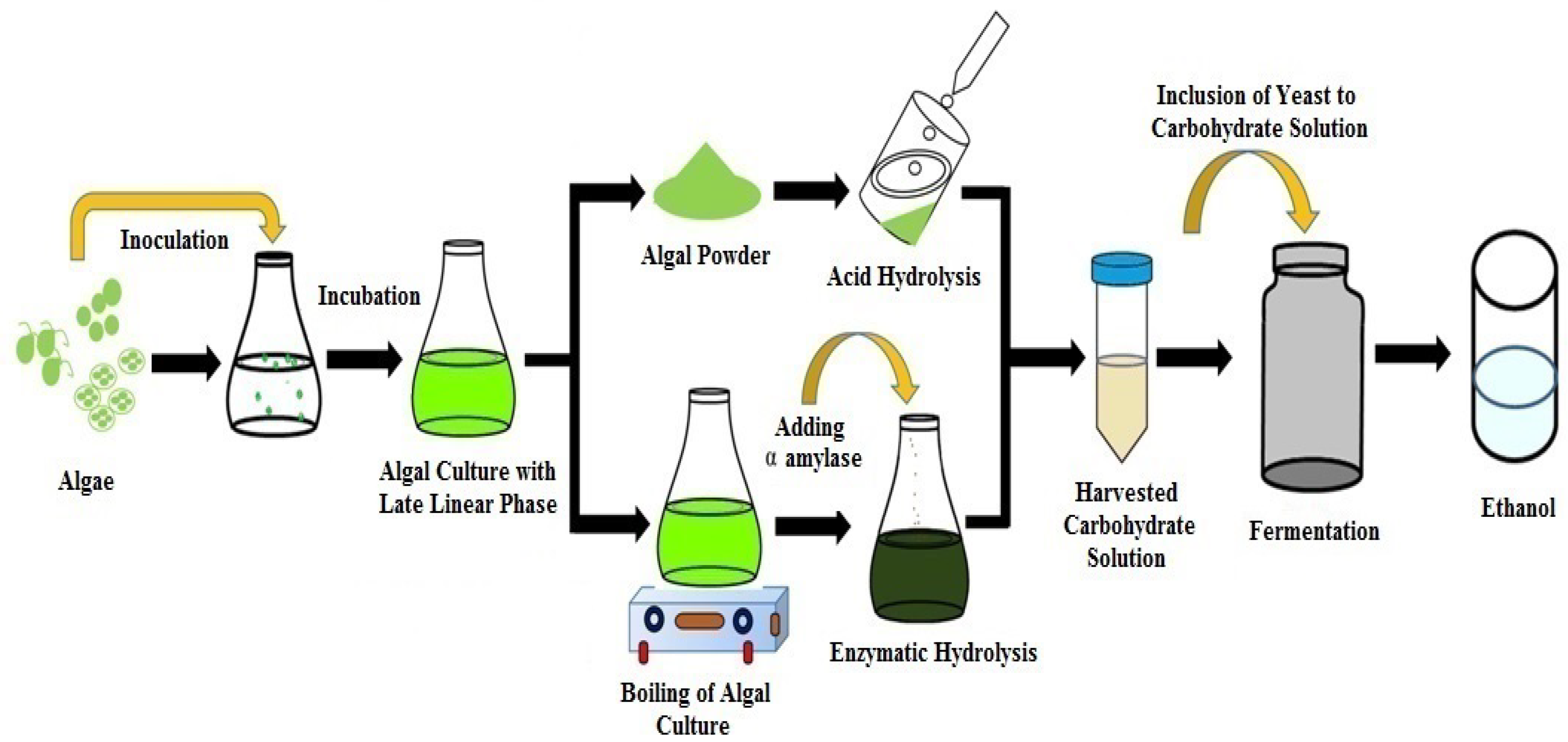
5. Production of Bioethanol from Various Algal Species
5.1. Production of Ethanol from Green Algae
5.2. Generation of Ethanol from Brown Algae
5.3. Production of Ethanol from Red Algae
5.4. Production of Ethanol from Blue–Green Algae
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomaa, M.R.; Mustafa, R.J.; Al-Dmour, N. Solar thermochemical conversion of carbonaceous materials into syngas byco-gasification. J. Clean. Prod. 2020, 248, 119185. [Google Scholar] [CrossRef]
- Outlook, S.A.E. World Energy Outlook Special Report; International Energy Agency: Paris, France, 2015; p. 135. [Google Scholar]
- Demirbas, A. Use of algae as biofuel sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Reddy, L.V.A.; Veda, A.S.; Wee, Y.J. Utilization of banana crop residue as an agricultural bioresource for the production of acetone-butanol-ethanol by Clostridium beijerinckii YVU1. Lett. Appl. Microbiol. 2020, 70, 36–41. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. Bioethanol production from acidic and enzymatic hydrolysates of mixed microalgae culture. Fuel 2017, 200, 380–386. [Google Scholar] [CrossRef]
- Gomaa, M.R.; Al-Dmour, N.; Al-Rawashdeh, H.A.; Shalby, M. Theoretical model of a fluidized bed solar reactor design with the aid of MCRT method and synthesis gas production. Renew. Energy 2020, 148, 91–102. [Google Scholar] [CrossRef]
- Onay, M.; Aladag, E. Productionanduse of Scenedesmus acuminatus biomass in synthetic municipal wastewater for integrated biorefineries. Environ. Sci. Pollut. Res. 2023, 30, 15808–15820. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Sherif, S.A.; Ghareeb, M.M. Bioethanol production by various hydrolysis and fermentation processes with micro and macro greenalgae. Waste Biomass Valorization 2018, 9, 1495–1501. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Hebbale, D. Bioethanol from macroalgae: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 117, 109479. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef]
- Ragasudha, N.; Varaprasad, D.; Bramhachari, P.V.; Sudhakar, P.; Chandrasekhar, T. Effects of various factors on biomass, bioethanol, and biohydrogen production in greenalga Chlamydomonas reinhardtii. J. Appl. Biol. Biotech. 2021, 9, 152–156. [Google Scholar]
- Manoj, B.S.; Sushma, S.; Mohan, C.; Arti, K. Successive production of biodiesel and bioethanol feedstock from the Cosmarium sp. Int. J. Chem. 2018, 6, 550–554. [Google Scholar]
- Varaprasad, D.; Ragasudha, N.; Paramesh, K.; Chandramati Shankar, P.; Nazaneen Parveen, S.; Chandrasekhar, T. Production ofbioethanol from greenalga Chlorellavulgaris: An important approach to utilize algal feedstock or waste. In Bioresource Utilization and Bioprocess; Springer: Singapore, 2020; pp. 57–65. [Google Scholar]
- Paramesh, K.; Reddy, N.L.; Shankar, M.V.; Chandrasekhar, T. Enhancement of biological hydrogen production using green alga Chlorococcum minutum. Int. J. Hydrogen Energy 2018, 43, 3957–3966. [Google Scholar] [CrossRef]
- Foong, T.M.; Morganti, K.J.; Brear, M.J.; da Silva, G.; Yang, Y.; Dryer, F.L. The octane numbers of ethanol blended with gasoline and its surrogates. Fuel 2014, 115, 727–739. [Google Scholar] [CrossRef]
- Matovic, M.D. Biomass Now: Sustainable Growth and Use; Intechopen: Rijeka, Croatia, 2013. [Google Scholar]
- Khuong, L.S.; Masjuki, H.H.; Zulkifli, N.W.; Mohamad, E.N.; Kalam, M.A.; Alabdulkarem, A.; Arslan, A.; Mosarof, M.H.; Syahir, A.Z.; Jamshaid, M. Effect of gasoline–bioethanol blends on the properties and lubrication characteristics of commercial engine oil. RSC Adv. 2017, 7, 15005–15019. [Google Scholar]
- Varaprasad, D.; Narasimham, D.; Paramesh, K.; Sudha, N.R.; Himabindu, Y.; Keerthi Kumari, M.; Nazaneen Parveen, S.; Chandrasekhar, T. Improvement of ethanol production using green alga Chlorococcum minutum. Environ. Technol. 2021, 42, 1383–1389. [Google Scholar] [CrossRef]
- Hidzir, N.S.; Som, A.S.; Abdullah, Z. Ethanol production via direct hydration of ethylene: A review. In ICGSCE 2014: Proceedings of the International Conference on Global Sustainability and Chemical Engineering, Kuala Lumpur, Malaysia, 20–22 August 2014; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–6. [Google Scholar]
- Katada, N.; Iseki, Y.; Shichi, A.; Fujita, N.; Ishino, I.; Osaki, K.; Torikai, T.; Niwa, M. Production of ethanol by vapor phase hydration of ethane over tungsta monolayer catalyst loaded on titania. Appl. Catal. A Gen. 2008, 349, 55–61. [Google Scholar] [CrossRef]
- Matar, S.; Hatch, L.F. Chemistry of Petrochemical Processes, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA, 2001; pp. 248–250. [Google Scholar]
- Yue, H.; Ma, X.; Gong, J. Analternativesyntheticapproachforefficientcatalyticconversionofsyngastoethanol. Acc. Chem. Res. 2014, 47, 1483–1492. [Google Scholar] [PubMed]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Varaprasad, D.; Raghavendra, P.; RagaSudha, N.; Subramanyam Sarma, L.; Nazaneen Parveen, S.; Sri Chandana, P.; Subhosh Chandra, M.; Chandrasekhar, T. Augmentation of ethanol production in greenalga Chlorococcum minutum through grapheme oxide-supported platinum-ruthenium(Pt-Ru/RGO) Nanoparticles. BioEnergy Res. 2022, 15, 280–288. [Google Scholar] [CrossRef]
- Sato, K.; Goto, S.; Yonemura, S.; Sekine, K.; Okuma, E.; Takagi, Y.; Hon-Nami, K.; Saiki, T. Effect of yeast extract and vitamin B12 on ethanol production from cellulose by Clostridium thermocellumI-1-B. Appl. Environ. Microbiol. 1992, 58, 734–736. [Google Scholar] [CrossRef]
- Sulfahri, M.S.; Sunarto, E.; Irvansyah, M.Y.; Utami, R.S.; Mangkoedihardjo, S. Ethanol production from algae Spirogyra with fermentation by Zymomonas mobilis and Saccharomyces cerevisiae. J. Basic Appl. Sci. Res. 2011, 1, 589–593. [Google Scholar]
- Salman, J.M.; Ali, M.A. Bioethanol production from greenalga Chlorella vulgaris under different concentrations of nitrogen. Asian J. Nat. Appl. Sci. 2014, 3, 27–36. [Google Scholar]
- Nakas, J.P.; Schaedle, M.; Parkinson, C.M.; Coonley, C.E.; Tanenbaum, S.W. System development forlinked-fermentation production of solvents from algal biomass. Appl. Environ. Microbiol. 1983, 46, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Progress Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Nat. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [PubMed]
- Chandel, A.K.; Chan, E.S.; Rudravaram, R.; Narasu, M.L.; Rao, L.V.; Ravindra, P. Economics and environmental impact of bioethanol production technologies: An appraisal. Biotechnol. Mol. Biol. Rev. 2007, 2, 14–32. [Google Scholar]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar]
- Alia, K.B.; Rasul, I.; Azeem, F.; Hussain, S.; Siddique, M.H.; Muzammil, S.; Riaz, M.B.; Liaqat, S.; Nadeem, H. Microbial production of ethanol. Microb. Fuel Cells Mater. Appl. 2019, 46, 307–334. [Google Scholar]
- Al-Bawwat, A.K.; Jurado, F.; Gomaa, M.R.; Cano, A. Availability and the possibility of employing wastes and biomass materials energy in Jordan. Sustainability 2023, 15, 5879. [Google Scholar]
- Ibeto, C.N.; Ofoefule, A.U.; Agbo, K.E. Aglobal overview of biomass potentials for bioethanol production: A renewable alternative fuel. Trends Appl. Sci. Res. 2011, 6, 410–425. [Google Scholar]
- Malinauskaite, J.; Jouhara, H.; Czajczyńska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colon, J.; Ponsá, S.; Al-Mansour, F.; et al. Municipal solid waste management and waste-to-energy in the context of a circular economy and energy recycling in Europe. Energy 2017, 141, 2013–2044. [Google Scholar] [CrossRef]
- Saladini, F.; Patrizi, N.; Pulselli, F.M.; Marchettini, N.; Bastianoni, S. Guidelines for energy evaluation offirst, second and third generation biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Shobana, S.; Nguyen, D.D.; Bhanu, J.R.; Sivagurunathan, P.; Chang, S.W.; Ponnusamy, V.K.; Kumar, G. Application of nanotechnology (nanoparticles) in dark fermentative hydrogen production. Int. J. Hydrogen Energy 2019, 44, 431–440. [Google Scholar] [CrossRef]
- Guo, H.; Daroch, M.; Liu, L.; Qiu, G.; Geng, S.; Wang, G. Biochemical features and bioethanol production of microalgae from coastal waters of Pearl River Delta. Bioresour. Technol. 2013, 127, 422–428. [Google Scholar] [PubMed]
- Adhikari, S.; Nam, H.; Chakraborty, J.P. Conversion of solid wastes to fuels and chemicals through pyrolysis. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 239–263. [Google Scholar]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and cropresidues. Biomass Bioener. 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Pimentel, D.; Patzek, T.W. Ethanol production using corn, switchgrass, and wood; biodiesel production using soybean and sunflower. Nat. Resour. Res. 2005, 14, 65–76. [Google Scholar] [CrossRef]
- Chen, X.; Khanna, M. Effect of corn ethanol production on conservation reserve program acres in the US. Appl. Ener. 2018, 225, 124–134. [Google Scholar] [CrossRef]
- Martinelli, L.A.; Filoso, S. Expansion of sugarcane ethanol production in Brazil: Environmental and social challenges. Ecol. Appl. 2008, 4, 885–898. [Google Scholar]
- Brinkman, M.L.; da Cunha, M.P.; Heijnen, S.; Wicke, B.; Guilhoto, J.J.; Walter, A.; Faaij, A.P.; van der Hilst, F. Interregional assessment of socio-economic effects of sugarcane ethanol production in Brazil. Renew. Sustain. Energy Rev. 2018, 88, 347–362. [Google Scholar] [CrossRef]
- Al-Bawwat, A.K.; Cano, A.; Gomaa, M.R.; Jurado, F. Availability of biomass and potential of nanotechnologies for bioenergy production in Jordan. Processes 2023, 11, 992. [Google Scholar] [CrossRef]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioener. 2017, 100, 10–16. [Google Scholar]
- Castro, E.; Nieves, I.U.; Rondón, V.; Sagues, W.J.; Fernández-Sandoval, M.T.; Yomano, L.P.; York, S.W.; Erickson, J.; Vermerris, W. Potential for ethanol production from different sorghum cultivars. Ind. Crops Prod. 2017, 109, 367–373. [Google Scholar]
- Álvarez, C.; Sáez, F.; González, A.; Ballesteros, I.; Oliva, J.M.; Negro, M.J. Production of xylooligo saccharides and cellulosic ethanol from steam-exploded barley straw. Holzforschung 2018, 73, 35–44. [Google Scholar] [CrossRef]
- Eckert, C.T.; Frigo, E.P.; Albrecht, L.P.; Albrecht, A.J.; Christ, D.; Santos, W.G.; Berkembrock, E.; Egewarth, V.A. Maize ethanol production in Brazil: Characteristics and perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3907–3912. [Google Scholar]
- Knauf, M.; Moniruzzaman, M. Lignocellulosic biomass processing: A perspective. Int. Sugar J. 2004, 106, 147–150. [Google Scholar]
- Shi, J.; Sharma-Shivappa, R.R.; Chinn, M.; Howell, N. Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioener. 2009, 33, 88–96. [Google Scholar]
- García, A.; Cara, C.; Moya, M.; Rapado, J.; Puls, J.; Castro, E.; Martín, C. Dilute sulphuric acid pretreatment and enzymatic hydrolysis of Jatropha curcas fruit shells for ethanol production. Ind. Crops Prod. 2014, 53, 148–153. [Google Scholar]
- Cheng, J.; Leu, S.Y.; Zhu, J.Y.; Gleisner, R. Hightiter and yield ethanol production from undetoxified whole slurry of Douglas-fir forest residue using pH profiling in SPORL. Biotechnol. Biofuels 2015, 8, 22. [Google Scholar] [CrossRef]
- Klinke, H.B.; Ahring, B.K.; Schmidt, A.S.; Thomsen, A.B. Characterization of degradation products from alkaline wet oxidation of wheat straw. Biores. Technol. 2002, 82, 15–26. [Google Scholar]
- Bercier, A.; Plantier-Royon, R.; Portella, C. Convenient conversion of wheat hemicelluloses pentoses (D-xylose and L-arabinose) into a common intermediate. Carbohydr. Res. 2007, 342, 2450–2455. [Google Scholar] [PubMed]
- Fu, C.C.; Hung, T.C.; Chen, J.Y.; Su, C.H.; Wu, W.T. Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Biores. Technol. 2010, 101, 8750–8754. [Google Scholar]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar]
- Sakuragi, H.; Kuroda, K.; Ueda, M. Molecular breeding of advanced microorganisms for biofuel production. BioMed Res. Int. 2011, 2011, 416931. [Google Scholar]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Progress Ener. Comb. Sci. 2012, 38, 522–550. [Google Scholar]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11, 3366. [Google Scholar]
- Sirajunnisa, A.R.; Surendhiran, D. Algae—A quintessential and positive resource of bioethanol production: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 66, 248–267. [Google Scholar]
- Kusekwa, M.A. Biomass conversion to energy in Tanzania: A critique. In New Developments in Renewable Energy; BoD–Books on Demand: Norderstedt, Germany, 2013; pp. 240–270. [Google Scholar]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar]
- Bibi, R.; Ahmad, Z.; Imran, M.; Hussain, S.; Ditta, A.; Mahmood, S.; Khalid, A. Algal bioethanol production technology: A trend towards sustainable development. Renew. Sustain. Energy Rev. 2017, 71, 976–985. [Google Scholar]
- Ueda, R.; Hirayama, S.; Sugata, K.; Nakayama, H. Process for the Ethanol Production from Microalgae. U.S. Patent 5,578,472, 26 November 1996. [Google Scholar]
- Umamaheswari, A.; Saranraj, P.; Rajesh Kanna, G.; Elumalai, S.; Sangeetha, T. Synthesis of bioethanol by dark fermentation using marine seaweed Acanthophora spicifera (Vahl.) borgesen as a cheap substrate. Bioenergetics 2017, 6, 146. [Google Scholar]
- Kim, K.H.; Choi, I.S.; Kim, H.M.; Wi, S.G.; Bae, H.J. Bioethanol production from the nutrient stress-induced microalga Chlorellavulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour. Technol. 2014, 153, 47–54. [Google Scholar] [CrossRef]
- Salmon, J.M.; Mauricio, J.C. Relationship between sugar uptake kinetics and total sugar consumption indifferent industrial Saccharomyces cerevisiaes trains during alcoholic fermentation. Biotechnol. Lett. 1994, 16, 89–94. [Google Scholar] [CrossRef]
- Blanch, H.W.; Clark, D.S. Principles of catalysis. In Biochemical Engineering; Marcel Dekker Inc.: New York, NY, USA, 1997; p. 702. [Google Scholar]
- Bandaru, V.V.; Somalanka, S.R.; Mendu, D.R.; Madicherla, N.R.; Chityala, A. Optimization of fermentation conditions forth eproduction of ethanol from sago starch by co-immobilized amyloglucosidase and cells of Zymomonas mobilis using response surface methodology. Enzym. Microb. Technol. 2006, 38, 209–214. [Google Scholar] [CrossRef]
- Phisalaphong, M.; Srirattana, N.; Tanthapanichakoon, W. Mathematical modeling to investigate temperature effect on kinetic parameters of ethanol fermentation. Biochem. Eng. J. 2006, 28, 36–43. [Google Scholar] [CrossRef]
- Fakruddin, M.; Quayum, M.A.; Ahmed, M.M.; Choudhury, N. Analysis of key factors affecting ethanol production by Saccharomyces cerevisiae IFST-072011. Biotechnology 2012, 11, 248–252. [Google Scholar] [CrossRef]
- Bajpai, P.; Margaritis, A. The effect of temperature and pH on ethanol production by free and immobilized cells of Kluyveromyces marxianus grown on Jerusalem artichoke extract. Biotechnol. Bioeng. 1987, 30, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, K.; Wang, H.; Zhang, J.; Tang, L.; Mao, Z. Control of pH by acetic acid and its effect on ethanol fermentation in an integrated ethanol–methane fermentation process. RSC Adv. 2016, 6, 57902–57909. [Google Scholar]
- Arif, A.R.; Natsir, H.; Rohani, H.; Karim, A. Effect of pH fermentation on production bioethanol from jackfruit seeds (Artocarpus heterophyllus) through separate fermentation hydrolysis method. Int. J. Phys. Conf. Ser. 2018, 979, 012015. [Google Scholar]
- Croft, M.T.; Warren, M.J.; Smith, A.G. Algae need their vitamins. Eukaryot. Cell 2006, 5, 1175–1183. [Google Scholar] [CrossRef]
- Alfenore, S.; Molina-Jouve, C.; Guillouet, S.; Uribelarrea, J.L.; Goma, G.; Benbadis, L. Improving ethanol production and viability of Saccharomyces cerevisiae by a vitamin feeding strategy during fed-batch process. Appl. Microbiol. Biotechnol. 2002, 60, 67–72. [Google Scholar]
- Tandon, P.; Jin, Q.; Huang, L. A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb. Cell Factories 2017, 16, 219. [Google Scholar]
- Ruangsomboon, S.; Sornchai, P.; Prachom, N. Enhanced hydrocarbon production and improved biodiesel qualities of Botryococcus braunii KMITL5 by vitamins thiamine, biotin and cobalamin supplementation. Algal Res. 2018, 29, 159–169. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Liu, X. An overview of algae bioethanol production. Int. J. Energy Res. 2014, 38, 965–977. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, S.E.; Lee, H.; Yun, J.Y. Enhancement of bioethanol production in syngas fermentation with Clostridium ljungdahlii using nanoparticles. Bioresour. Technol. 2014, 159, F446–F450. [Google Scholar] [CrossRef]
- Ueno, Y.; Kurano, N.; Miyachi, S. Ethanol production by dark fermentation in the marine greenalga, (Chlorococcum littorale). J. Ferment. Bioeng. 1998, 86, 38–43. [Google Scholar] [CrossRef]
- Hirayama, S.; Ueda, R.; Ogushi, Y.; Hirano, A.; Samejima, Y.; Hon-Nami, K.; Kunito, S. Ethanol production from carbon dioxide by fermentative microalgae. Stud. Surf. Sci. Catal. 1998, 114, 657–660. [Google Scholar]
- Shirai, F.; Kunii, K.; Sato, C.; Teramoto, Y.; Mizuki, E.; Murao, S.; Nakayama, S. Cultivation of microalgae in the solution from the desalting process of soysauce waste treatment and utilization of the algal biomass for ethanol fermentation. World J. Microbiol. Biotechnol. 1998, 14, 839–842. [Google Scholar] [CrossRef]
- Matsumoto, M.; Yokouchi, H.; Suzuki, N.; Ohata, H.; Matsunaga, T. Saccharification of marine microalgae using marine bacteria for ethanol production. Appl. Biochem. Biotechnol. 2003, 105, 247–254. [Google Scholar] [CrossRef]
- Bush, R.A.; Hall, K.M. Process for the Production of Ethanol from Algae. U.S. Patent 7,135,308, 14 November 2006. [Google Scholar]
- Nguyen, M.T.; Choi, S.P.; Lee, J.W.; Lee, J.H.; Sim, S.J. Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J. Microbiol. Biotechnol. 2009, 19, 161–166. [Google Scholar] [PubMed]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar]
- Harun, R.; Jason, W.S.Y.; Cherrington, T.; Danquah, M.K. Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl. Energy 2011, 88, 3464–3467. [Google Scholar] [CrossRef]
- Eshaq, F.S.; Ali, M.N.; Mohd, M.K. Production of bioethanol from next generation feed-stock alga Spirogyra species. Int. J. Eng. Sci. Technol. 2011, 3, 1749–1755. [Google Scholar]
- Lee, S.; Oh, Y.; Kim, D.; Kwon, D.; Lee, C.; Lee, J. Converting carbohydrates extracted from marine algae in to ethanol using various ethanolic Escherichia coli strains. Appl. Biochem. Biotechnol. 2011, 164, 878–888. [Google Scholar]
- Choi, J.A.; Hwang, J.H.; Dempsey, B.A.; Abou-Shanab, R.A.; Min, B.H. Enhancement of fermentative bioenergy (ethanol/hydrogen) production using ultrasonication of Scenedesmuso bliquus YSW15 cultivated in swine wastewater effluent. Energy Environ. Sci. 2011, 4, 3513–3520. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Enzymatic hydrolysis of microalgal biomass for bioethanol production. Chem. Eng. J. 2011, 168, 1079–1084. [Google Scholar] [CrossRef]
- Miranda, J.R.; Passarinho, P.C.; Gouveia, L. Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour. Technol. 2012, 104, 342–348. [Google Scholar] [CrossRef]
- Sulfahri, S.; Ni, M.; Manuhara, Y.S.W. Optimization of the bioconversion of Spirogyra hyaline hydrolysates to become ethanol using Zymomonas mobilis. J. Appl. Environ. Biol. Sci. 2012, 2, 374–379. [Google Scholar]
- Miranda, J.R.; Passarinho, P.C.; Gouveia, L. Bioethanol production from Scenedesmus obliquus sugars: The influence of photobioreactors and culture conditions on biomass production. Appl. Microbiol. 2012, 96, 555–564. [Google Scholar]
- Asada, C.; Doi, K.; Sasaki, C.; Nakamura, Y. Efficient extraction of starch from microalgae using ultrasonic homogenizer and its conversion into ethanol by simultaneous saccharification and fermentation. Nat. Res. 2012, 3, 175–179. [Google Scholar]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.K.; Kim, A.L.; Seong, D.H.; Lee, C.G.; Jung, Y.T.; Lee, J.W.; Lee, E.Y. Chemo-enzymatic saccharification and bioethanol fermentation of lipid-extracted residual biomass of the microalga, Dunaliella tertiolecta. Bioresour. Technol. 2013, 132, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Moncada, J.; Jaramillo, J.J.; Higuita, J.C.; Younes, C.; Cardona, C.A. Production of bioethanol using Chlorella vulgaris cake: A technoeconomic and environmental assessment in the colombian context. Ind. Eng. Chem. Res. 2013, 52, 16786–16794. [Google Scholar]
- Cheng, Y.S.; Zheng, Y.; Labavitch, J.M.; VanderGheynst, J.S. Virus infection of Chlorella variabilis and enzymatic saccharification of algal biomass for bioethanol production. Bioresour. Technol. 2013, 137, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Jensen, N.; Thygesen, A.; Leipold, F.; Thomsen, S.T.; Roslander, C.; Lilholt, H.; Bjerre, A.B. Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol-comparison of five pretreatment technologies. Bioresour. Technol. 2013, 140, 36–42. [Google Scholar] [CrossRef]
- Ho, S.H.; Li, P.J.; Liu, C.C.; Chang, J.S. Bioprocess development on microalgae-based CO2 fixation and bioethanol production using Scenedesmus obliquus CNW-N. Bioresour. Technol. 2013, 145, 142–149. [Google Scholar] [CrossRef]
- Trivedi, N.; Gupta, V.; Reddy, C.R.K.; Jha, B. Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour. Technol. 2013, 150, 106–112. [Google Scholar] [CrossRef]
- Yoza, B.A.; Masutani, E.M. The analysis of macroalgae biomass found around Hawaii for bioethanol production. Environ. Technol. 2013, 34, 1859–1867. [Google Scholar] [CrossRef]
- Scholz, M.J.; Riley, M.R.; Cuello, J.L. Acid hydrolysis and fermentation of microalgal starches to ethanol by the yeast Saccharomyces cerevisiae. Biomass Bioenergy 2013, 48, 59–65. [Google Scholar]
- Wang, H.; Ji, C.; Bi, S.; Zhou, P.; Chen, L.; Liu, T. Joint production of biodiesel and bioethanol from filamentous oleaginous microalgae Tribonema sp. Bioresour. Technol. 2014, 172, 169–173. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Thiruvenkadam, S. Particulate size of microalgal biomass affects hydrolysate properties and bioethanol concentration. BioMed Res. Int. 2014, 2014, 435631. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem. Eng. J. 2015, 262, 939–945. [Google Scholar] [CrossRef]
- Lee, O.K.; Oh, Y.K.; Lee, E.Y. Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour. Technol. 2015, 196, 22–27. [Google Scholar] [CrossRef]
- El Harchi, M.; Kachkach, F.F.; Elmtili, N. Bioethanol production from acid hydrolysate of Ulva rigida C. Agardh by using Pachysolen tannophilus and Zymomonas mobilis. Moroc. J Biol. 2015, 12, 27–34. [Google Scholar]
- Ashokkumar, V.; Salam, Z.; Tiwari, O.N.; Chinnasamy, S.; Mohammed, S.; Ani, F.N. An integrated approach for biodiesel and bioethanol production from Scenedesmus bijugatus cultivated in a vertical tubular photobioreactor. Energy Convers. Manag. 2015, 101, 778–786. [Google Scholar] [CrossRef]
- Costa, R.L.; Oliveira, T.V.; de Souza Ferreira, J.; Cardoso, V.L.; Batista, F.R.X. Prospective technology on bioethanol production from photofermentation. Bioresour. Technol. 2015, 181, 330–337. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Yeheia, D.S.; Hussein, M.H.; Hamzah, H.A. Removal of heavy metals and production of bioethanol by green alga Scenedesmus obliquus grown in different concentrations of wastewater. Sains Malays. 2016, 45, 467–476. [Google Scholar]
- Sulfahri, A.M.; Sumitro, S.B.; Saptasari, M. Bioethanol production from algae Spirogyra hyalina using Zymomonas mobilis. Biofuels 2016, 7, 621–626. [Google Scholar] [CrossRef]
- Yahmed, N.B.; Jmel, M.A.; Alaya, M.B.; Bouallagui, H.; Marzouki, M.N.; Smaali, I. A biorefinery concept using the green macroalgae Chaetomorpha linum for the coproduction of bioethanol and biogas. Energy Conv. Manag. 2016, 119, 257–265. [Google Scholar] [CrossRef]
- Bhooshan Kumar, V.; Pulidindi, I.N.; Kinel-Tahan, Y.; Yehoshua, Y.; Gedanken, A. Evaluation of the potential of Chlorella vulgaris for bioethanol production. Energy Fuels 2016, 30, 3161–3166. [Google Scholar] [CrossRef]
- Karatay, S.E.; Erdoğan, M.; Dönmez, S.; Dönmez, G. Experimental investigations on bioethanol production from halophilic microalgal biomass. Ecol. Eng. 2016, 95, 266–270. [Google Scholar] [CrossRef]
- Varela-Bojórquez, N.; Vélez-de la Rocha, R.; Angulo, M.Á. Production of bioethanol from biomass of microalgae Dunaliella tertiolecta. Int. J. Environ. Agri. Res. 2016, 2, 110–116. [Google Scholar]
- Hwang, J.H.; Kabra, A.N.; Ji, M.K.; Choi, J.; El-Dalatony, M.M.; Jeon, B.H. Enhancement of continuous fermentative bioethanol production using combined treatment of mixed microalgal biomass. Algal Res. 2016, 17, 14–20. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Kurade, M.B.; Abou-Shanab, R.A.; Kim, H.; Salama, E.S.; Jeon, B.H. Long-term production of bioethanol in repeated-batch fermentation of microalgal biomass using immobilized Saccharomyces cerevisiae. Bioresour. Technol. 2016, 219, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Sherif, S.A.; Dawoud, G.; Ghareeb, M.M. Enhancement of bioethanol production from Ulva fasciata by biological and chemical saccharification. Rend. Lincei 2016, 27, 665–672. [Google Scholar] [CrossRef]
- Neifar, M.; Chatter, R.; Chouchane, H.; Genouiz, R.; Jaouani, A.; Masmoudi, A.S.; Cherif, A. Optimization of enzymatic saccharification of Chaetomorpha linum biomass for the production of macroalgae-based third generation bioethanol. AIMS Bioeng. 2016, 3, 400–411. [Google Scholar] [CrossRef]
- Sulfahri, N.M.; Manuhara, S.W. Studies on the hungate technique for ethanol fermentation of algae Spirogyra hyalina using Saccharomyces cerevisiae. Biofuels 2017, 8, 367–372. [Google Scholar] [CrossRef]
- Rizza, L.S.; Smachetti, M.E.S.; Nascimento, M.D.; Salerno, G.R.; Curatti, L. Bioprospecting for native microalgae as an alternative source of sugars for the production of bioethanol. Algal Res. 2017, 22, 140–147. [Google Scholar] [CrossRef]
- Agwa, O.; Nwosu, I.; Abu, G. Bioethanol production from Chlorella vulgaris biomass cultivated with Plantain (Musa paradisiaca) peels extract. Adv. Biosci. Biotechnol. 2017, 8, 478–490. [Google Scholar] [CrossRef]
- Chng, L.M.; Lee, K.T.; Chan, D.C.J. Evaluation on microalgae biomass for bioethanol production. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012018. [Google Scholar] [CrossRef]
- Reyimu, Z.; Özçimen, D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar]
- Ramachandran, S.; Incharoensakdi, A. Utilization of microalgae feedstock for concomitant production of bioethanol and biodiesel. Fuel 2018, 217, 458–466. [Google Scholar]
- El Harchi, M.; FakihiKachkach, F.Z.; El Mtili, N. Optimization of thermal acid hydrolysis for bioethanol production from Ulva rigida with yeast Pachysolen tannophilus. S. Afr. J. Bot. 2018, 115, 161–169. [Google Scholar]
- Phwan, C.K.; Chew, K.W.; Sebayang, A.H.; Ong, H.C.; Ling, T.C.; Malek, M.A.; Ho, Y.C.; Show, P.L. Effects of acids pre-treatment on the microbial fermentation process for bioethanol production from microalgae. Biotechnol. Biofuels 2019, 12, 191. [Google Scholar] [CrossRef]
- Augastini, N.W.S.; Hidayathi, N.; Wibisono, S.A. Effect of hydrolysis time and acid concentration on bioethanol production of microalga Scenedesmus sp. IOP Conf. Ser. Earth Environ. Sci. 2019, 308, 012029. [Google Scholar]
- Ngamsirisomsakul, M.; Reungsang, A.; Liao, Q.; Kongkeitkajorn, M.B. Enhanced bio-ethanol production from Chlorella sp. biomass by hydrothermal pretreatment and enzymatic hydrolysis. Renew. Energy 2019, 141, 482–492. [Google Scholar] [CrossRef]
- Alam, M.S.; Yuan, T.; Xiong, W.; Zhang, B.; Lv, Y.; Xu, J. Process optimization for the production of high-concentration ethanol with Scenedesmus raciborskii biomass. Bioresour. Technol. 2019, 294, 122219. [Google Scholar] [CrossRef]
- Sulfahri, S.; Husain, D.R.; Tassakka, A.C.M.; Wulandari, D.P.; Taufan, W.L. Bioethanol production from algae Spirogyra peipingensis using Saccharomyces cerevisiae, Pichia kudriavzevii and Kluyveromyces thermotolerans. J. Phys. Conf. Ser. 2019, 1341, 022004. [Google Scholar] [CrossRef]
- Yu, K.L.; Chen, W.-S.; Sheen, H.-K.; Chang, J.-S.; Lin, C.-S.; Ong, H.C.; Show, P.L.; Ling, T.C. Bioethanol production from acid pretreated microalgal hydrolysate using microwave-assisted heating wet torrefaction. Fuel 2020, 279, 118435. [Google Scholar]
- Gengiah, K.; Moses, G.L.P.; Baskar, G. Bioethanol production from Codium tomentosum residue. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–10. [Google Scholar] [CrossRef]
- Seon, G.; Kim, H.S.; Cho, J.M.; Kim, M.; Park, W.-K.; Chang, Y.-K. Effect of post-treatment process of microalgal hydrolysate on bioethanol production. Sci. Rep. 2020, 10, 16698. [Google Scholar] [CrossRef] [PubMed]
- Chandra, N.; Shukla, P.; Mallick, N. Role of cultural variables in augmenting carbohydrate accumulation in the green microalga Scenedesmus acuminatus for bioethanol production. Biocatal. Agric. Biotechnol. 2020, 26, 101632. [Google Scholar] [CrossRef]
- Bader, A.N.; Rizza, L.S.; Consolo, V.B.; Curatti, L. Efficient saccharification of microalgal biomass by Trichoderma harzianum enzymes for the production of ethanol. Algal Res. 2020, 48, 101926. [Google Scholar] [CrossRef]
- Gohain, M.; Hasin, M.; Eldiehy, K.S.H.; Bardhan, P.; Laskar, K.; Phukon, H.; Mandal, M.; Kalita, K.; Deka, D. Bio-ethanol production: A route to sustainability of fuels using bio-based heterogeneous catalyst derived from waste. Process Safety Environ. Prot. 2021, 146, 190–200. [Google Scholar]
- Kaur, A.; Taggar, M.S.; Anu, K.; Singh, M. Nitrate-induced carbohydrate accumulation in Chlorella sorokiniana and its potential for ethanol production. BioEnergy Res. 2022, 15, 253–263. [Google Scholar] [CrossRef]
- Condor, B.E.; de Luna, M.D.G.; Chang, Y.H.; Chen, J.H.; Leong, Y.K.; Chen, P.T.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Bioethanol production from microalgae biomass at high-solids loadings. Bioresour. Technol. 2022, 363, 128002. [Google Scholar] [CrossRef]
- Acebu, P.I.G.; de Luna, M.D.G.; Chen, C.Y.; Abarca, R.R.M.; Chen, J.H.; Chang, J.S. Bioethanol production from Chlorella vulgaris ESP-31 grown in unsterilized swine wastewater. Bioresour. Technol. 2022, 352, 127086. [Google Scholar] [CrossRef]
- Gengiah, K.; Rajendran, N.; Al-Ghanim, K.A.; Govindarajan, M.; Gurunathan, B. Process and technoeconomic analysis of bioethanol production from residual biomass of marine macroalgae Ulva lactuca. Sci. Total Environ. 2023, 868, 161661. [Google Scholar] [CrossRef]
- Adams, J.M.; Gallagher, J.A.; Donnison, I.S. Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J. Appl. Phycol. 2009, 21, 569. [Google Scholar] [CrossRef]
- Ji-Yeon, H.; Seo, H.B.; Oh, S.H.; Choi, W.S.; Kang, D.H.; Lee, H.Y.; Jung, K.H. Bioethanol production from hydrolysate of seaweed Sargassum sagamianum. KSBB J. 2010, 25, 283–288. [Google Scholar]
- Fasahati, P.; Liu, J.J. Process simulation of bioethanol production from brown algae. IFAC Proc. Vol. 2012, 45, 597–602. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.H. Ethanol fermentation for main sugar components of brown-algae using various yeasts. J. Ind. Eng. Chem. 2012, 18, 16–18. [Google Scholar] [CrossRef]
- Lee, J.Y.; Li, P.; Lee, J.; Ryu, H.J.; Oh, K.K. Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour. Technol. 2013, 127, 119–125. [Google Scholar] [CrossRef]
- Borines, M.G.; de Leon, R.L.; Cuello, J.L. Bioethanol production from the macroalgae Sargassum spp. Bioresour. Technol. 2013, 138, 22–29. [Google Scholar] [CrossRef]
- Enquist-Newman, M.; Faust, A.M.E.; Bravo, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Yoshikuni, Y. Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 2014, 505, 239–243. [Google Scholar] [CrossRef]
- Hou, X.; Hansen, J.H.; Bjerre, A.B. Integrated bioethanol and protein production from brown seaweed Laminaria digitata. Bioresour. Technol. 2015, 197, 310–317. [Google Scholar] [CrossRef]
- Obara, N.; Okai, M.; Ishida, M.; Urano, N. Bioethanol production from mixed biomass (waste of Undaria pinnatifida processing and paper shredding) by fermentation with marine-derived Saccharomyces cerevisiae. Fish. Sci. 2015, 81, 771–776. [Google Scholar]
- Obata, O.; Akunna, J.; Bockhorn, H.; Walker, G. Ethanol production from brown seaweed using non-conventional yeasts. Bioethanol 2016, 2, 134–145. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Salim, M.R.; Salam, Z.; Sivakumar, P.; Chong, C.T.; Elumalai, S.; Suresh, V.; Ani, F.N. Production of liquid biofuels (biodiesel and bioethanol) from brown marine macroalgae Padina tetrastromatica. Energy Conv. Manag. 2017, 135, 351–361. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Bleathman, G.; Thomas, D.; Gallagher, J.A. The effect of mechanical pre-processing and different drying methodologies on bioethanol production using the brown macroalga Laminaria digitata (Hudson) JV Lamouroux. J. Appl. Phycol. 2017, 29, 2463–2469. [Google Scholar]
- Lamb, J.; Sarker, S.; Hjelme, D.; Lien, K. Fermentative bioethanol production using enzymatically hydrolysed Saccharina latissima. Adv. Microbiol. 2018, 8, 378–389. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, Y.; Liu, Z.; Wang, M. Ethanol production from Colpomenia sinuosa by an alginate fermentation strain Meyerozyma guilliermondii. Indian J. Microbiol. 2022, 62, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Kang, J.Y.; Jeong, G.T.; Koo, H.M.; Park, S.M.; Hong, Y.K. Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). J. Appl. Phycol. 2012, 24, 857–862. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Babujanarthanam, R.; Kavitha, P. Simultaneous saccharification and fermentation of dilute acid pretreated red algae (Gelidiella acerosa) for bioethanol production. Energy Sources Part A Recovery Util. Environ. Effects 2014, 36, 1305–1314. [Google Scholar]
- Wu, C.H.; Chien, W.C.; Chou, H.K.; Yang, J.; Lin, H.T.V. Sulfuric acid hydrolysis and detoxification of red alga Pterocladiella capillacea for bioethanol fermentation with thermotolerant yeast Kluyveromyces marxianus. J. Microbiol. Biotechnol. 2014, 24, 1245–1253. [Google Scholar] [CrossRef]
- Kim, H.M.; Wi, S.G.; Jung, S.; Song, Y.; Bae, H.J. Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour. Technol. 2015, 175, 128–134. [Google Scholar]
- Lee, H.J.; Kim, S.J.; Yoon, J.J.; Kim, K.H.; Seo, J.H.; Park, Y.C. Evolutionary engineering of Saccharomyces cerevisiae for efficient conversion of red algal biosugars to bioethanol. Bioresour. Technol. 2015, 191, 445–451. [Google Scholar] [CrossRef]
- Kim, H.M.; Oh, C.H.; Bae, H.J. Comparison of red microalgae (Porphyridium cruentum) culture conditions for bioethanol production. Bioresour. Technol. 2017, 233, 44–50. [Google Scholar] [CrossRef]
- Alfonsín, V.; Maceiras, R.; Gutiérrez, C. Bioethanol production from industrial algae waste. Waste Manag. 2019, 87, 791–797. [Google Scholar] [CrossRef]
- Hessami, M.J.; Cheng, S.F.; Ambati, R.R.; Yin, Y.H.; Phang, S.M. Bioethanol production from agarophyte red seaweed, Gelidium elegans, using a novel sample preparation method for analysing bioethanol content by gas chromatography. 3Biotech 2019, 9, 25. [Google Scholar]
- Wadi, A.; Ahmad, A.; Tompo, M.; Hasyim, M.; Tuwo, A.; Nakajima, M.; Karim, H. Production of bioethanol from seaweed, Gracilaria verrucosa and Eucheuma cottonii, by simultaneous saccharification and fermentation methods. J. Phys. Conf. Ser. 2019, 1341, 032031. [Google Scholar]
- Park, Y.R.; Yang, J.W.; Sunwoo, I.Y.; Jang, B.K.; Kim, S.R.; Jeong, G.T.; Kim, S.K. Enhancement of catabolite regulatory genes in Saccharomyces cerevisiae to increase ethanol production using hydrolysate from red seaweed Gloiopeltis furcata. J. Biotechnol. 2021, 333, 1–9. [Google Scholar] [CrossRef]
- Kim, J.; Sunwoo, I.; Jo, H.; Kim, Y.; Kim, S.K.; Jeong, G.T. Enhancement of galactose uptake for bioethanol production from Eucheuma denticulatum hydrolysate using galactose-adapted yeasts. Bioprocess Biosyst. Eng. 2023, 46, 839–850. [Google Scholar]
- Kampfe, S.K. Processing and Conversion of Algae to Bioethanol. Master’s Thesis, William & Mary, Williamsburg, VA, USA, 2010. [Google Scholar]
- Kim, J.D.; Chae, G.W.; Seo, H.J.; Chaudhary, N.; Yoon, Y.H.; Shin, T.S.; Kim, M.Y. Bioalcohol production with microalgae, Microcystis aeruginosa. KSBB J. 2012, 27, 335–340. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Nerantzis, E.; Georgakakis, D. Bioethanol production by carbohydrate-enriched biomass of Arthrospira (Spirulina) platensis. Energies 2013, 6, 3937–3950. [Google Scholar] [CrossRef]
- Hossain, M.N.B.; Basu, J.K.; Mamun, M. The production of ethanol from micro-algae Spirulina. Procedia Eng. 2015, 105, 733–738. [Google Scholar] [CrossRef]
- Khan, M.I.; Lee, M.G.; Seo, H.J.; Shin, J.H.; Shin, T.S.; Yoon, Y.H.; Kim, M.Y.; Choi, J.; Kim, J.D. Enhancing the feasibility of Microcystis aeruginosa as a feedstock for bioethanol production under the influence of various factors. BioMed Res. Int. 2016, 2016, 4540826. [Google Scholar] [CrossRef]
- Khan, M.I.; Lee, M.G.; Shin, J.H.; Kim, J.D. Pretreatment optimization of the biomass of Microcystis aeruginosa for efficient bioethanol production. AMB Exp. 2017, 7, 19. [Google Scholar]
- Tourang, M.; Baghdadi, M.; Torang, A.; Sarkhosh, S. Optimization of carbohydrate productivity of Spirulina microalgae as a potential feedstock for bioethanol production. Int. J. Environ. Sci. Technol. 2019, 16, 1303–1318. [Google Scholar]
- Tsolcha, O.N.; Patrinou, V.; Economou, C.N.; Dourou, M.; Aggelis, G.; Tekerlekopoulou, A.G. Utilization of biomass derived from Cyanobacteria-based agro-industrial wastewater treatment and raisin residue extract for bioethanol production. Water 2021, 13, 486. [Google Scholar]
- Papadopoulos, K.P.; Economou, C.N.; Stefanidou, N.; Moustaka-Gouni, M.; Genitsaris, S.; Aggelis, G.; Tekerlekopoulou, A.G.; Vayenas, D.V. A semi-continuous algal-bacterial wastewater treatment process coupled with bioethanol production. J. Environ. Manag. 2023, 326, 116717. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.J.; Li, H.; Jung, K.; Chang, H.N.; Lee, P.C. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour. Technol. 2011, 102, 7466–7469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Um, B.H.; Kim, T.H. Bioethanol production from micro-algae, Schizocytrium sp., using hydrothermal treatment and biological conversion. Korean J. Chem. Eng. 2012, 29, 209–214. [Google Scholar] [CrossRef]
- Gupta, R.; Biswas, K.; Mishra, I.; Suthidiran, K. Ethanol production from marine algae using yeast fermentation. Biosci. Technol. 2012, 1, 17–22. [Google Scholar]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. Enzymatic hydrolysis of microalgal cellulose for bioethanol production, modeling and sensitivity analysis. Fuel 2018, 228, 30–38. [Google Scholar] [CrossRef]
- Amamou, S.; Sambusiti, C.; Monlau, F.; Dubreucq, E.; Barakat, A. Mechano-enzymatic deconstruction with a new enzymatic cocktail to enhance enzymatic hydrolysis and bioethanol fermentation of two macroalgae species. Molecules 2018, 23, 174. [Google Scholar]
- Soliman, R.M.; Younis, S.A.; El-Gendy, N.S.; Mostafa, S.S.M.; El-Temtamy, S.A.; Hashim, A.I. Batch bioethanol production via the biological and chemical saccharification of some Egyptian marine macroalgae. J. Appl. Microbiol. 2018, 125, 422–440. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S. Kinetic study on bioethanol production from enzymatic hydrolysates of microalgal biomass. Modares J. Biotech. JMBS 2019, 10, 61–68. [Google Scholar]
- Fetyan, N.A.H.; El-Sayed, A.E.B.; Ibrahim, F.M.; Attia, Y.A.; Sadik, M.W. Bioethanol production from defatted biomass of Nannochloropsis oculata microalgae grown under mixotrophic conditions. Environ. Sci. Pollut. Res. 2022, 29, 2588–2597. [Google Scholar] [CrossRef]

| S.No | Class of Biofuel | Involved Organisms |
|---|---|---|
| 1 | First generation | Edible crops: Corn, Sugarcane, Maize, Barley, Oat, etc. |
| 2 | Second generation | Non-edible crops: Jatropha, Cotton, Switch grass etc. |
| 3 | Third generation | Sea weeds, Algae, Diatoms etc. |
| 4 | Fourth generation | Genetically engineered algae, microorganisms etc. |
| S.No | Ethanol Production Work | Name of the Algae | Reference |
|---|---|---|---|
| 1 | Both conventional and intracellular anaerobic fermentation were practiced using microalgae, and the formergenerated more ethanol | Chlorella vulgaris, Chlamydomonas reinhardtii | Hirano et al., 1997 [23] |
| 2 | Using the marine alga, ethanol was generated under dark fermentation | Chlorococum littorale | Ueno et al., 1998 [83] |
| 3 | Screening for carbon dioxide fixation; subsequently, ethanol production by fermentative microalgae was carried out | 200 strains including Chlamydomonas YA-SH-1 | Hirayama et al., 1998 [84] |
| 4 | Ethanol fermentation studies using microalgae grown in a solution from the desalting process of soy sauce waste treatment | Chlamydomonas sps Dunaliella sps, Nannochloropsis oculata, Tetraselmis tetrathele | Shirai et al., 1998 [85] |
| 5 | The saccharification of marine microalgae was carried out for ethanol production using the bacterium Pseudoalterimonas undina NKMB 0074, which possesses the marine amylase | Green microalgae (NKG120701) | Matsumoto et al., 2003 [86] |
| 6 | The production of ethanol using the algae was established | Different algae (Zygnemataceae, Cladophoraceae, Oedogoniales etc.) | Bush and Hall, 2006 [87] |
| 7 | Algal biomass was pretreated with sulfuric acid for glucose release and, in turn, ethanol production | Chlamydomonas reihardtii UTEX 90 | Nguyen et al., 2009 [88] |
| 8 | Ethanol was produced using the biomass of green alga with enzymatic pretreatment | Chlamydomonas reinhardtii UTEX 90 | Choi et al., 2010 [89] |
| 9 | Ethanol production carried out using the microalgal biomass | Chlorococcum | Harun et al., 2010 [90] |
| 10 | NaOH treatment was carried out for algal biomass for bioethanol production | Chlorococcum infusionum | Harun et al., 2011 [91] |
| 11 | Green alga was used for ethanol production through fermentation by both bacterium and yeast (Zymomonas mobilis and Saccharomyces cerevisiae) | Spirogyra | Sulfahri et al., 2011 [26] |
| 12 | Algal species was used for ethanol production; Aspergillus niger MTCC 2196 and Saccharomyces cerevisiae MTCC 170 were used for saccharification and fermentation | Spirogyra | Eshaq et al., 2011 [92] |
| 13 | Ethanol was extracted from marine algae carbohydrates using E. coli strains | Undaria pinnatifida, Chlorella vulgaris, Chlamydomonas reinhardtii | Lee et al., 2011 [93] |
| 14 | Improvement of ethanol production was noticed with sonicated green alga cultivated in swine wastewater effluent. | Scenedesmus obliquus YSW15 | Choi et al., 2011 [94] |
| 15 | Acid-pretreated microalgal biomass enhanced the bioethanol production | Chlorococcum humicola | Harun and Danquah, 2011 [95] |
| 16 | Improved ethanol production was observed by enzymatic hydrolysis of microalgal biomass | Chlorococcum sps | Harun and Danquah, 2011 [96] |
| 17 | Acid-treated dried biomass of Scenedesmus obliquus resulted efficient sugar extraction | Scenedesmus obliquus | Mirinda et al., 2012 [97] |
| 18 | Ethanol production was achieved by the conversion of hydrolysates of algal species with Zymomonas mobilis | Spirogyra hyalina | Sulfhari et al., 2012 [98] |
| 19 | Ethanol production achieved through the influence of photobioreactors and culture conditions with Scenedesmus obliquus | Scenedesmus obliquus | Miranda et al., 2012 [99] |
| 20 | Optimal starch extraction by ultrasonic homogenizer and ethanol production through conversion withSSF method | Chlamydomonas fasciata Ettl 437 | Asada et al., 2012 [100] |
| 21 | Ethanol production was carried out using different hydrolysis and fermentation methods | Chlorella vulgaris | Ho et al., 2013 [101] |
| 22 | Bioethanol was generated from a green alga by enzymatic saccharification along with yeast | Dunaliella tertiolecta | Lee et al., 2013 [102] |
| 23 | Ethanol production was carried out using the cake of green alga along with cost of the experiment and environmental safety aspects | Cholerlla vulgaris | Moncada et al., 2013 [103] |
| 24 | Sugars were released due to virus infection in green alga, which were used for bioethanol production through E.coli | Chlorella variabilis NC64A | Cheng et al., 2013 [104] |
| 25 | Eighteen algal strains isolated in Pearl River delta were used for ethanol production; pretreatment was carried with dilute acid and cellulase | Mychonastes afer PKUAC 9 and Scenedesmus abundans PKUAC 1 | Guo et al., 2013 [39] |
| 26 | Five pretreatments were conducted with macroalgal biomass for ethanol production | Chaetomorpha linum | Schultz-Jensen et al., 2013 [105] |
| 27 | Microalgae cultivated in two stages for more carbohydrates, which were used for ethanol production | Scenedesmus obliquus CNW-N | Ho et al., 2013 [106] |
| 28 | Macrophytic green alga was used for ethanol production through enzymatic hydrolysis | Ulva fasciata | Trivedi et al., 2013 [107] |
| 29 | Green alga from Hawaii was used for ethanol production by saccharification cellulase of Trichoderma reesei followed by fermentation | Ulva reticulata | Yoza and Masutani, 2013 [108] |
| 30 | Wall-deficient mutant model alga used for ethanol production by acid hydrolysis and fermentation of starch through yeast | Chlamydomonas reinhardtii mutant cw15 | Scholz et al., 2013 [109] |
| 31 | Less ethanol was generated in the cultures without nitrogen content | Chlorella vulgaris | Salman and Ali, 2014 [27] |
| 32 | Improved ethanol was noticed from the nutrient stress-induced microalga by enzymatic hydrolysis and immobilized yeast fermentation | Chlorella vulgaris | Kim et al., 2014 [68] |
| 33 | Ethanol production was carried out with filamentous green alga | Tribonema sps | Wang et al., 2014 [110] |
| 34 | Green alga biomass particle size was altered and small particles resulted more ethanol | Chlorococcum infusionum | Harun et al., 2014 [111] |
| 35 | Physical, chemical and enzymatic pretreatments for saccharification of carbohydrates were practiced for ethanol production in microalgal species | Chlorella sorokiniana, Nannochloropsis gaditana | Hernandez et al., 2015 [112] |
| 36 | Ethanol was generated from green alga after lipid extraction | Chlorella sps KR-1 | Lee et al., 2015 [113] |
| 37 | Ethanol production was achieved in a marine alga using two fermentation organisms, i.e., Pachysolen tannophilus and Zymomonas mobilis, and the earlier one resulted more yield | Ulva rigida | El Harchi et al., 2015 [114] |
| 38 | Both ethanol and diesel were prepared using a green microalga cultivated in tubular photobioreactor | Scenedesmus bijugatus | Ashokkumar et al., 2015 [115] |
| 39 | Ethanol generation was studied using model alga and also using a hybrid system | Chlamydomonas reinhardtii | Costa et al., 2015 [116] |
| 40 | Ethanol production and heavy metal removal was carried out using green alga grown in various concentrations of wastewater | Scenedesmus obliquus | Hamouda et al., 2016 [117] |
| 41 | Ethanol was produced from green alga using Zymomonas mobilis | Spirogyra hyalina | Sulfhari et al., 2016 [118] |
| 42 | Ethanol and methane were produced from a green macroalga using a biorefinery concept | Chaetomorpha linum | Yahmed et al., 2016 [119] |
| 43 | Green alga was tested for ethanol production using HCl hydrolysis and yeast fermentation | Chlorella vulgaris | Bhooshan Kumar et al., 2016 [120] |
| 44 | Ethanol was produced using various experimental conditions and pretreatments from halophilic microalgal biomass | Dunaliella sps | Karatay et al., 2016 [121] |
| 45 | Efficient ethanol was produced from marine alga in a pilot scale model | Dunaliella tertiolecta | Varela-Bojórquez et al., 2016 [122] |
| 46 | Both Cyclotella and green algal species generated more ethanol in SHE treatment in yeast fermentation | Chlorella vulgaris YSL001 Uronema belkae | Hwang et al., 2016 [123] |
| 47 | Repeated batch SSF was proved as best method for long-term ethanol production with microalgal biomass using immobilized Saccharomyces cerevisiae | Chlamydomonas mexicana | El-Dalatony et al., 2016 [124] |
| 48 | Improvement of ethanol generation was observed with green alga through chemical and biological saccharification | Ulva fasciata | Hamouda et al., 2016 [125] |
| 49 | Enhancement of enzymatic saccharification of algal biomass resulted more ethanol production | Chaetomorpha linum | Neifar et al., 2016 [126] |
| 50 | Hungate technique for ethanol fermentation of algae using Saccharomyces cerevisiae | Spirogyra hyalina | Sulfahri et al., 2016 [127] |
| 51 | Algal strains grown under nitrogen deficiency act as potential feedstock for ethanol production | 17 strains Desmodesmus sps SP2-3 | Rizza et al., 2017 [128] |
| 52 | Efficient ethanol production was noticed from green alga grown in plantain peel extract with increased fermentation time | Chlorella vulgaris | Agwa et al., 2017 [129] |
| 53 | Pretreated green microalga generated more and cost-effective ethanol | Scenedesmus dimorphus | Chng et al., 2017 [130] |
| 54 | Cultivation of marine microalgae in treated municipal wastewater toward bioethanol production | Nannochloropsis oculata, Tetraselmis suecica | Reyimu and Ozcimen, 2017 [131] |
| 55 | Ethanol was produced from defatted residues of freshwater green alga along with biodiesel | Cosmarium sps | Manoj et al., 2018 [12] |
| 56 | Green alga was used for concomitant production of both ethanol and diesel | Scenedesmus sp. | Ramachandran and Incharoensakdi, 2018 [132] |
| 57 | Both micro- and macroalgal species were used for ethanol production through different hydrolysis and fermentation methods | Ulva fasciata, Chlorella vulgaris | Hamouda et al., 2018 [8] |
| 58 | Enhanced ethanol was obtained through acid hydrolysis of green alga | Ulva rigida | El Harchi et al., 2018 [133] |
| 59 | Bioethanol was generated from green alga by acidpre-treatment on the microbial fermentation process | Chlorella sps | Phwan et al., 2019 [134] |
| 60 | Effect of hydrolysis time and acid concentration on ethanol generation from microalga | Scenedesmus sps | Agustini et al., 2019 [135] |
| 61 | Augmented ethanol production from green algal biomass by hydrothermal pretreatment and enzymatic hydrolysis | Chlorella sps | Ngamsirisomsakul et al., 2019 [136] |
| 62 | Ethanol produced through fed-batch method using green algal species | Scenedesmus raciborskii | Alam et al., 2019 [137] |
| 63 | Ethanol production from algal species using Saccharomyces cerevisiae, Pichia kudriavzevii and Kluyveromyces thermotolerans | Spirogyra peipingensis | Sulfahri et al., 2019 [138] |
| 64 | Ethanol production from acid-pretreated microalgal hydrolysate using microwave-assisted heating wet torrefaction | Chlorella vulgaris ESP-31 | Yu et al., 2020 [139] |
| 65 | Ethanol production from green seaweed residue | Codium tomentosum | Gengaiah et al., 2020 [140] |
| 66 | Ethanol from green algal feedstock using both light and dark fermentation | Chlorella vulgaris | Varaprasad et al., 2020 [13] |
| 67 | Different hydrolysis and post-treatment processes of microalgal hydrolysate on bioethanol production | Chlorella ABC-001 | Seon et al., 2020 [141] |
| 68 | Supplementation of lysine and magnesium resulted more carbohydrates and, in turn, ethanol content | Scenedesmus acuminatus | Chandra et al., 2020 [142] |
| 69 | Production of ethanol in green alga using efficient saccharification of biomass by Trichoderma harzianum enzymes | Chlamydomonas reinhardtii | Bader et al., 2020 [143] |
| 70 | Bioethanol was produced using deoiled biomass of green alga using eco-friendly bio-based heterogeneous catalysts and hydrolysates | Scenedesmus obliquus | Gohain et al., 2021 [144] |
| 71 | Influence of different factors on biomass, bioethanol and biohydrogen generation in green alga | Chlamydomonas reinhardtii | Ragasudha et al., 2021 [11] |
| 72 | Vitamins improved the ethanol in green alga | Chlorococcum minutum | Varaprasad et al., 2021 [18] |
| 73 | Limiting the nitrate content in algal culture enhanced the total carbohydrate and starch, and thus, ethanol production | Chlorella sorokiniana | Kaur et al., 2022 [145] |
| 74 | Ethanol generation from green alga through Pt-Ru/RGO nanoparticles | Chlorococcum minutum | Varaprasad et al., 2022 [24] |
| 75 | Microalgae biomass was used for ethanol generation at high-solids loadings | Chlorella vulgaris FSP-E | Condor et al., 2022 [146] |
| 76 | Ethanol production from green alga grown in unsterilized swine wastewater | Chlorella vulgaris ESP-31 and Chlorella sorokiniana AK-1 | Acebu et al., 2022 [147] |
| 77 | Process and technoeconomic analysis of ethanol production from residual biomass of marine macroalga | Ulva lactuca | Gengaiah et al., 2023 [148] |
| 78 | Production and use of biomass in synthetic municipal wastewater for integrated biorefineries | Scenedesmus acuminatus CCALA436 | Onay and Aladag, 2023 [7] |
| S.No | Ethanol Production Work | Name of the Algae | Reference |
|---|---|---|---|
| 1 | Fermentation of brown alga was carried out for ethanol production considering various pretreatments | Saccharine lasissima (Laminaria) | Adams et al., 2009 [149] |
| 2 | A brown alga hydrolysate was used to generate bioethanol with six Pichia stipitis strains | Sargassum sagamianum | Ji-Hyeon et al., 2010 [150] |
| 3 | Simulations of processes of bioethanol generation were conducted using brown alga | Saccharina japonica | Fasahati and Liu, 2012 [151] |
| 4 | Various yeast strains were used for ethanol production from brown algae | Laminaria japonica | Lee and Lee, 2012 [152] |
| 5 | Brown alga was used to generate ethanol through low-acid pretreatment followed by simultaneous saccharification and fermentation | Saccharina japonica | Lee et al., 2013 [153] |
| 6 | Macroalgal species was used for ethanol production through acid pretreatment and fermentation | Sargassum sps | Borines et al., 2014 [154] |
| 7 | Improved ethanol production from brown macroalgae sugars was achieved by a synthetic yeast platform along with re-engineering of catabolic pathways | Brown macroalgae | Enquist-Newman et al., 2014 [155] |
| 8 | Both ethanol and protein generation were carried out from brown alga | Laminaria digitata | Hou et al., 2015 [156] |
| 9 | Ethanol was produced by mixing the saccharified waste of brown alga and paper shredder scrap using 19 strains of Saccharomyces cerevisiae | Undaria pinnatifida | Obara et al., 2015 [157] |
| 10 | Brown seaweed was used for ethanol using non-conventional yeasts | Ascophylum nodosum and Laminaria digitata | Obata et al., 2016 [158] |
| 11 | Brown marine macroalgae used for liquid biofuel source. Ethanol was generated after lipid extraction | Padina tetrastromatica | Ashokkumar et al., 2017 [159] |
| 12 | Improved ethanol was noticed with brown alga using simple pre-processing and drying methods | Laminaria digitata | Adams et al., 2017 [160] |
| 13 | Ethanol production was carried out using the enzymatic hydrolysate of brown alga | Saccharina latissima | Lamb et al., 2018 [161] |
| 14 | Ethanol production from brown macroalga by an alginate fermentation strain, Meyerozyma guilliermondii | Colpomenia sinuosa | Zhang et al., 2022 [162] |
| S.No | Ethanol Production Work | Name of the Algae | Reference |
|---|---|---|---|
| 1 | The acid hydrolysates of red algae were used for ethanol production | Red algae including Kappaphycus alvarezii | Meinita et al., 2012 [163] |
| 2 | Ethanol production was achieved with red alga in a biorefinery approach | Gracilaria verrucosa | Kumar et al., 2013 [164] |
| 3 | Ethanol was generated using red alga through simultaneous saccharification and fermentation of dilute-acid-pretreated samples | Gelidiella acerosa | Babujanarthanam and Kavitha, 2014 [165] |
| 4 | Ethanol was produced from acid hydrolysis and detoxification methods, and fermentation with thermotolerant Kluyveromyces marxianus | Pterocladiella capillacea | Wu et al., 2014 [166] |
| 5 | Red algal species was used for ethanol production by autoclaving and SHF and SSF | Gelidium amansii | Kim et al., 2015 [167] |
| 6 | Rapid ethanol production was achieved through mutant yeast using biomass of red alga | Gelidium amansii | Lee et al., 2015 [168] |
| 7 | Enzymatic hydrolysateof freshwater red alga generated more ethanol with SSF method than seawater red alga | Porphyridium cruentum | Kim et al., 2017 [169] |
| 8 | Ethanol was generated from industrial algae waste | Eucheuma spinosum | Alfonsin et al., 2019 [170] |
| 9 | Ethanol production from agarophyte red seaweed using a novel sample preparation method for analyzing ethanol content by GC | Gelidium elegans | Hessami et al., 2019 [171] |
| 10 | Production of bioethanol from red seaweed by SSF method | Gracilaria verrucosa Eucheuma cottonii | Wadi et al., 2019 [172] |
| 11 | Enhanced ethanol production was achieved with hydrolysate of red alga by improvement of catabolite regulatory genes in yeast | Gloiopeltis furcata | Park et al., 2021 [173] |
| 12 | Ethanol production was improved using galactose-adapted yeasts with red alga | Eucheuma denticulatum | Kim et al., 2021 [174] |
| S.No | Ethanol Production Work | Name of the Algae | Reference |
|---|---|---|---|
| 1 | Ethanol was produced using wild algae along with blue–green alga | Wild algae along with Spirulina | Kampfe, 2010 [175] |
| 2 | Ethanol production was achieved with microalga using different media compositions and Saccharomyces cerevisiae, Pichia stipitis and Zymomonas mobilis | Microcystis aeruginosa | Kimet al., 2012 [176] |
| 3 | Blue–green alga was used for ethanol production with four acids for saccharification | Spirulina platensis | Markou et al., 2013 [177] |
| 4 | Microalgal species was used for the production of ethanol | Spirulina | Hossain et al., 2015 [178] |
| 5 | Blue–green alga was used for ethanol production with various factors along with three fermentation organisms | Microcystis aeruginosa | Khan et al., 2016 [179] |
| 6 | CaO pretreatment and combination of four microorganisms for fermentation improved the ethanol production in blue–green alga | Microcystis aeruginosa (KMMCC-1135) | Khan et al., 2017 [180] |
| 7 | Augmented ethanol was achieved through optimization of carbohydrate productivity in blue-green alga | Spirulina | Tourang et al., 2019 [181] |
| 8 | Ethanol was produced from cyanobacteria-based agro-industrial wastewater treatment and raisin residue extract | Leptolynbgya sps | Tsolcha et al., 2021 [182] |
| 9 | Ethanol was generated along with semi-continuous algal–bacterial wastewater treatment | Cyanobacteria | Papadopoulos et al., 2023 [183] |
| S.No | Ethanol Production Work | Name of the Algae | Reference |
|---|---|---|---|
| 1 | Marine algal hydrolysates were used for ethanol production using Escherichia coli KO11 | Ulva lactuca, Gelidium amansii, Laminaria japonica, Sargassum fulvellum | Kim et al., 2011 [184] |
| 2 | Hydrothermal treatment and biological conversion were applied for ethanol production | Schizochytrium sps | Kim et al., 2012 [185] |
| 3 | Marine algae was used for ethanol production by yeast fermentation | Marine algae | Gupta et al., 2012 [186] |
| 4 | Ethanol content was greater with enzymatic hydrolysis than acid hydrolysis | Mixed microalgae | Shokrkar et al., 2017 [5] |
| 5 | Modeling and sensitivity analysis were carried out for ethanol production through enzymatic hydrolysis of microalgal cellulose | Mixed microalgae | Shokrkar et al., 2018 [187] |
| 6 | Both red and green macroalgal species were used for ethanol production using mechano-enzymatic deconstruction | Ulva lactuca, Gelidium sesquipedale | Amamou et al., 2018 [188] |
| 7 | Batch ethanol production through the biological and chemical saccharification of some Egyptian marine macroalgae | Jania rubens, Ulva lactuca, Sargassum latifolium | Soliman et al., 2018 [189] |
| 8 | A kinetic model was developed on ethanol production from enzymatic hydrolysates of microalgal biomass | Microalgae | Shokrkar and Ebrahimi, 2019 [190] |
| 9 | Ethanol production from defatted biomass ofmicroalgae grown under mixotrophic conditions | Nannochloropsis oculata | Fetyan et al., 2022 [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekhar, T.; Varaprasad, D.; Gnaneswari, P.; Swapna, B.; Riazunnisa, K.; Anu Prasanna, V.; Korivi, M.; Wee, Y.-J.; Lebaka, V.R. Algae: The Reservoir of Bioethanol. Fermentation 2023, 9, 712. https://doi.org/10.3390/fermentation9080712
Chandrasekhar T, Varaprasad D, Gnaneswari P, Swapna B, Riazunnisa K, Anu Prasanna V, Korivi M, Wee Y-J, Lebaka VR. Algae: The Reservoir of Bioethanol. Fermentation. 2023; 9(8):712. https://doi.org/10.3390/fermentation9080712
Chicago/Turabian StyleChandrasekhar, Thummala, Duddela Varaprasad, Poreddy Gnaneswari, Battana Swapna, Khateef Riazunnisa, Vankara Anu Prasanna, Mallikarjuna Korivi, Young-Jung Wee, and Veeranjaneya Reddy Lebaka. 2023. "Algae: The Reservoir of Bioethanol" Fermentation 9, no. 8: 712. https://doi.org/10.3390/fermentation9080712
APA StyleChandrasekhar, T., Varaprasad, D., Gnaneswari, P., Swapna, B., Riazunnisa, K., Anu Prasanna, V., Korivi, M., Wee, Y.-J., & Lebaka, V. R. (2023). Algae: The Reservoir of Bioethanol. Fermentation, 9(8), 712. https://doi.org/10.3390/fermentation9080712







