Abstract
We studied the changes induced in pH, acidity, brix, reducing sugar, soluble protein, nutritional components, primary metabolites, and antioxidant activities of isoflavone-enriched soybean leaf during the different stages involved in Tricholoma matsutake mycelia fermenting. We found that total fatty acid contents increased sequentially in dried soybean leaf, sterilized soybean leaf, and fermented soybean leaf (413.8, 420.3, and 909.4 mg/100 g, respectively). Particularly, linoleic acid content was 5-fold higher in the fermented soybean leaf than in the previous stages. The total free amino acid contents were decreased with progressing processing stages (2389.71, 1860.90, and 1434.25 mg/100 g). However, glutamic acid and lysine contents were highest in fermented soybean leaves. Total mineral contents increased with progressing processing stages (40.30, 41.72, and 55.32 mg/100 g). Water-soluble vitamins, riboflavin, and niacin were about 26-fold and 2.6-fold higher, respectively, in fermented soybean leaf. Comprehensive data analysis of primary metabolites detected changes in a total of 28 metabolites, including, amino acids, organic acids, carbohydrates, and fatty acid metabolites. Antioxidant activities were measured by 2,2-diphenyl-1-picrylhydrazyl, 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), and hydroxyl radical scavenging activities and ferric-reducing antioxidant power. Overall, the antioxidant activities increased with progressing processing stages. Thus, we show that T. matsutake mycelia fermented isoflavone-enriched soybean leaf products have excellent nutritional value.
1. Introduction
Soybean (Glycine max L. Merrill) leaf is used as an edible food in Korea and Japan. It contains functional compounds such as amino acids, phenolic acids, coumestrol, and pterocarpan [,,]. Functional compounds found in soybean leaf improve perimenopause disorders via α-glucosidase inhibition, cholesterol reduction, anti-diabetes, and anti-obesity activities [,,,]. However, to the best of our knowledge, while most countries value soybean seeds, they consider soybean pods, stems, roots, and leaves as by-products. Nevertheless, soybean leaves are valuable in the food, cosmetics, bio, and medicinal industries. Recently, plant metabolites have been modulated by stimulating defense and metabolic pathways to increase their crop value [,]. When the representative plant signal transducing hormone, ethylene, is applied to soybean leaves, the myeloblastosis (MYB) gene is expressed which promotes the synthesis of secondary metabolites in the isoflavonoid biosynthesis pathways. This method significantly increases isoflavone derivatives []. Recently, Ban et al. [] reported a 5 to 6-fold increase in the free amino acid contents of isoflavone-enriched soybean leaves (IESL) compared to common soybeans. Also, IESL has benefits in mitigating perimenopause disorders, such as osteoporosis relief and obesity prevention [,].
Fermentation, historically the oldest food processing technology, improves the nutrition and sensual quality of foods []. During fermentation, microbial enzymes biochemically act on food constituents. Thus, large proteins, polysaccharides, and triglycerides are decomposed into low-molecular peptides, amino acids, monosaccharides, and fatty acids. This helps improve the digestion and absorption of the food; and the physiological activity of the food is increased, thus, affecting the food characteristics []. Among various fermentation methods, fungal fermentation brings about cellulose decomposition, glycolytic hydrolysis, and secondary metabolite change. Thus, it is used in various industries such as biogas production, wastewater purification, soil purification, and high-value-added food production industries []. Tricholoma matsutake is one of the most popular edible mushrooms in East Asia, including Japan, China, and Korea. It has effective antioxidant, anti-inflammatory, and immune control activities []. However, this fungus is incapable of artificial sporocarp production and can produce sporocarp from starch and inulin substrates alone, and not from other polysaccharides []. The enzymes produced by this strain include α-amylase, α-glucosidase, and β-glucosidase and are positively involved in the fermenting and/or activating of functional substances in food [,]. Various studies have evaluated fermented foods for metabolic changes, antioxidant improvement, and other beneficial properties [,,]. Among them, Lee et al. [] have reported that when soybeans are solid-state fermented, beneficial fermentation properties such as isoflavone aglycones conversion, phenolics, and antioxidant activities increase by T. matsutake mycelium and significantly enhance nutritional components. The components of IESL fermented by lactic acid bacteria have previously been evaluated []. However, the components of IESL fermented by the fungus have not been evaluated and changes in nutrients and primary metabolites have not been reported to date. We did this based on the need to consider how the nutritional components of fermented IESL by the T. matsutake mycelium will change.
Thus, this is the first study to research fermentation of IESL using T. matsutake mycelia in order to analyze the changes in its pH, acidity, brix, reducing sugar, soluble protein, nutritional components (fatty acids, amino acids, minerals, and vitamins), primary metabolites, and antioxidant activities. Our goal is to demonstrate how processing IESL, which is considered a by-product, might increase the value of food by altering and modulating nutritional composition and physiological activities.
2. Materials and Methods
2.1. Experiment with Plants and Microorganisms
The IESL used in this study was grown and provided in a dried state by JCN Farm Co., Ltd. (Namhae-gun, Gyeongsangnam-do, Republic of Korea) in 2018. The T. matsutake mycelium was provided by the Wood Chemistry Research Laboratory (Department of Forest Engineering, Gyeongsang National University, South Korea) and cultured using potato dextrose broth/agar (PDB/PDA, BD-Difco, Sparks, MD, USA).
2.2. Reagents and Analytical Instruments
The reagents used in this study—CuSO4-5H2O, KnaC4H4O6-4H2O, 5-sulfosalicylic acid dihydrate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2-thiobarbituric acid (TBA), trichloroacetic acid (TCA), and 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ)—were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Organic solvents (methanol, acetonitrile, and water) used for extract preparation, physiological activities testing, and device analysis were purchased from J.T. Baker (Phillipsburg, NJ, USA), and other reagents were purchased and used as necessary. The Ph and brix were measured using a Ph meter (Orion Star A211, Thermo Fisher Scientific Inc., Waltham, MA, USA) and a brix meter (A83450, ATAGO, Saitama, Japan). Free amino acids were measured using an automatic amino acid analyzer (L-8900, Hitachi High-Technologies Corp., Tokyo, Japan). Fatty acids were analyzed by gas chromatography (GC) system (Agilent 7890A system, Agilent Technologies Inc., Wilmington, DE, USA) equipped with SP-2560 capillary column (100 m × 0.25 mm i.d., 0.25-μm film thickness, Sigma-Aldrich Co., St. Louis, MO, USA) and flame ion detector (FID). Mineral content was measured using a microwave high-pressure digestion system (Ultrawave 2.0, Milestone SRL, Sorisole BG, Italy) and an inductively coupled plasma-optical emission spectroscopy (ICP-OES, OPTIMA 8300DV, PerkinElmer Inc., Waltham, MA, USA). Vitamins were measured using a high-performance liquid chromatography (HPLC) system (Agilent 1200 system, Agilent Technologies Inc., Waldbronn, Germany) equipped with a diode array detector (Agilent 1260 series, Agilent Co., Santa Clara, CA, USA). The metabolite analysis was performed using GC-2010 plus (Tokyo, Japan) equipped with DB-5 MS capillary column (30 m × 0.25 mm, 0.25-μm, Agilent J-W, Santa Clara, CA, USA) and gas chromatography-mass spectrometry (Shimadzu GCMS-TQ8030, Tokyo, Japan). A 0.45 μm-membrane filter (Dismic25CS, Toyoroshikaisha, Ltd., Tokyo, Japan) and a rotary evaporator (Labconco, Kansas City, MO, USA) were used to concentrate and filter the extract. A spectrophotometer (UV-1800 240V, Shimadzu Corp., Kyoto, Japan) was used to measure the absorbance of in vitro activities.
2.3. Preparation of IESL by Processing Stages
In a previous study, fermentation patterns were compared by mixing dried IESL and brown rice (Jinju, Republic of Korea) at ratios of 10:0, 9:1, 8:2, and 7:3. As a result, the dried IESL mixture was set to 8:2 condition, which was the most effective in the growing of T. matsutake mycelium.
For processing, distilled water (DW) was added 5-fold (500 mL) to the dried IESL (DIESL) mixture (100 g) and hydrated for 1 h, and the mixture was sterilized for 1 h at 121 ± 1 °C. Then, T. matsutake mycelium was inoculated into sterilized rice medium (10 g of rice + 50 mL of DW) and cultivated in a shaking incubator (IS-971R, Jeio Tech Co., Ltd., Daejeon, Republic of Korea) at 25 °C for five days, and fermentation was performed at 25 ± 1 °C for eight days after inoculating the sterilized IESL (SIESL) mixture with 5% (v/v) of T. matsutake mycelium. Thereafter, the fermented IESL (FIESL) mixture was dried at 50 ± 2 °C for three days, then pulverized and stored in a −70 ± 1 °C deep freezer. The samples were divided into DIESL, SIESL, and FIESL according to the processing stages and used in the following experiments (Figure 1).
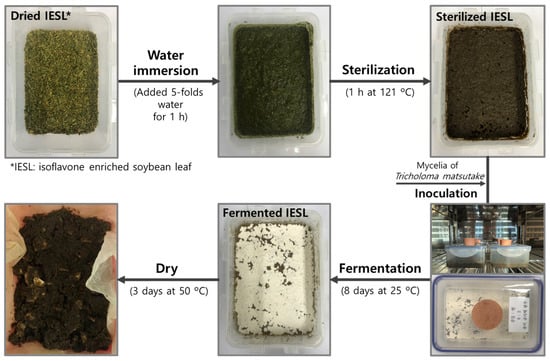
Figure 1.
Photograph of food processing with isoflavone-enriched soybean leaf (IESL) by mycelia of Tricholoma matsutake.
2.4. pH, Acidity, Brix, Reducing Sugars, and Soluble Proteins
The pH and total acidity measurement methods reported in previous studies were used []. Briefly, pH was measured using a pH meter after diluting 1 g of sample powders to 10 mL with DW. Total acidity was estimated by titrating against 0.1 N NaOH; 1 g sample powders diluted with 50 mL of DW were neutralized using 0.1 N NaOH to Ph 8.2 ± 0.1. The lactic acid concentration was calculated using the following formula:
where “1.002” is the factor for 0.1 N NaOH, “0.1” is the molarity of NaOH, and “0.09” is the lactic acid correction factor of the sample
Sample extracts were prepared for measuring brix, reducing sugars, and water-soluble proteins. Thereafter, 10 g of the sample powders were added to 200 mL of 50% EtOH and extracted for 14 ± 2 h. Then, sample extracts were concentrated to 20 mL using a rotary evaporator at 60 ± 1 °C. Brix was measured by dispensing 500 mL of sample extracts into a brix meter. Reducing sugar contents was measured using a slightly modified dinitrosalicylic acid (DNS) method. Briefly, sample extracts (100 mL) and 3,5-dinitrosalicylic acid reagent (1 mL) were dispensed in a test tube, and the absorbance of the colored sample was measured at 570 nm after heating at 100 ± 1 °C for 10 min. Thereafter, the absorbance value was converted into reducing sugar contents using a glucose standard curve. Soluble proteins were quantified using a slightly modified Biuret method. Biuret reagent was prepared as follows: 1.5 g of CuSO4-5H2O and 6.0 g of KNaC4H4O6-4H2O were dissolved in 500 mL of DW, 300 mL of 10% NaOH was added, and then diluted to 1 L with DW. Then, 4 mL of Biuret reagent was added to 1 mL of sample extracts, incubated at 37 ± 1 °C for 30 min, and then the absorbance was measured at 540 nm. Thereafter, the absorbance value was used to quantify the water-soluble proteins using a formula obtained from a protein standard curve (bovine serum albumin).
2.5. Analysis of Fatty Acids
Fatty acid contents were estimated using a slightly modified previously reported method []. Briefly, sample powders (1 g) and 0.5 N methanolic NaOH (3 mL) mixtures were heated at 100 ± 1 °C for 10 min in test tubes, to hydrolyze fatty acids and glycerol. Then, methyl esterification was carried out by heating the mixture for an additional 30 min in the presence of boron trifluoride (BF3) (of 2 mL). After methyl esterification, 1 mL of isooctane was added, shaken, and centrifuged to recover only the isooctane layer, which was dehydrated with anhydrous sodium sulfate and filtered through a 0.45 μm-membrane filter. Thereafter, fatty acids analysis was performed using GC; nitrogen gas was used as the mobile phase gas, and the gas speed was maintained at 1 mL/min. As for the analysis conditions, the temperature of the oven was maintained at 140 °C for 5 min, then raised by 20 °C per min to 180 °C and maintained for 2 min. Thereafter, the temperature was increased by 5 °C per min to a final temperature of 230 °C, maintained for 35 min.
2.6. Analysis of Free Amino Acids
For free amino acids analysis, a slightly modified previously reported method was used in this study []. An aqueous solution of sample powders (0.1 g in 5 mL of DW) was hydrolyzed at 60 ± 1 °C for 1 h. The solution was then incubated with 10% 5-sulfosalicylic acid dihydrate (1 mL) for 2 h at 4 °C and filtered with a syringe filter under reduced pressure. The solution was then concentrated with a rotary evaporator, dissolved in lithium buffer (pH 2.2, 2 mL), and filtered through a 0.45 μm-membrane filter. Thereafter, filtered samples were subjected to quantitative analysis using an automatic amino acid analyzer.
2.7. Analysis of Minerals
Mixtures of sample powders (0.5 g) and 70% nitric acid (10 mL) were decomposed using a microwave. DW was added to a final volume of 50 mL. In addition, to estimate sodium, 0.5 g of each sample powder was incinerated at 550–600 °C, and the final mixture (30 mL) in a 3% nitric acid solution was prepared. Subsequently, samples were quantitatively analyzed by ICP-OES [].
2.8. Analysis of Water-Soluble Vitamins
Analysis of water-soluble vitamins was performed using a previously reported method with slight modifications []. Extracts were prepared by incubating powder samples (10 g) with 70% MeOH (20 mL) for 30 min, centrifuging at 4000× g, and filtering the supernatant through a 0.45 μm-membrane filter. The filtered extracts were analyzed using HPLC. Mobile phase A was 0.2% acetic acid in water, mobile phase B was 0.2% acetic acid in acetonitrile, and a LiChrospher® 100 RP C18 column (4.6 × 250 mm, 5 μm, Merck, Germany) was used as the analytical column. The sample injection amount was 20 mL, the flow rate was 1 mL/min, the column temperature was 30 °C, and the analysis wavelength was 256 nm. The linear gradient of solvent B was 0% (0 to 5 min), 0–75% (5 to 15 min), and 75% (15 to 25 min).
2.9. Metabolic Derivatization and Gas Chromatography-Mass Spectrometry Analysis
Metabolite derivatization and GC-MS analysis methods previously reported were used []. Sample powders (0.1 g) were dissolved in DW (5 mL), hydrolyzed at 60 ± 2 °C for 1 h, and centrifuged at 3500× g. The supernatant (30 µL) was completely dried in a rotary evaporator at 40 ± 1 °C. Methoximation was carried out by incubating dried samples with 200 µL of methoxyamine hydrochloride containing 20 mg/mL of pyridine at 37 ± 1 °C for 90 min. Then, 80 µL of N, O-bis(trimethylsilyl)trifluoroacetamide (BSTFA), and 1% trimethylchlorosilane (TMCS) was added and derivatized at 70 ± 1 °C for 30 min. Thereafter, 1 mL of the derivatized sample was injected into the capillary column in split mode (40:1, v/v); the helium flow rate was 1 mL/min, the injector temperature was 200 °C, and the transfer line and ion source temperatures were 230 °C and 280 °C, respectively. The oven temperature was maintained at 70 °C for 2 min, then raised from 70 °C to 210 °C at a rate of 7 °C/min, held at 210 °C for 7 min, and then raised from 210 °C to 320 °C at a rate of 10 °C/min. Finally, the ions in the derivatized sample were detected using a mass spectrometer in electron ionization (EI) mode (70 eV) and monitored in scan mode at 45–800 m/z.
2.10. Processing of Data
GC-MS was used to analyze the MS data, which included data on mass spectra deconvolution, data collection, alignment, and normalization (Shimadzu, Tokyo, Japan). An area threshold of 4000 was used to deconvolute and collect metabolite peaks. An internal standard of dicyclohexyl phthalate was utilized to standardize all MS results. The Wiley and NIST mass spectrum databases, the published RIs, and reliable standards were used to compare the mass spectra and retention indices (RI) of the metabolites with those obtained using a variety of n-alkanes (C8–C40).
2.11. Extraction and Analysis of Antioxidant Activities
2.11.1. Preparation of Sample Extracts for Analysis of Antioxidant Activities
Sample extracts were prepared by incubating solutions of sample powders (20 g) and 50% ethanol (EtOH, 1000 mL) at room temperature for 14 ± 2 h using a stirrer. Subsequently, the sample extracts were centrifuged at 5000× g, and the supernatant was filtered through a 0.45 μm-membrane filter. The sample extracts were concentrated using a rotary evaporator at 60 ± 1 °C, lyophilized to a powder, and prepared using a freeze dryer (FD-1000, TOKYO RIKAKIKAI Co., Ltd., Tokyo, Japan) at −55 °C. Finally, the lyophilized powders were diluted with 50% EtOH, as necessary, to analyze the antioxidant activities.
2.11.2. Analysis of Antioxidant Activities
Here, slightly modified radical (DPPH, ABTS, and hydroxyl) scavenging activities and ferric-reducing antioxidant power (FRAP) evaluation methods were used []. Briefly, DPPH radical scavenging activity was measured at an absorbance of 525 nm by adding 0.2 mL of the sample to 0.8 mL of 1.5 × 10−4 M DPPH solution and reacting for 30 min under dark conditions. ABTS radical scavenging activity was determined by mixing 7 mM ABTS+ solution and 2.45 mM K2S2O8 at a ratio of 1:3 and leaving it in the dark for 14 ± 2 h to form ABTS+, then diluting with methanol to obtain an absorbance of 0.7 ± 0.02 at 732 nm. After adding 0.1 mL of the sample extract to 0.9 mL of the ABTS+ solution, the mixture was reacted for 3 min in the dark and the absorbance was measured at 732 nm. Hydroxyl radical scavenging activity was measured by adding 0.2 mL of 10 mM FeSO4-EDTA solution, 0.2 mL of 10 mM 2-deoxyribose, 0.2 mL of 10 mM H2O2, and 1.4 mL of the sample extract to a test tube, reacting at 37 ± 1 °C for 4 h, and then adding 1% TBA and 2.8 mL of 1% TBA. After adding 1 mL each of TCA, the color was developed at 100 ± 1 °C for 20 min, and then the absorbance was measured at 520 nm. In this experiment, the negative control was tested by taking pH 7.2 phosphate-buffered saline. FRAP assay was obtained by adding 5 mL each of 10 mM TPTZ and 20 mM FeCl3 to 50 mL of 300 mM sodium acetate buffer (pH 3.6) to obtain a 10:1:1 (v/v/v) and pre-reacted at 37 ± 1 °C for 15 min to prepare a FRAP reagent. After adding 0.05 mL of the sample extract to 0.95 mL of the FRAP reagent, the main reaction was performed at 37 ± 1 °C for 15 min, and then the absorbance was measured at 593 nm. In this study, the lyophilized powders were diluted to concentrations of 2, 1, and 0.5 mg/mL using 50% EtOH. Trolox (Sigma Aldrich Co., St. Louis, MO, USA) was used as a standard to measure the radical (DPPH, ABTS, and hydroxyl) scavenging activities and FRAP, and the result was represented as Trolox mg/g by inserting the calculation formula derived from the standard curve.
2.12. Statistical Analysis
The results of all experiments are presented as mean ± standard derivation (SD) of triplicate measurements. Statistical Analysis System (SAS) software (ver. 9.4; SAS Institute, Cary, NC, USA) was used to perform Tukey’s multiple tests (p < 0.05) to identify the significant differences between the data.
3. Results and Discussion
3.1. Changes in pH, Acidity, Brix, Reducing Sugars, and Soluble Proteins
Overall, there was a side effect of T. matsutake mycelium not growing due to a lack of carbon sources in IESL. To compensate, brown rice was added as a substrate that can help the growth of T. matsutake mycelium. In addition, preliminary experiment results for the best fermentation conditions of IESL and brown rice showed the best growth in the 8:2 (IESL:brown rice) mixture. However, since brown rice can affect the detection data, it has been added at the same rate to all samples (DIESL, SIESL, and FIESL) to reduce the parameter impact of brown rice.
The processing-stages-dependent (DIESL, SIESL, and FIESL) changes in pH, acidity, brix, reducing sugars, and soluble proteins are shown in Table 1. The pH increased with progressing processing stages: pH for DIESL, SIESL, and FIESL was 5.76, 5.83, and 6.33, respectively. Total acidity decreased as follows (%): DIESL (0.09) > SIESL (0.08) > FIESL (0.07). Brix value did not change depending on processing stages (%): DIESL, SIESL, and FIESL were 18.5, 18.5, and 18.6, respectively. However, significant changes were observed in the reducing sugar (mg/mL): DIESL (0.8) < SIESL (1.3) < FIESL (1.5). Soluble protein tended to decrease as follows (mg/mL): DIESL (2.26) > SIESL (2.17) > FIESL (2.09).

Table 1.
Change in pH, acidity, brix, reducing sugars, and soluble proteins during food processing of isoflavone-enriched soybean leaf (IESL) by mycelia of Tricholoma matsutake.
Generally, during fungal fermentation, pH increases due to the increase in soluble nitrogen concentration []. Agreeingly, here, the observed changes in pH, presumably, occurred due to the increase in soluble nitrogen caused by fermentation with T. matsutake mycelium. The decrease in acidity as processing stages progress is related to an increase in pH by the T. matsutake mycelium. Usually, sugars, polysaccharides, and oligosaccharides are hydrolyzed to reduce sugars during fermentation, and then consumed by the microorganisms in the late stage of fermentation [,]. Here, we fermented the soybean leaves for 8 days, which corresponds to its initial fermentation stage. Therefore, the reduced sugar content appears to increase. During fermentation, soluble and other protein components are hydrolyzed to peptides and amino acids. Thus, soluble protein content decreases as fermentation progresses []. Therefore, it is necessary to closely examine the decrease in soluble protein and change in the amino acid contents of IESL during fermentation.
3.2. Changes in Fatty Acid Compositions
The processing-stages-dependent changes in fatty acid compositions are shown in Table 2. The total fatty acid content of IESL increased steeply during fermentation (in mg/100 g): DIESL (413.8) < SIESL (420.3) < FIESL (909.4). Palmitic acid increased by approximately 2-fold during fermentation: 177.5 to 303.6 mg/100 g. In addition, stearic acid increased significantly during fermentation: 69.7 to 98.1 mg/100 g. Unsaturated fatty acids, such as oleic acid, linoleic acid, and α-linolenic acid, increased by approximately 2 to 5-fold during fermentation: 32.0 to 77.7 mg/100 g for oleic acid, 56.7 to 274.9 mg/100 g for linoleic acid, and 47.2 to 87.8 mg/100 g for linoleic acid (Table 2).

Table 2.
Change in fatty acid contents during food processing of isoflavone-enriched soybean leaf (IESL) by mycelia of Tricholoma matsutake.
Lipase enzyme produced by fermenting microorganisms such as Rhizopus oligosporus increases the fatty acid contents by hydrolysis of glycerol and fatty acids bound to triglycerides []. These results suggest that palmitic acid, oleic acid, linoleic acid, and α-linolenic acid are the main fatty acid components that account for more than 80% of the fermented soybean product []. Consistently, we fermented IESL products that were also detected as main fatty acids such as palmitic acid, oleic acid, linoleic acid, and α-linolenic acid in high contents. According to Lee et al. [], fermenting soybeans using T. matsutake mycelia leads to high oleic acid and linoleic acid content in the fermented product. Here, fermenting IESL with T. matsutake mycelia leads to a similar increase in oleic acid and linoleic acid contents. Therefore, the fatty acid content of IESL can be effectively increased by fermenting with T. matsutake mycelium.
3.3. Changes in Free Amino Acid Compositions
The processing-stages-dependent changes in free amino acid composition are shown in Table 3. Here, 8 essential and 24 non-essential amino acids were detected. Total free amino acids at different stages of processing were as follows (in mg/100 g): FIESL (1434.25) < SIESL (1860.90) < DIESL (2389.71); fully fermented stage had the lowest total free amino acid content. DIESL (with the highest total free amino acid content) had the highest concentration of aspartic acid-NH2 (asparagine), followed by arginine, aspartic acid, proline, and γ-aminobutyric acid. In addition, most amino acids in SIESL showed a slight decrease compared to that in DIESL. In FIESL, a significant number of amino acids were decreased, however, significant enrichment was observed in the glutamic acid content (in mg/100 g): FIESL (211.65) > DIESL (121.53) > SIESL (65.57). In addition, non-essential amino acids such as aminoadipic acid, glycine, citrulline, and cystine tended to increase about 1.5 to 2-fold in FIESL. Among essential amino acids, lysine showed a significant increase after fermentation (in mg/100 g): SIESL (30.50) < DIESL (48.54) < FIESL (82.02). In addition, threonine, methionine, and leucine tended to increase by approximately 1.2 to 2-fold after fermentation (Table 3).

Table 3.
Change in free amino acid contents during food processing of isoflavone-enriched soybean leaf (IESL) by mycelia of Tricholoma matsutake.
Changes in amino acid contents during food processing are due to proteases secreted by microorganisms during fermentation which degrade polyproteins and release them as peptides or amino acids []. During fermentation, the mycelium of mushrooms can change the amino acid content and composition, and improve the flavor by producing glutamic acid and aspartic acid []. Lee et al. [] found that glutamic acid and lysine were significantly increased during solid-state fermentation of soybeans with T. matsutake mycelium. Consistently, fermented IESL products demonstrate a significant increase in glutamic acid and lysine by T. matsutake mycelium. Ali et al. [] suggested a significant change in free amino acids in fermented leaf crops. Therefore, here we conjecture that T. matsutake mycelium affects the production of specific free amino acids during fermentation.
3.4. Changes in Mineral Compositions
The processing-stages-dependent changes in mineral compositions are shown in Table 4. The total mineral content tended to increase with progressing processing stages (in mg/100 g): DIESL (40.30) < SIESL (41.72) < FIESL (55.32) (Table 4). The elements, potassium (K) and calcium (Ca) tended to increase in the order of processing stages (in mg/100 g): DIESL (19.96) < SIESL (20.23) < FIESL (26.44) and DIESL (11.35) < SIESL (11.73) < FIESL (15.45) for K and Ca, respectively. And other elements, P, S, Mg, Fe, Zn, Mn, Al, B, Si, and Na showed similar increases in contents with fermentation.

Table 4.
Change in mineral contents during food processing of isoflavone-enriched soybean leaf (IESL) by mycelia of Tricholoma matsutake.
The bioavailability of minerals in food is low because they are bound in complex matrices comprising proteins and carbohydrates. However, as these proteins and carbohydrates are decomposed by phytases, proteases, and lipases produced by microorganisms during fermentation, the minerals bound to the complex matrices are eluted, increasing bioavailability, and increasing the total mineral content []. In addition, significant increases have been reported for K, Ca, Mg, and Fe during soybean fermentation; their increase plays a positive role in growth, bone health, and metabolic activity [,]. Therefore, we postulate that the fermentation of IESL with T. matsutake mycelium will enhance the bioavailability and concentration of free minerals in the fermented products.
3.5. Changes in Water-Soluble Vitamin Compositions
Here, the processing-stages-dependent changes in water-soluble vitamin compositions were studied (Table 5). Riboflavin (B2), niacin (B3), pantothenic acid (B5), folic acid (B9), and ascorbic acid (C) composition were analyzed. The total water-soluble vitamin contents increased with the progressing processing stage (in mg/100 g): 8.4, 12.4, and 32.2 for DIESL, SIESL, and FIESL, respectively. In addition, the contents of vitamin B2 and vitamin B3 increased by about 26-fold and 2.6-fold, respectively, after the fermentation of IESL. However, dried and sterilized stages would not have detected vitamins B5, B9, and C by high-temperature drying and sterilization at 121 °C. Thereafter, it was determined that, at the fermented stage, vitamins B5, B9, and C were not increased by T. matsutake (Table 5).

Table 5.
Change in water-soluble vitamin contents during food processing of isoflavone-enriched soybean leaf (IESL) by mycelia of Tricholoma matsutake.
Water-soluble vitamins (vitamin C or B complex) are not stored in the body. They act as antioxidants and aid in the metabolism of energy, amino acids, fatty acids, and the biosynthesis of pentose sugars []. Fermentation improves the vitamin B complex bioavailability of several grains and crops []. In addition, fermenting microorganisms may contribute to vitamin B complex enhancement [,]. Although information on the water-soluble vitamins related to T. matsutake mycelium is lacking, many documents have reported the presence of a significant amount of vitamin B complexes in fungi related to edible mushrooms [,]. Our study showed a significant increase in vitamin B complex content in FIESL. Thus, noteworthily, fermentation with T. matsutake mycelium effectively reinforces food with vitamins.
3.6. Changes in Metabolite Compositions
We screened for a total of 30 metabolites to understand the processing-stages-dependent changes in primary metabolites. We could detect 28 metabolites comprising 11 amino acids (alanine, valine, leucine, isoleucine, proline, serine, threonine, aspartic acid, pyroglutamic acid, phenylalanine, asparagine, and tyrosine), 9 organic acids (lactic acid, oxalic acid, malonic acid, succinic acid, fumaric acid, malic acid, cyanuric acid, glutamic acid, and citric acid), 5 carbohydrates (pinitol, fructose, glucose, myo-inositol, and sucrose), and 3 fatty acids (myristic acid, palmitic acid, and stearic acid) (Figure 2). Table 6 shows the average peak area GC-MS data, which changed significantly with progressing processing stages.
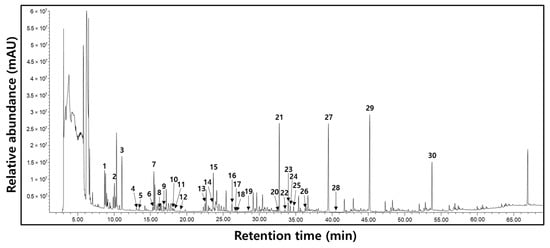
Figure 2.
Typical base peak intensity (BPI) chromatogram of processing stages with isoflavone-enriched soybean leaf (IESL) extracts using GC-MS. 1, Lactic acid; 2, Alanine; 3, Oxalic acid; 4, Malonic acid; 5, Valine; 6, Leucine; 7, Isoleucine; 8, Proline; 9, Succinic acid; 10, Fumaric acid; 11, Serine; 12, Threonine; 13, Malic acid; 14, Aspartic acid; 15, Pyroglutamic acid; 16, Cyanuric acid; 17, Glutamic acid; 18, Phenylalanine; 19, Asparagine; 20, Citric acid; 21, Pinitol; 22, Myristic acid; 23, Fructose-1; 24, Fructose-2; 25, Glucose; 26, Tyrosine; 27, Palmitic acid; 28, Myo-inositol; 29, Stearic acid; and 30, Sucrose.

Table 6.
Change in metabolite contents during food processing of isoflavone-enriched soybean leaf (IESL) by mycelia of Tricholoma matsutake.
Most amino acids tended to decrease after sterilization. In the fermentation stage, alanine and aspartic acid tended to decrease, and other amino acids showed no significant changes (Table 6). Organic acids play an important role in changing the properties of food. Non-volatile organic acids are substrates for microorganisms during fermentation which are converted to volatile organic acids such as acetic acid, propanoic acid, and butanoic acid []. In addition, the changes occurring in organic acid’s composition during food fermentation impart a unique aroma and taste to the food [,]. Oxalic acid comprises about 70% of the total organic acids content in soybean leaves. Oxalic acid content increases from 99.21 → 108.10 → 119.58 μg/g with progressing processing stage. Citric acid, malonic acid, and succinic acid, which are the intermediates of the TCA cycle, mostly decreased with progressing processing stage (Table 6). The increase in monosaccharide carbohydrates, such as fructose and glucose, during the sterilization stage, caused the decrease of the disaccharide and sucrose. In addition, the natural cyclitols sugars (or cyclic polyols) such as pinitol and myo-inositol are found in various plants including soybeans; pinitol and myo-inositol are phytates derivatives [,]. However, negatively charged phytates and cationic are chelated and decrease the bioavailability of minerals. Thus, methods such as soaking, steaming, and fermentation are used to decrease phytate contents and improve the bioavailability of minerals []. Here, pinitol and myo-inositol decreased in the sterilization stage. Moreover, fermentation did not, further, affect their levels, indicating that they are decomposed by heat treatment during sterilization. Therefore, IESL processing is expected to increase the bioavailability of minerals.
3.7. Changes in Antioxidant Activities
Radical (DPPH, ABTS, and hydroxyl) scavenging activities and FRAP were investigated using in vitro methods. In this study, the sample extracts were diluted to concentrations of 2, 1, and 0.5 mg/mL. The antioxidant activities were stronger at higher concentrations. Processing increased the antioxidant activities of IESL (Figure 3). The DPPH radical scavenging activity increased as follows (Trolox mg/g): DIESL as 6.44, SIESL as 6.80, and FIESL as 7.59 at a sample extract concentration of 2 mg/mL. The DPPH radical scavenging activity increased by 1.15 in FIESL compared to DIESL. The ABTS radical scavenging activity increased as follows (Trolox mg/g): DIESL as 20.04, SIESL as 21.01, and FIESL as 23.13 at a sample extract concentration of 1 mg/mL; it was 3.09 higher in FIESL. The hydroxyl radical scavenging activity increased as follows (Trolox mg/g): DIESL as 1.59, SIESL as 1.84, and FIESL as 2.23 at a sample extract concentration of 2 mg/mL. The hydroxyl radical scavenging activity for FIESL was 0.64 higher than that for DIESL. FRAP values increased with progressing processing stages (Trolox mg/g): DIESL as 1.21, SIESL as 1.56, and FIESL as 1.89 at a sample extract concentration of 2 mg/mL. It was found that the FIESL had a greater effect on the FRAP value than the DIESL (Figure 3).
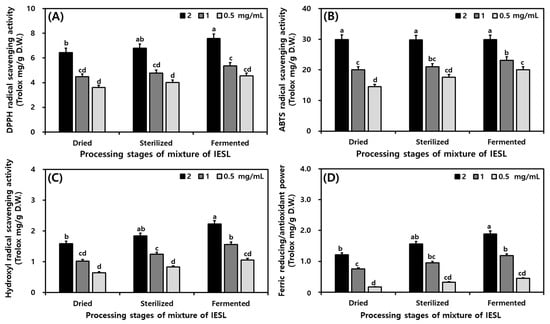
Figure 3.
Change in antioxidant activity during food processing of isoflavone-enriched soybean leaf (IESL) by mycelia of Tricholoma matsutake. (A) DPPH radical scavenging activity; (B) ABTS radical scavenging activity; (C) Hydroxyl radical scavenging activity; and (D) Ferric-reducing/antioxidant power. All values are presented as the mean ± SD of triplicate determination, different letters (a–d) above the columns present significant differences (p < 0.05) between different processing stages (dried, sterilized, and fermented) and concentrations (2, 1, and 0.5 mg/mL).
The DPPH radical scavenging activity measurement is one of the most widely used methods to gauge antioxidant activity. It is recorded as the reduction in absorbance by phenolic compounds due to free radical activity []. Generally, the radical scavenging activity estimates using the ABTS method are more precise than those measured by the DPPH method. Probably, this is because, while the DPPH method estimates the radical scavenging activity of hydrogen-donating antioxidants, the ABTS method estimates the scavenging activities of hydrogen-donating and chain-breaking antioxidants in the hydrolyzed sample extract. Thus, radical scavenging activity measured using the ABTS method more accurately estimates the antioxidant levels []. Bai et al. [] proved that apart from phenolics, flavanols and isoflavones are also involved in the free radical scavenging activity and strongly affect antioxidant activity due to hydroxylation and glycosylation of their B ring. FRAP assay was proposed by Benzie and Strain [] for measuring antioxidant activity because of rapid and reproducible results. Lee et al. [] measured the time-dependent change in the radical scavenging activities of soybeans during solid-state fermentation with T. matsutake mycelium. The radical scavenging activity of soybean gradually increased with increasing fermentation duration due to the positive correlation between bio-transformed secondary metabolites and antioxidant activity. Also, Lee et al. [] studied the time course of the antioxidant activities’ change of IESL during solid lactic acid fermentation and showed that the highest antioxidant activities occurred at 72 h of fermentation. Consistently, here, we observed a consistent increase in antioxidant activities in the fermentation stage. Therefore, we inferred that the increase in antioxidant activities was brought about by the increased nutritional components and metabolites caused by IESL fermenting.
4. Conclusions
Our research aims to increase added value by adding food processing technology to IESL, and not only functional components but also nutrients that are vital factors to consider when assessing food components. The pH, acidity, brix, reducing sugar, and soluble protein, nutritional components (fatty acids, free amino acids, minerals, and water-soluble vitamins), primary metabolites, and antioxidant activities (DPPH, ABTS, hydroxyl radical scavenging activities, and FRAP) were investigated according to the processing stages of IESL by mycelia of T. matsutake. pH and acidity changed to basicity in the final fermentation stage according to the processing stages, and reducing sugar increased, and water-soluble protein tended to decrease. When comparing nutrients, both saturated and unsaturated fatty acids increased during fermentation processing, glutamic acid and lysine content of free amino acids increased about 2-fold, mineral contents increased slightly in the order of processing stages, and water-soluble vitamin contents increased in vitamins B2 and B3. When the primary metabolites were qualitatively quantified using GC-MS, 28 metabolites were detected in amino acids, organic acids, carbohydrates, and fatty acids, showing significant changes during the processing. As a result of measuring antioxidant activities, it showed an increasing effect during the processing process. Therefore, in this research, fermentation of IESL by the mycelium of T. matsutake not only increased nutrition but also had an influence on antioxidant activities. This study can be used as basic data for the development of new materials with potential in the food industry by investigating functional metabolites in the future.
Author Contributions
Conceptualization, M.A.H. and K.-M.C.; methodology, D.-Y.C., H.-Y.L. and J.-H.L. (Jin-Hwan Lee); software, D.-Y.C., J.-B.J. and M.-Y.J.; validation, D.-Y.C., J.-B.J. and K.-M.C.; formal analysis, D.-Y.C., J.-H.L. (Ji-Ho Lee), G.-Y.L. and M.-Y.J.; investigation, D.-Y.C., J.-H.L. (Ji-Ho Lee), G.-Y.L. and K.-M.C.; resources, J.-H.L. (Jin-Hwan Lee), J.-H.L. (Ji-Hyun Lee) and K.-M.C.; data curation, D.-Y.C. and K.-M.C.; writing—original draft preparation, D.-Y.C.; writing—review and editing, H.-Y.L., M.A.H. and K.-M.C.; visualization, D.-Y.C., H.-Y.L. and J.-H.L. (Ji-Hyun Lee); supervision, K.-M.C.; project administration, K.-M.C.; funding acquisition, K.-M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education (Grant number 2016R1D1A1B01009898 and RS-2023-00245096), Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data reported in this study is contained within the article. The underlying raw data is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ban, Y.J.; Song, Y.H.; Kim, J.Y.; Cha, J.Y.; Ali, I.; Baiseitova, A.; Shah, A.B.; Kim, W.Y.; Park, K.H. A significant change in free amino acids of soybean (Glycine max L. Merr) through Ethylene Application. Molecules 2021, 26, 1128. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.J.; Curtis-Long, M.J.; Ryu, H.W.; Jang, K.C.; Seo, W.D.; Kim, J.Y.; Kang, K.Y.; Park, K.H. Pterocarpan profiles for soybean leaves at different growth stages and investigation of their glycosidase inhibitions. J. Agric. Food Chem. 2011, 59, 12683–12690. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.J.; Lee, J.H.; Curtis-Long, M.J.; Lee, J.W.; Kim, Y.S.; Ryu, H.W.; Park, C.G.; Jeong, T.S.; Park, K.H. The most abundant polyphenol of soy leaves, coumestrol, displays potent α-glucosidase inhibitory activity. Food Chem. 2011, 126, 1057–1063. [Google Scholar] [CrossRef]
- Kim, J.E.; Jeon, S.M.; Park, K.H.; Lee, W.S.; Jeong, T.S.; McGregor, R.A.; Choi, M.S. Does Glycine max leaves or Garcinia Cambogia promote weight-loss or lower plasma cholesterol in overweight individuals: A randomized control trial. Nutr. J. 2011, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, H.S.; Kang, J.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Lee, C.H.; Lee, I.K.; Yun, B.S.; Jeong, T.S. Soy leaf extract containing kaempferol glycosides and pheophorbides improves glucose homeostasis by enhancing pancreatic β-Cell function and suppressing hepatic lipid accumulation in db/db mice. J. Agric. Food Chem. 2015, 63, 7198–7210. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, J.H.; Han, J.M.; Cho, M.H.; Chung, Y.J.; Park, K.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Jeong, T.S. Anti-obesity effects of soy leaf via regulation of adipogenic transcription factors and fat oxidation in diet-induced obese mice and 3T3-L1 adipocytes. J. Med. Food. 2015, 18, 899–908. [Google Scholar] [CrossRef]
- Yuk, H.J.; Song, Y.H.; Curtis-Long, M.J.; Kim, D.W.; Woo, S.G.; Lee, Y.B.; Uddin, Z.; Kim, C.Y.; Park, K.H. Ethylene induced a high accumulation of dietary isoflavones and expression of isoflavonoid biosynthetic genes in soybean (Glycine max) leaves. J. Agric. Food Chem. 2016, 64, 7315–7324. [Google Scholar] [CrossRef]
- Xie, C.L.; Kang, S.S.; Cho, K.M.; Park, K.H.; Lee, D.H. Isoflavone-enriched soybean (Glycine max) leaves prevents ovariectomy-induced obesity by enhancing fatty acid oxidation. J. Funct. Foods. 2018, 43, 165–172. [Google Scholar] [CrossRef]
- Xie, C.L.; Park, K.H.; Kang, S.S.; Cho, K.M.; Lee, D.H. Isoflavone-enriched soybean leaves attenuate ovariectomy-induced osteoporosis in rats by anti-inflammatory activity. J. Sci. Food Agric. 2020, 101, 1499–1506. [Google Scholar] [CrossRef]
- Jung, S.; Chae, S.W.; Shin, D.H. Fermented foods of Korea and their functionalities. Fermentation 2022, 8, 645. [Google Scholar] [CrossRef]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT 2020, 125, 109264. [Google Scholar] [CrossRef]
- Alhomodi, A.F.; Zavadil, A.; Berhow, M.; Gibbons, W.R.; Karki, B. Application of cocultures of fungal mycelium during solid-state fermentation of canola meal for potential feed application. J. Am. Oil Chem. Soc. 2021, 98, 509–517. [Google Scholar] [CrossRef]
- You, L.; Gao, Q.; Feng, M.; Yang, B.; Ren, J.; Gu, L.; Cui, C.; Zhao, M. Structural haracterization of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013, 138, 2242–2249. [Google Scholar] [CrossRef]
- Onuma, H.; Hara, K.; Sugita, K.; Kano, A.; Fukuta, Y.; Shirasaka, N. Purification and characterization of a glycoside hydrolase family 5 endoglucanase from Tricholoma matsutake grown on barley based solid-state medium. J. Biosci. Bioeng. 2019, 128, 669–676. [Google Scholar] [CrossRef]
- Kusuda, M.; Ueda, M.; Konishi, Y.; Yamanaka, K.; Terashita, T.; Miyatake, K. Effects of carbohydrate substrate on the vegetative mycelial growth of an ectomycorrhizal mushroom, Tricholoma matsutake, isolated from Quercus. Mycoscience 2007, 48, 358–364. [Google Scholar] [CrossRef]
- Kusuda, M.; Ueda, M.; Miyatake, K.; Terashita, T. Characterization of the carbohydrase productions of an ectomycorrhizal fungus, Tricholoma matsutake. Mycoscience 2008, 49, 291–297. [Google Scholar] [CrossRef]
- Xu, M.; Qu, Y.; Li, H.; Tang, S.; Chen, C.; Wang, Y.; Wang, H. Improved extraction yield, water solubility, and antioxidant activity of lentinan from Lentinula edodes via Bacillus subtilis natto fermentation. Fermentation 2023, 9, 333. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, C.E.; Son, K.S.; Cho, K.M. Comparisons of nutritional constituents in soybeans during solid state fermentation times and screening for their glucosidase enzymes and antioxidant properties. Food Chem. 2019, 272, 362–371. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Singh, D.; Oh, J.Y.; Jeon, E.J.; Ryu, H.S.; Lee, D.W.; Kim, B.S.; Lee, C.H. Comparative evaluation of microbial diversity and metabolite profiles in doenjang, a fermented soybean paste, during the two different industrial manufacturing processes. Food Chem. 2017, 221, 1578–1586. [Google Scholar] [CrossRef]
- Lee, H.Y.; Cho, D.Y.; Jang, K.J.; Lee, J.H.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Haque, M.A.; Cho, K.M. Changes of γ-aminobutyric acid, phytoestrogens, and biofunctional properties of the isoflavone-enriched soybean (Glycine max) leaves during solid lactic acid fermentation. Fermentation 2022, 8, 525. [Google Scholar] [CrossRef]
- Hwang, C.E.; Kim, S.C.; Kim, D.H.; Lee, H.Y.; Suh, H.K.; Cho, K.M.; Lee, J.H. Enhancement of isoflavone aglycone, amino acid, and CLA contents in fermented soybean yogurts using different strains: Screening of antioxidant and digestive enzyme inhibition properties. Food Chem. 2021, 340, 128199. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Bioprocessing of pea protein can enhance fortified Fe but reduce Zn in vitro bioaccessibility. J. Agric. Food Chem. 2022, 70, 1241–1251. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, B.K.; Bhattacharjee, S.; Seal, T. Nutritional composition, mineral content, antioxidant activity and quantitative estimation of water soluble vitamins and phenolics by RP-HPLC in some lesser used wild edible plants. Heliyon 2019, 5, 14–31. [Google Scholar] [CrossRef]
- Muzdalifah, D.; Athaillah, Z.A.; Nugrahani, W.; Devi, A.F. Colour and pH changes of tempe during extended fermentation. AIP Conf. Proc. 2017, 1803, 020036. [Google Scholar]
- Lee, S.Y.; Lee, S.; Lee, S.; Oh, J.Y.; Jeon, E.J.; Ryu, S.H.; Lee, C.H. Primary and secondary metabolite profiling of doenjang, a fermented soybean paste during industrial processing. Food Chem. 2014, 165, 157–166. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Ruiz-Teran, F.; Owens, J.D. Chemical and enzymic changes during the fermentation of bacteria-free soya bean tempe. J. Sci. Food Agric. 1996, 71, 523–530. [Google Scholar] [CrossRef]
- Park, M.K.; Cho, I.H.; Lee, S.; Choi, H.K.; Kwon, D.Y.; Kim, Y.S. Metabolite profiling of Cheonggukjang, a fermented soybean paste, during fermentation by gas chromatography-mass spectrometry and principal component analysis. Food Chem. 2010, 122, 1313–1319. [Google Scholar] [CrossRef]
- Shi, H.; Yang, E.; Li, Y.; Chen, X.; Zhang, J. Effect of solid-state fermentation on nutritional quality of leaf flour of the drumstick tree (Moringa oleifera Lam.). Front. Bioeng. Biotechnol. 2021, 9, 626628. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, O.; Huang, Z.; Wang, Y.; Roubik, H.; Yang, K.; Cai, M.; Peilong Sun, P. Solid-state fermentation of soybean meal with edible mushroom mycelium to improve its nutritional, antioxidant capacities and physicochemical properties. Fermentation 2023, 9, 322. [Google Scholar] [CrossRef]
- Ali, M.W.; Ilays, M.Z.; Saeed, M.T.; Shin, D.H. Comparative assessment regarding antioxidative and nutrition potential of Moringa oleifera leaves by bacterial fermentation. J. Food Sci. Technol. 2019, 57, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Puniya, A.K.; Dhawa, T. Enhancing micronutrients bioavailability through fermentation of plant-based foods: A concise review. Fermentation 2021, 7, 63. [Google Scholar] [CrossRef]
- Cai, J.S.; Feng, J.Y.; Ni, Z.J.; Ma, R.H.; Thakur, K.; Wang, S.; Hu, F.; Zhang, J.G.; Wei, Z.J. An update on the nutritional, functional, sensory characteristics of soy products, and applications of new processing strategies. Trends Food Sci. Technol. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- Olanipekun, B.; Adelakun, O. Nutritional and microbiological attributes of soybean (Glycine max) during fermentation with Rhizopus oligosporus. Food Sci. Qual. Manag. 2015, 39, 111–118. [Google Scholar]
- Yaman, M.; Çatak, J.; Uğur, H.; Gürbüz, M.; Belli, İ.; Tanyıldız, S.N.; Yıldırım, H.; Cengiz, S.; Yavuz, B.B.; Kişmiroğlu, C.; et al. The bioaccessibility of water-soluble vitamins: A review. Trends Food Sci. Technol. 2021, 09, 552–563. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Thakur, K.; Feng, J.Y.; Cai, J.S.; Zhang, J.G.; Hu, F.; Russo, P.; Spano, G.; Wei, Z.J. Riboflavin-overproducing lactobacilli for the enrichment of fermented soymilk: Insights into improved nutritional and functional attributes. Appl. Microbiol. Biotechnol. 2020, 104, 5759–5772. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogon, K.; Bernas, E.; Duda-Chodak, A. Nutraceuticals and antioxidant activity of prepared for consumption commercial mushrooms Agaricus bisporus and Pleurotus ostreatus. J. Food Qual. 2015, 38, 111–122. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Bernaś, E.; Skrzypczak, A.; Kapusta, I. Vitamins, phenolics and antioxidant activity of culinary prepared Suillus luteus (L.) Roussel mushroom. LWT 2014, 59, 701–706. [Google Scholar] [CrossRef]
- Razali, S.; Khan, A.Y.F.; Khatib, A.; Ahmed, Q.U.; Wahab, R.A.; Zakaria, Z.A. An in vitro anticancer activity evaluation of Neolamarckia cadamba (Roxb.) bosser leaves’ extract and its metabolite profile. Front. Pharmacol. 2021, 12, 741683. [Google Scholar] [CrossRef]
- Dumschott, K.; Richter, A.; Loescher, W.; Merchant, A. Post photosynthetic carbon partitioning to sugar alcohols and consequences for plant growth. Phytochemistry 2017, 144, 243–252. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta 2007, 71, 1741–1751. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, Y.; Wang, B.; Li, S.; Guo, F.; Hua, H.; Zhao, Y.; Yu, Z. Comparison of phenolic compounds, antioxidant and antidiabetic activities between selected edible beans and their different growth periods leaves. J. Funct. Foods 2017, 35, 694–702. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).