Abstract
In this study, the data of the antagonistic action of the enzyme L-lysine-α-oxidase in relation to clinical isolates of multiresistant uropathogenic Escherichia coli isolated from patients aged 2 to 17 years of various genders with an established diagnosis of infectious urethritis and/or cystitis are presented. According to the results obtained, the top priority drugs for the treatment of infectious urethritis and/or cystitis are FO (Fosfomycin, 200 µg/disc), IMP (Imipenem, 10 mg/disc), and CIP (Ciprofloxacin, 30 µg/disc). It was found that out of 70 clinical isolates, only 36 of them formed biofilms using the plate method, which is equal to 51.4% of the total number of isolates studied. Despite polyresistance, clinical isolates of E. coli have moderate sensitivity to both the homogeneous enzyme and the culture fluid of the producer. The introduction of an enzyme or culture liquid at an early stage of strain cultivation significantly inhibits the formation of biofilms (91–100%). When introduced at later stages of the experiment—24 h and 48 h—inhibition is less pronounced—20–36% and 5–22%, respectively.
1. Introduction
The formation of antibiotic resistance in bacteria is an adaptive survival mechanism that creates a global health problem [1,2,3,4,5,6]. The wide prevalence of E. coli and its ability to quickly acquire resistance to antibacterial drugs and disinfectants, including through the formation of biofilms and horizontal gene transfer, allow us to consider multidrug-resistant uropathogenic E. coli (UPEC) as a dangerous infectious agent that can cause urinary tract infections (UTIs), purulent infections, septicemia, and many other diseases [7,8,9,10,11].
At present, according to scientific publications, UPEC is one of the leading etiological factors in the development of UTIs [10,11,12,13,14]. This is due to several factors. First, rapid migration from the neighboring ecological niche, the gut [13,14]. Secondly, the presence of many adhesion factors that contribute to the rapid colonization of the urinary tract [13,14]. Thirdly, UPEC actively forms biofilms on various biogenic and abiogenic surfaces [8,15,16] and, as a result, is difficult to treat, which makes it difficult to eliminate the pathogen and leads to chronic or recurrent infections. It is known that the mechanism of biofilm formation by microorganisms is regulated by QS molecules and depends on environmental factors, availability of nutrients, hydrodynamic conditions, etc. [17,18]. Several studies have shown that E. coli strains associated with recurrent UTIs are more efficient at forming biofilms on abiotic surfaces [19,20].
According to studies by Maharjan G., the least active antibacterial drugs against UPEC are ampicillin and amoxicillin with clavulanic acid [21]. E. coli is most sensitive to piperacillin and imipenem [21]. Most E. coli strains can form biofilms, which correlates with the multidrug resistance of E. coli isolates [21]. In 2021, Z. Naziri et al. found that 99% of the E. coli isolates they studied could form biofilms in vitro [19,22]. In the same year, L. D. Buck described the genes responsible for the formation of biofilms, which can help predict the severity of UTI at the stage of laboratory screening [2]. Recent studies have shown that E. coli biofilms are sensitive to N-acetylcysteine, which not only inhibits biofilm formation but can also destroy already formed E. coli biofilms [8,23,24,25]. In a 2019 study by F. M. Carvalho and colleagues, it was found that some probiotic bacterial strains and metabolites can affect biofilms formed by E. coli [26].
All the above shows the necessity of further study of UPEC to find and justify new approaches to the treatment of UTIs.
L-lysine-α-oxidase is an enzyme that belongs to the class of oxidoreductases of the essential amino acid L-lysine. For the first time, L-lysine-α-oxidase was isolated by Japanese researcher H. Kusakabe [27]. Producers are classified as imperfect fungi. Taxonomy: domain: Eukaryotes; kingdom: Fungi; division: Ascomycota; class: Sordariomycetes; order: Hypocreales; family: Hypocreaceae; genus: Trichoderm. The genus includes more than 90 species. Representatives of the genus Trichoderma are saprophytic and phytopathogenic mold fungi. In the struggle for an ecological niche and a substrate for nutrition, fungi of the Trichoderma genus produce many metabolites, antibiotics (such as viridin, gliotoxin, chitinase, etc.), as well as enzymes that destroy polysaccharides (cellulose, hemicellulose, chitin) and some other polymer structures.
The enzyme consists of two tunnel-like structures in space c, formed between the substrate-binding domain and the helical domain; they are located on the isoalloxazine ring. The amino acid backbone of L-lysine interacts with the binding domain in the same way as with other similar enzymes. The α-carboxyl group of L-lysine forms hydrogen bonds with the enzyme. Hydrogen bonds of the α-amino group with carbonyl oxygen (Figure 1).
Figure 1.
The structure of L-lysine-α-oxidase [28].
Representatives of the genus Trichoderma are saprophytic and phytopathogenic mold fungi. According to research, the enzyme has antitumor [29,30] and antifungal activity [31].
The aim of this study was to investigate the effect of Trichoderma harzianum Rifai culture liquid containing the enzyme L-lysine-α-oxidase and purified homogeneous enzyme L-lysine-α-oxidase on the formation of biofilms by multidrug-resistant uropathogenic E. coli.
2. Materials and Methods
2.1. Microorganisms and Enzyme
The homogeneous enzyme L-lysine-α-oxidase (LO) (obtained from the production of the Institute of Biochemical Physics of the Russian Academy of Sciences named after G.K. Skriabin) and a culture liquid (CL) concentrate of the producer L-lysine-α-oxidase Trichoderma harzianum Rifai have been used in this study.
The cultivation of the producer was carried out on the equipment of a pilot technological plant of the Institute of Biochemical Physics of the Russian Academy of Sciences, K.I. Scriabin. We used a fermenter (BIOR-01, OKB TBM, Kirishi, Russia) with a volume of 100 L and a filling factor of 0.6. The preparation of the nutrient medium was carried out in the apparatus itself: 60 L of distilled water contained fine wheat bran in the amount of 3% (1800 g) of the final medium volume. Wheat bran was presoaked in 10 L of distilled water for 4 h then sterilized in an autoclave at 121 °C 1.1 atm. within 60 min. After that, 1.3% (780 g) of ammonium sulfate (NH4)2SO4 and 20 mL of adecanol were added to the fermenter. For the final pH of the medium (5.8–6.0), a solution of 10% hydrochloric acid was added. The prepared medium was sterilized again in the autoclave at 121 °C 1.1 atm. within 60 min.
For sterility control, samples of the medium were taken from the fermenter before inoculation. The samples were inoculated on meat-peptone agar, Sabouraud agar, and meat-peptone broth. The inoculum of the culture grown at the preliminary stage in flasks on Sabouraud medium was introduced into the prepared fermenter for 12 days in a shaker-incubator (Heidolph Unimax 1010, Heidolph, Schwabach, Germany) at a temperature of 27 °C and 80–100 rpm, for constant aeration of the medium and mixing of the substrate. The inoculation dose was not less than 5–10%. The culture was grown under the following conditions: temperature, 28 ± 1 °C; medium pH, 5.8–6.0; rate of change, 250 rpm; aeration, 30 L/min. When the pH rose above 7.0, solution of 10% hydrochloric acid was automatically added to the fermenter. The process was characterized by pH and L-lysine-α-oxidase activity. Samples were taken every 24 h. The fermentation duration was 94–98 h.
At the end of fermentation, the culture liquid was sent for preliminary purification. The mycelium of fungi and coarse particles of the medium were separated from the liquid part by vacuum filtration on a suction filter. The solution was concentrated by ultrafiltration through an XM300 membrane (>300,000 MW; Millipeore XM300 quantily-25, France) to cut off large molecular fibers with a molecular weight above 300 kDa. Filtration mode: pressure increased from 7 psi to 20 psi (0.5–1.5 atm.), temperature 25–30 °C. The retentate was purified by dialysis-washing five times with phosphate buffer (pH 7.1) and purification control by the optical density using a spectrophotometer (SF-2000, Russia) in the range of wavelengths of 200–1000 nm with a step of 0.1 nm. The purity control was the complete absence of protein in the permeate after washing. The retentate was removed and the permeate subsequently passed through two membranes with circuit flush identification using an XM150 (150,000 MW; Millipeore XM150 Quantity-25, France) under the following conditions: filtration mode, pressure increased from 7 psi to 25 psi (0.5–2.0 atm.); temperature, 25–30 °C. Then, a UM20E (>15–25,000 MW; Millipeore UM20E quantity-25, France) was used under the following conditions: filtration mode, pressure increased from 15 psi up to 75 psi (1.0–5.0 atm.); temperature, 25–30 °C.
The presence of the enzyme in the CL of the final product was detected by electrophoresis in PAGE [32]. For processing aliquots, denaturing solution was used with the following composition: 2.4 g SDS (dry); 7.5 mL 1 M Tris buffer (pH = 6.8); 0.24 mL 0.5 M EDTA; 15.1 g glycerin (12 mL); 3.0 mL β-mercaptoethanol; 1.2 mg bromophenol blue. For protein separation, a 12.5% agarose gel was used with the following composition: 12.5 mL of 30% acrylamide; 9.7 mL H2O; 7.50 mL 1.5 M Tris buffer (pH = 8.8); 0.30 mL 10% SDS. As a control of molecular weights, a marker with a mass range of 118.0–19.0 kDa was used under the following conditions: 118 kDa; 90.0 kDa; 50.0 kDa; 34.0 kDa; 26.0 kDa; 19.0 kDa (Fermentas PureExtreme, Vilnius, Lithuania). A homogeneous enzyme purified from impurities was used as a positive control. For electrophoresis, we used PowerPac Basic (Bio-Rad), with the voltage set to 9 mA.
The concentrate of CL (cCL) was obtained using Amocon Ultra 100 kDa centrifuge concentrators (Merck Millipore, France).
The activity of the enzyme L-lysine-α-oxidase in the culture liquid of the producer Trichoderma harzianum Rifai was determined at reference values of 0.54–0.58 U/mg according to the method described by us earlier [9]. The activity of the enzyme L-lysine-α-oxidase in cCL was determined in the range from 2.84 to 2.88 U/mg. For the purification of the enzyme L-lysine-α-oxidase, isolated by high performance liquid chromatography from the culture liquid, the activity was determined in terms of values of 50 U/mg.
2.2. Antibiotics Resistance Tests
The antimicrobial activity of cCL containing the L-lysine-α-oxidase enzyme and solutions of the homogeneous L-lysine-α-oxidase enzyme was studied on uropathogenic E. coli (UPEC, n = 70) by the disk-diffusion method, resistant to three or more of the following antibiotics:
- Imipenem, 10 mg/disk (Imipenem, IPM10–SD073, HiMedia, Mumbai, India);
- Ceftazidime, 30 µg/disk (Ceftazidime, CAZ30–SD062, HiMedia, Mumbai, India);
- Ceftriaxone, 30 µg/disk (Ceftriaxone, CTR30–SD065, HiMedia, Mumbai, India);
- Ceftazidime with clavulanic acid, 30/10 µg/disc (Ceftazidime/Clavulanic acid, CAC30/10–SD207, HiMedia, Mumbai, India);
- Amoxiclav, 30 µg/disk (Amoxyclav, AMC30–SD063, HiMedia, Mumbai, India);
- Nitrofurantoin, 200 µg/disk (Nitrofurantoin, NIT200–SD090, HiMedia, Mumbai, India);
- Ampicillin, 25 µg/disk (Ampicillin, AMP25–SD077, HiMedia, Mumbai, India);
- Trimethoprim, 30 µg/disk (Trimethoprim, TR30–SD149, HiMedia, Mumbai, India);
- Ciprofloxacin, 30 µg/disk (Ciprofloxacin, CIP30–SD142, HiMedia, Mumbai, India);
- Tetracycline, 30 µg/disk (Tetracycline, TE30–SD037, HiMedia, Mumbai, India);
- Ceftriaxone, 30 µg/disk (Ceftriaxone, CTR30–SD065, HiMedia, Mumbai, India).
During the work, the following E. coli strains were studied: 884, 1072, 1260, 1252, 1522, 1524, 1534, 1579, 1629, 1664, 1735, 2181, 2183, 2260, 2323, 2376, 2595, 2667, 2 757, 2841, 6508, 6387, 70-19, 118-19, 151-19, 226-19 721, 4183, 4184, 4269, 4461, 4770, 6123, 4840, 7527, 5045, 5193, 5221, 5628, 5629, 5632, 5633, 5795, 5843, 5846, 5878, 5886, 5896, 5962, 5964, 7362, 6955, 6993, 6941, 7561, 7534, and 6080. Escherichia coli ATCC 25,922 and Escherichia coli—M17 acted as reference strains.
The antibacterial activity of cCL and LO against UPEC was determined using the Kirby–Bauer disk-diffusion method [33,34]. For the study, E. coli isolates were grown in heart–brain broth (HIMEDIA® M210, India) for 24 h at 37 °C. After cultivation, the broth culture was centrifuged for 10 min at 2.4 × 103 rpm (ELMI SkyLine CM-6M centrifuge). The bacterial suspension for inoculation was prepared from the microbial sediment according to the turbidity standard 0.5 (McFarland, HIMEDIA) equal to 1.5–3.0 × 108 CFU/mL in saline (0.9% NaCl). The resulting bacterial suspension with a volume of 100 µL was inoculated by the “lawn” culture method on Mueller–Hinton agar (HiMedia M173, India). After inoculation, wells were made in agar with a sterile metal punch. Well size: d = 5 mm, V = 15 ± 1 µL. Trichoderma harzianum Rifai cCL and LO solution was added to the wells. A 0.9% sodium chloride solution was chosen as a negative control, and a test disk with fosfomycin (FO200 (200 µg/disk) HIMEDIA®, India) was chosen as a positive control.
2.3. Biofilm Formation Ability
The biofilm formation of UPEC and the effect of LO/cCL on the suppression of biofilm formation were assessed using the microplate culture method. For this, the studied cultures of E. coli were grown for 24 h at a temperature of 37 °C on the Mueller–Hinton broth medium in a volume of 5 mL. At the end of the day, the studied E. coli cultures were centrifuged at 2.4 × 103 rpm, and a bacterial suspension was prepared in accordance with the 0.5 McFarland turbidity standard (HiMedia, Mumbai, India) using a densitometer (Biosan, DEN-1B, Riga, Latvia). The resulting suspension was diluted in a solution of 0.9% sodium chloride at a ratio of 1/20 to obtain an inoculum. In the wells of a sterile polystyrene 96-well plate, the Mueller–Hinton broth medium with a volume of 180 μL was added. Next, previously prepared inoculums of the studied E. coli cultures were added in a volume of 20 μL (final dilution of the inoculum 1:10). Further, a solution of LO with a final concentration of 0.1 mg/mL and cCL with a final dilution of 1:10 were added to the corresponding wells. Solutions of LO and cCL were introduced in three variants: at the beginning of the experiment, after 24 h, and after 48 h from the beginning of the cultivation. The total cultivation time for E. coli, regardless of the time of application of the test substances, was 72 h at 37 °C. After cultivation, the wells of the tablet were washed three times with phosphate buffer (PB) and dried for 15 min. A 1% solution of crystalline violet was added to each well. The wells were incubated for 15 min, washed again with PB solution, and dried for 15 min. After that, 150 µL of 96% ethanol was added to each well; then, each well was incubated for 15 min and the result was recorded on the microplate reader at a wavelength of 492 nm.
2.4. Statistical Analysis
Experiments were performed in triplicate, and results were expressed as means ± standard deviation. We used Spearman’s correlation to analyze the correlation dependence. All the results obtained were processed in the XLSTAT (Premium v2016.02.28451) program.
3. Results
Figure 2 shows the data on the distribution of resistance for the studied cultures of E. coli (n = 70). According to the results, 45 studied E. coli cultures were found to be resistant to amoxiclav (30 µg/disk); 41 studied E. coli cultures were resistant to ampicillin (25 µg/disk); and 31 studied E. coli cultures were resistant to cefazolin (30 µg/disk). But at the same time, none of the 70 UPEC isolates studied in the experiment had resistance to fosfomycin (200 µg/disk), imipenem (10 µg/disk), and ciprofloxacin (30 µg/disk).
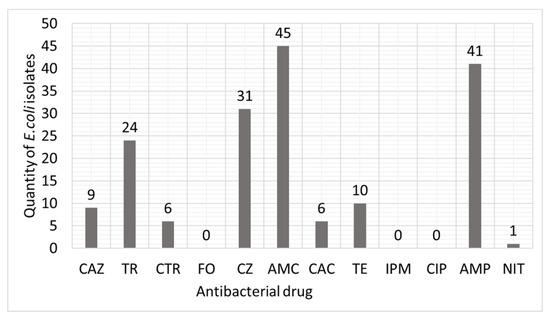
Figure 2.
Distribution of resistance in clinical isolates of UPEC to antibacterial drugs.
CAZ—Ceftazidime 30 mg/disk; TR—Trimethoprim 30 µg/disk; CTR—Ceftriaxone 30 µg/disk; FO—Fosfomycin 200 µg/disk; CZ—Cefazolin 30 µg/disk; AMC—Amoxiclav 30 µg/disk; CAC—Ceftazidime with clavulanic acid 30/10 µg/disk; TE—Tetracycline 30 µg/disk; IMP—Imipenem 10 µg/disk; CIP—Ciprofloxacin 30 µg/disk; AMP—Ampicillin 25 µg/disk; NIT—Nitrofurantoin 200 µg/disk.
As a result of determining the antibacterial activity of cCL and LO against UPEC using the Kirby–Bauer well-diffusion method, it was found that a homogeneous enzyme produces a larger zone of growth inhibition than cCL. The largest number of isolates of UPEC—23 strains—produced a zone of growth inhibition of 10 mm. A total of 15 strains produced a zone of 11 mm, and 14 more strains produced a zone with a diameter of 12 mm. Only one strain of E. coli demonstrated the highest value—16 mm. The culture liquid concentrate showed less pronounced results: 11 mm for 2 strains, 10 mm for 11 strains, 9 mm for 23 strains, and 8 mm for 20 strains.
Antibacterial activity against UPEC of the two objects of study is shown in Figure 3.
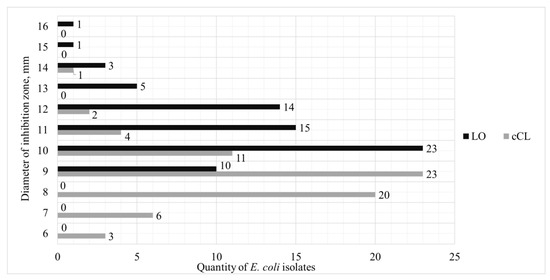
Figure 3.
Distribution of antibacterial activity of cCL and LO in relation to strains of UPEC.
To confirm the effectiveness of the samples of multidrug-resistant UPEC, we calculated the multidrug resistance index (MDRI) for 70 strains and compared it with the effectiveness of the samples (Figure 4).
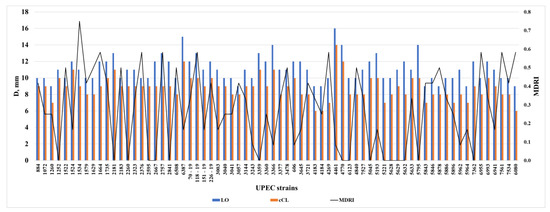
Figure 4.
Analysis of the results of the obtained zones of growth inhibition under the action of LO and cCL with a multidrug resistance index (MDRI) in clinical strains of UPEC.
When analyzing the correlation dependence of the antibacterial action of a homogeneous enzyme and culture fluid concentrate on the severity of antibiotic resistance of UPEC strains, the correlation was calculated by Student’s method. At p = 0.005, the correlation between MDRI and LO, as well as MDRI and cCL, is significant and negative with SCC (Spearman correlation coefficient) equal to −0.358 (p = 0.002) and −0.315 (p = 0.008), respectively, which is a very good result and tells us that the more antibiotics the E. coli isolate is resistant to, the more pronounced the antibacterial activity of the homogeneous enzyme and culture fluid concentrate on the microorganism. The data is presented in Figure 3.
It is very important that LO and cCL had an effect on multidrug-resistant UPEC, given the fact that all strains used in the experiment were absolutely resistant to at least three of the antibiotics, and some to seven or more antibiotics (Figure 4).
When analyzing the formation of biofilms in the studied isolates of UPEC by the method of microplate cultivation on Mueller–Hinton broth, after 72 h of cultivation on a microplate, it was revealed that only 36 out of 70 strains formed a biofilm. According to the results of the experiment, the enzyme has a pronounced effect on the suppression of biofilms (Figure 5).
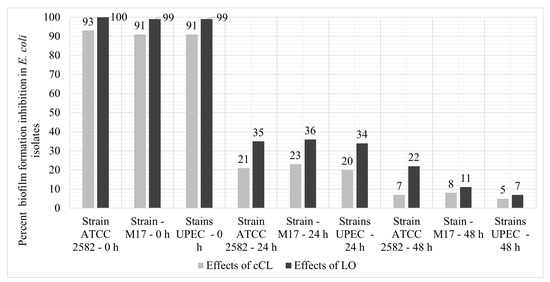
Figure 5.
The level of suppression of the formation of biofilms with the addition of LO and cCL upon the introduction of the experiment after 24 h and after 48 h.
But such inhibition of biofilm formation is observed only when LO/cCL is introduced at the early stages of biofilm formation (up to 24 h of cultivation). When introduced at later stages—after 24 and 48 h of cultivation–the studied samples either suppressed the formation several times less or it had almost no effect on the formation process.
4. Discussion
The question of the resistance formation of uropathogenic Escherichia coli attracts many researchers. The resistance formation is ongoing as an adaptive mechanism, which continues to pose a risk to patients with UTIs [31,35]. Alternative ways to combat UPEC include the development and further theoretical application like vaccines, receptor analogs, pilicides and curlicides, bacterial interference, or phage therapy [31,33]. Perhaps the use of enzymes can also be added to this list.
In our study, LO and cCL showed a moderate antibacterial effect in the modified Kirby–Bauer well-diffusion method. At the same time, these results may indicate that too low of a concentration of the enzyme in the solution was added to the wells. It is possible that when higher concentrations are applied to the wells, the enzyme will show a more pronounced antibacterial effect. This issue requires further study, since the problem of treating diseases caused by polyresistant UPEC remains relevant and the mechanisms that contribute to the formation of antibiotic resistance in bacteria are evolving.
However, despite a moderately pronounced antibacterial effect, it is worth noting a statistically significant relationship: the higher the MDRI of the strain, the larger the zone of inhibition and, hence, the sensitivity to the enzyme. This are very interesting and promising data which give us some hope for the fight against super-bacteria resistant to all currently used antibacterial drugs. The authors of this study suggest that such results are associated with the limitations of the prokaryotic genome and the phenomenon of “crowding out”; that is, the more antibiotic resistance genes a given microorganism has, the more likely it is that the housekeeping gene—which is responsible, for example, for alternative pathway synthesis of L-lysine—is crowded out.
Biofilm formation in UPEC plays an important role in chronic disease in patients. The process of biofilm formation is a multistage and complex process that includes the transition of bacteria from a free planktonic form to an adhesive form, which is the initial stage of biofilm formation. At the same time, it is important to develop methods aimed at both the prevention of the formation of biofilms and at the irradiation of biofilms that are formed.
The theoretical mechanism of enzyme action is that L-lysine-α-oxidase catalyzes the reaction of oxidative deamination of the essential amino acid L-lysine with the formation of four reaction products: α-keto-α-aminocaproic acid, ammonia, δ-piperidine-2-carboxylic acid (which can return to α-keto-α-aminocaproic acid), and hydrogen peroxide.
L-lysine itself is an essential amino acid in UPEC metabolism. The amino acid is synthesized in E. coli via the diaminopimelate pathway and is involved in protein malonylation. This leads to the inhibition of protein synthesis on ribosomes, and the authors of this study suggest that it is this process that underlies the inhibition of biofilm formation, namely, the inhibition of the synthesis of matrix proteins that act as the basis for biofilm formation or signal molecules involved in QS. This assumption is indirectly confirmed by a pronounced decrease in the effect of the introduction of LO and cCL at later stages of biofilm formation. Also, because of the action of the enzyme, the synthesis of the cell wall is suppressed due to the destruction of L-lysine, which is part of the latter.
On the other hand, the acids formed during the reaction also, theoretically, have a bactericidal effect, but in this case, it is rather difficult to predict the amount of reaction products due to multiple uncontrolled conditions during the experiment itself.
It is possible that hydrogen peroxide, which forms during the reaction and has a bactericidal effect, can suppress the formation of biofilms. When hydrogen peroxide interacts with a substrate, reactive oxygen species are released that damage cell membranes. This action may underlie the inhibition of biofilm formation by this chemical in other studies [36,37,38].
According to the research results, LO had a slightly more pronounced effect compared to cCL, which may indicate that it was the enzyme itself at a slightly higher concentration that had an inhibitory effect on biofilm formation and not another metabolite produced in cCL. However, the difference in activity between LO and cCL is not large, which, theoretically, shows the possibility of using both to suppress the formation of biofilms. It should be noted that the inhibitory effect was manifested upon the introduction of LO and cCL at the beginning of the experiment, which, in the future, provides us an assumption about the possibility of using the enzyme as a “protective” coating that prevents the formation of biofilms on it.
5. Conclusions
As a result of the studies, it was found that the highest priority drugs for the treatment of infectious urethritis and/or cystitis are FO (Fosfomycin, 200 µg/disc), IMP (Imipenem, 10 mg/disc), and CIP (Ciprofloxacin, 30 µg/disc) since none of the studied strains did not have resistance to these drugs. It should be considered that the enzyme and the culture liquid influenced multiresistant UPEC, given that almost all strains used in the experiment were resistant to at least one of the antibiotics, and some to seven antibiotics or more.
Despite polyresistance, clinical isolates of UPEC have moderate sensitivity to both the homogeneous enzyme and the culture fluid of the producer—the largest number of isolates of uropathogenic Escherichia coli. A total of 23 strains produced a zone of growth inhibition of 10 mm, 15 strains produced a zone of growth inhibition of 11 mm, and 14 more strains produced a zone of growth inhibition with a diameter of 12 mm.
It was found that out of 70 clinical isolates, only 36 of them formed biofilms using the plate method, which is equal to 51.4% of the total number of isolates studied.
The introduction of an enzyme or culture liquid at an early stage of strain cultivation significantly inhibits the formation of biofilms (91–100%). At the same time, the results with the introduction of LO were equal to 99–100% and, with the introduction of cCL, they ranged from 91% to 93%. When introduced at later stages of the experiment, after 24 h and 48 h, the inhibition was less pronounced: 20–36% and 5–22%, respectively.
Author Contributions
Conceptualization, formal analysis, investigation—A.S.; writing—original draft—M.D.; writing—review and editing; N.S., V.S., O.K. and A.I. were responsible for project administration, data analysis, and data cleaning. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by the Peoples’ Friendship University of Russia named after Patrice Lumumba Strategic Academic Leadership Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon request, the data will be made available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.D.; Paladino, M.M.; Nagashima, K.; Brezel, E.R.; Holtzman, J.S.; Urso, S.J.; Ryno, L.M. Temperature-Dependent Influence of FliA Overexpression on PHL628 E. coli Biofilm Growth and Composition. Front. Cell. Infect. Microbiol. 2021, 11, 775270. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F. Threats of antibiotic resistance: An obliged reappraisal. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2021, 24, 499–506. [Google Scholar] [CrossRef]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. Link Between Antibiotic Persistence and Antibiotic Resistance in Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 900848. [Google Scholar] [CrossRef]
- Gabibov, A.G.; Dontsova, O.A.; Egorov, A.M. Overcoming Antibiotic Resistance in Microorganisms: Molecular Mechanisms. Biochem. Biokhimiia 2020, 85, 1289–1291. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ikeda, M.; Okada, Y.; Higurashi, Y.; Okugawa, S.; Moriya, K. Clinical and Microbiological Characteristics of Recurrent Escherichia coli Bacteremia. Microbiol. Spectr. 2021, 9, e0139921. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, H.; Bi, D.; Khaledi, A.; Qiao, M. A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections. Microb. Pathog. 2020, 144, 104196. [Google Scholar] [CrossRef]
- Senyagin, A.N.; Larichev, A.F.; Smirnova, I.P.; Podoprigora, I.V. A Novel Express Method to Determine Activity of Antitumor Enzyme L-Lysine-α-Oxidase of Trichoderma harzianum Rifai F-180. Bull. Exp. Biol. Med. 2020, 169, 119–121. [Google Scholar] [CrossRef]
- Kot, B. Antibiotic Resistance among Uropathogenic Escherichia coli. Pol. J. Microbiol. 2019, 68, 403–415. [Google Scholar] [CrossRef]
- Asadi Karam, M.R.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67. [Google Scholar] [CrossRef]
- Lemke, P.; Zoheir, A.E.; Rabe, K.S.; Niemeyer, C.M. Microfluidic cultivation and analysis of productive biofilms. Biotechnol. Bioeng. 2021, 118, 3860–3870. [Google Scholar] [CrossRef]
- Raeispour, M.; Ranjbar, R. Antibiotic resistance, virulence factors and genotyping of Uropathogenic Escherichia coli strains. Antimicrob. Resist. Infect. Control. 2018, 7, 118. [Google Scholar] [CrossRef]
- York, A. Uropathogenic E. coli creates a memory. Nat. Rev. Microbiol. 2023, 21, 345. [Google Scholar] [CrossRef]
- Rathi, B.; Gupta, S.; Kumar, P.; Kesarwani, V.; Dhanda, R.S.; Kushwaha, S.K.; Yadav, M. Anti-biofilm activity of caffeine against uropathogenic E. coli is mediated by curli biogenesis. Sci. Rep. 2022, 12, 18903. [Google Scholar] [CrossRef]
- Ranjani, S.; Kathun, U.R.; Hemalatha, S. Silver Decorated Myconanoparticles Control Growth and Biofilm Formation in Uropathogenic E. coli. Appl. Biochem. Biotechnol. 2022, 194, 504–516. [Google Scholar] [CrossRef]
- Pousti, M.; Zarabadi, M.P.; Abbaszadeh Amirdehi, M.; Paquet-Mercier, F.; Greener, J. Microfluidic bioanalytical flow cells for biofilm studies: A review. Analyst 2018, 144, 68–86. [Google Scholar] [CrossRef]
- Henly, E.L.; Norris, K.; Rawson, K.; Zoulias, N.; Jaques, L.; Chirila, P.G.; Parkin, K.L.; Kadirvel, M.; Whiteoak, C.; Lacey, M.M.; et al. Impact of long-term quorum sensing inhibition on uropathogenic Escherichia coli. J. Antimicrob. Chemother. 2021, 76, 909–919. [Google Scholar] [CrossRef]
- Naziri, Z.; Kilegolan, J.A.; Moezzi, M.S.; Derakhshandeh, A. Biofilm formation by uropathogenic Escherichia coli: A complicating factor for treatment and recurrence of urinary tract infections. J. Hosp. Infect. 2021, 117, 9–16. [Google Scholar] [CrossRef]
- Katongole, P.; Nalubega, F.; Florence, N.C.; Asiimwe, B.; Andia, I. Biofilm formation, antimicrobial susceptibility and virulence genes of Uropathogenic Escherichia coli isolated from clinical isolates in Uganda. BMC Infect. Dis. 2020, 20, 453. [Google Scholar] [CrossRef]
- Maharjan, G.; Khadka, P.; Siddhi Shilpakar, G.; Chapagain, G.; Dhungana, G.R. Catheter-Associated Urinary Tract Infection and Obstinate Biofilm Producers. Can. J. Infect. Dis. Med. 2018, 2018, 7624857. [Google Scholar] [CrossRef]
- Raad, N.; Tandon, D.; Hapfelmeier, S.; Polacek, N. The stationary phase-specific sRNA FimR2 is a multifunctional regulator of bacterial motility, biofilm formation and virulence. Nucleic Acids Res. 2022, 50, 11858–11875. [Google Scholar] [CrossRef] [PubMed]
- Nwabuife, J.C.; Omolo, C.A.; Govender, T. Nano delivery systems to the rescue of ciprofloxacin against resistant bacteria “E. coli; P. aeruginosa; Saureus; and MRSA” and their infections. J. Control. Release Off. J. Control. Release Soc. 2022, 349, 338–353. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Kárpáti, K.; Nagy, Á.L.; Gugolya, M.; Stájer, A.; Burián, K. Association between biofilm-production and antibiotic resistance in Escherichia coli isolates: A laboratory-based case study and a literature review. Acta Microbiol. Immunol. Hung. 2021, 68, 217–226. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Mergulhão FJ, M.; Gomes, L.C. Using Lactobacilli to Fight Escherichia coli and Staphylococcus aureus Biofilms on Urinary Tract Devices. Antibiotics 2021, 10, 1525. [Google Scholar] [CrossRef]
- Kusakabe, H.; Kodama, K.; Kuninaka, A.; Yoshino, H.; Misono, H.; Soda, K. A new antitumor enzyme, L-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J. Biol. Chem. 1980, 255, 976–981. [Google Scholar] [CrossRef]
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: Possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist. Updates 2020, 51, 100695. [Google Scholar] [CrossRef]
- Lukasheva, E.V.; Babayeva, G.; Karshieva, S.S.; Zhdanov, D.D.; Pokrovsky, V.S. L-Lysine α-Oxidase: Enzyme with Anticancer Properties. Pharmaceuticals 2021, 14, 1070. [Google Scholar] [CrossRef]
- Pokrovsky, V.S.; Chepikova, O.E.; Davydov, D.Z.; Zamyatnin, A.A.; Lukashev, A.N., Jr.; Lukasheva, E.V. Amino Acid Degrading Enzymes and their Application in Cancer Therapy. Curr. Med. Chem. 2019, 26, 446–464. [Google Scholar] [CrossRef]
- Sobowale, A.; Uzoma, L.; Aduramigba-Modupe, A.; Bamkefa, B. Fungitoxicity of Trichoderma longibrachiatum (Rifai) Metabolites against Fusarium oxysporum, Aspergillus niger and Aspergillus tamarii. Am. J. Plant Sci. 2022, 13, 984–993. [Google Scholar] [CrossRef]
- Brunelle, J.L.; Green, R. One-dimensional SDS-polyacrylamide gel electrophoresis (1D SDS-PAGE). Methods Enzymol. 2014, 541, 151–159. [Google Scholar] [CrossRef]
- Olabode, I.R.; Sachivkina, N.; Karamyan, A.; Mannapova, R.; Kuznetsova, O.; Bobunova, A.; Zhabo, N.; Avdonina, M.; Gurina, R. In Vitro Activity of Farnesol against Malassezia pachydermatis Isolates from Otitis Externa Cases in Dogs. Animals 2023, 13, 1259. [Google Scholar] [CrossRef]
- Sachivkina, N.; Vasilieva, E.; Lenchenko, E.; Kuznetsova, O.; Karamyan, A.; Ibragimova, A.; Zhabo, N.; Molchanova, M. Reduction in Pathogenicity in Yeast-like Fungi by Farnesol in Quail Model. Animals 2022, 12, 489. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B.M.; Piątek, R.J. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains. Acta Biochim. Pol. 2019, 66, 129–138. [Google Scholar] [CrossRef]
- Koroleva, E.A.; Soloveva, A.V.; Morgunova, E.Y.; Kapotina, L.N.; Luyksaar, S.I.; Luyksaar, S.V.; Bondareva, N.E.; Nelubina, S.A.; Lubenec, N.L.; Zigangirova, N.A.; et al. Fluorothiazinon inhibits the virulence factors of uropathogenic Escherichia coli involved in the development of urinary tract infection. J. Antibiot. 2023, 76, 279–290. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy KR, R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Singh, K.; Gujju, R.; Bandaru, S.; Misra, S.; Babu, K.S.; Puvvada, N. Facet-Dependent Bactericidal Activity of Ag3PO4Nanostructures against Gram-Positive/Negative Bacteria. ACS Omega 2022, 7, 16616–16628. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).