Abstract
Cellulase and xylanase have been widely studied for bioconversion processes and applied in various industries. The high cost of these enzymes remains to be the major bottleneck for large-scale commercial application of lignocellulosic biorefinery. The use of agroindustrial residues and weeds as fermentation substrates is an important strategy to increase cellulolytic enzymes production and reduce costs. Penicillium crustosum was newly isolated and selected to study its enzyme production during solid-state fermentation (SSF). Natural and pretreated water hyacinth (WH) biomass was used as support, substrate and inducer of cellulases and xylanases. Thermochemical pretreatments of WH biomass at 121 °C and sulfuric acid at three concentrations (0.2, 0.6 and 1 M) were assayed. The pretreatments of WH biomass released mono- and oligo-saccharides that favored fungal growth and enzymes production on SSF. WH is a cost-effective substrate-support and inducer, which to be used as a solid medium, was impregnated with a saline solution, containing only (NH4)2SO4, KH2PO4 y MgCl2. Maximum cellulases (carboxymethylcellulase (CMCase)) and xylanases productions of P. crustosum cultured on SSF were reached using the WH pretreated biomass with H2SO4 0.6 M and 121 °C. The simultaneous CMCase and xylanases production reached (647.51 and 4257.35 U/g dry WH, respectively) are among the highest values ever reported.
1. Introduction
Water hyacinth (Eichhornia crassipes), an indigenous plant of the Amazon region, has spread to many other tropical and subtropical regions, invading water bodies [1,2]. Water hyacinth (WH) is considered one of the most invasive free-floating aquatic plants in the world. WH can cover an entire body of water in a densely packed mat in only two or so weeks [2,3,4,5]. The growth of other aquatic organisms is hampered and native species are wiped out by this dense mat, which prevents sunlight and oxygen from penetrating below the water surface [4,6,7].
Mechanical removal remains the most effective method for controlling WH populations, although it is expensive and labor-intensive [8,9]. If the WH biomass is not properly managed after harvesting, it can result in ecological issues wherever it is discarded. In this context, numerous studies have been conducted on the conversion and potential use of WH biomass to develop value-added products [10,11,12,13,14]. The challenge of solid waste management could be turned into an opportunity to make mechanical removal a profitable and economically viable approach to controlling these populations [4,15].
On a dry weight basis, water hyacinth consists of cellulose (18.2 to 19%), hemicellulose (48.7 to 50%), lignin (3.5 to 3.8%), and crude protein (13 to 13.5%) [16]. WH biomass is regarded as a desirable feedstock due to its environmentally friendly conversion into liquid fuels and chemical products, accomplished through biorefinery processes [10,11,12,13,14]. Additionally, as an aquatic plant, it does not compete with agricultural crops for land.
In lignocellulosic materials, the cellulose forms highly crystalline microfibrils embedded in a hemicellulose and lignin matrix. Due to the recalcitrant nature of lignocellulosic biomass, a pretreatment is required in order to open up the fibers and decrease the crystallinity of cellulose [17,18], thereby increasing its accessibility to enzyme attack [19,20,21]. Acid-thermal pretreatment is one of the most widely used methods due to its simplicity and efficient performance; it removes the lignin portion, hydrolyzes the hemicellulose at relatively moderate temperatures with high sugar yields and low formation of degradation products, and enhances the access of lignocellulosic enzymes to the inner space of the pretreated biomass [22,23,24,25,26,27,28].
The enzymatic hydrolysis of cellulosic and hemicellulosic materials requires the synergic actions of three types of cellulases (endo-1,4-β glucanase, exo-1,4-β glucanase, and β-glucosidases; two types of xylanases (endo-1,4-β xylanases and β-xylosidases); and other accessory enzymes (α-glucuronidase, acetylxylan esterase, α-L-arabinofuranosidases, p-coumaric esterase, and ferulic acid esterase) [29,30].
Cellulases and xylanases share several industrial applications, such as the production of paper and pulp, textiles, juice clarification and extraction, food additives, beer manufacturing, animal feed production, and bioethanol production [29,31,32,33,34]. For industrial applications, cellulases and xylanases are essential in large quantities [29,34,35]. Although cellulases in particular play a crucial role in the hydrolysis of lignocellulosic materials, the cost of these enzymes remains the main obstacle to the development of large-scale lignocellulosic biorefineries for commercial purposes [31]. To reduce the costs of enzymes, the following aspects should be considered: (i) selection of a potent producer microorganism; (ii) improvement in the production and productivity by optimizing culture conditions (including media components) [29]; (iii) use of cheaper feedstocks (including agricultural residues and weeds) as the main carbon sources and inducers for microbial culture [36]; and (iv) the use of solid-state fermentation (SSF) as a cost-effective fermentation process that can achieve higher cellulase production compared to submerged fermentations [30,37]. Remarkably, Penicillium species have been extensively used for the simultaneous production of cellulases and xylanases by SSF; e.g., P. roqueforti, P. citrinum, P. oxalicum, and P. echinulatum, with a wide variety of lignocellulosic materials as carbon sources, like sugar cane bagasse, agave bagasse, rice husks, and elephant grass, among others [38,39,40,41]. Cellulases and xylanases have been widely applied in saccharification processes [42,43,44,45]. In this regard, the aim of this work was the isolation of a filamentous fungus producing high levels of cellulases and xylanases (Penicillium crustosum); the study of some cultural conditions which could improve enzyme production; the use of a weed (water hyacinth) as a support source of carbon and energy for fungal growth as well as an inducer of the synthesis of cellulases and xylanases; and, finally, the implementation of SSF as a cultural technique that is particularly advantageous for the production of fungal lignocellulolytic enzymes and enables the direct use of lignocellulosic materials as solid substrates.
2. Materials and Methods
2.1. Sampling and Treatment of Water Hyacinth
Water hyacinth plants were collected from the Santiago River in Ocotlán Jalisco, Mexico (20°20′39.4″ N 102°46′29.6″ W). Plants were rinsed with tap water to eliminate dirt and then fractioned into three parts: roots, leaves, and stems. The wet plants were cut into smaller pieces and dried at 70 °C for 72 h in a hot air oven (Terlab, Zapopan, Mexico). For use as a support, carbon source, and inducer for inoculum preparation and solid-state fermentation (SSF), water hyacinth dry stems were milled in a blade mill (Vayco, Mexico) and sieved to provide two particle size ranges: medium (between 0.841 and 2.00 mm) and fine (between 0.420 and 0.841 mm).
2.2. Isolation of Cellulases and Xylanases Producing Fungi
The culture medium for the isolation of filamentous fungi had the following composition (g/L): fresh water hyacinth (stems and leaves) 100, glucose 5, NaNO3 6.07, (NH4)2SO4 4.73, KH2PO4 1.98, MgSO4∙7H2O 0.51, CaCl2 0.39, NaCl 0.13, FeSO4 0.05, trace element solution 4 mL, agar 15, and chloramphenicol 100 ppm. The water hyacinth, together with all the components of the culture medium (except the agar), was ground in a blender, and the pH was adjusted to 6.5. Subsequently, the medium was heated and the agar added, sterilized at 121 °C for 15 min, and distributed into Petri dishes.
The trace element solution consisted of (g/L): MnCl2∙4H2O 1.98, CoCl2∙6H2O 2.378, CuSO4∙5H2O 0.25, ZnSO4∙7H2O 0.29, EDTA 10.
Fifteen decaying water hyacinth plants were collected from the shores of Lake Chapala, Jalisco, Mexico (20°17′24.6″N 103°11′44.1″W). In addition, 30 fresh water hyacinth (WH) plants were collected in the water of Lake Chapala, placed inside of a plastic bag, and incubated at room temperature for one week. Seven moldy plants were selected from this bag to isolate fungi.
Fragments (approximately 1 cm) of each moldy plant were aseptically placed in the center of four Petri dishes containing the isolation culture medium. The Petri dishes (in duplicate) were incubated at 30 °C and 45 °C for 48 h. Subsequently, colonies with different morphologies were subcultured for further purification using the streaking method. To obtain axenic cultures, seven successive propagations were carried out using new Petri dishes containing the aforementioned medium and incubated at 30 °C or 45 °C for 48 h. The isolated fungal strains were preserved at 4 °C.
2.3. Inoculum Preparation
The culture medium for inoculum preparation had the same composition as the culture medium used for the isolation of the fungi, except that no chloramphenicol was added and the WH was not fresh, but dried and ground into a fine particle size (between 0.420 and 0.841 mm), adding 10 g/L. The pH was adjusted to 6.5, and 50 mL samples of the culture medium were placed into 250 mL Erlenmeyer flasks and sterilized at 121 °C for 15 min. The medium surface was inoculated using a spore suspension of the fungal strain preserved at 4 °C. Flasks were incubated at 30 °C for five days. The spores were then harvested by adding 50 mL of a Tween 80 solution (0.01% w/v) with magnetic stirring for 30 min. Spores were counted with a Neubauer chamber using the microscope (40-X).
2.4. Screening of Fungal Strains Producing Cellulases and Xylanases
Enzyme production was tested by culturing the recently isolated fungal strains on solid-state fermentations (SSF), using water hyacinth as the support, with a particle size ranging from 0.841 to 2 mm. The culture medium utilized was the aforementioned isolation medium, omitting WH, agar, and chloramphenicol. A blue-green fungal strain (with a velvety to powdery surface texture) was selected on the basis of CMCase and xylanases production. The isolated fungus was preserved on slants at 4° C and sub-cultured every three months. The culture medium for preservation was that mentioned above (isolation medium).
2.5. Molecular Identification of the Isolated Fungal Strain
For identification, the selected fungal strain (labeled as ABQ1) was grown in 250 mL Erlenmeyer flasks containing 50 mL of potato dextrose broth at 30 °C and 170 rpm for 48 h. Subsequently, mycelium in the form of pellets was vacuum-filtered through a Buchner funnel containing No. 1 Whatman filter paper, washed with sterile distilled water, and stored at −20 °C. Genomic DNA was extracted from the pellets using a commercial DNA extraction kit (GenElute, Sigma-Aldrich). Molecular identification was performed by PCR amplification of the ITS1-5.8S-ITS2 rDNA region. The amplified products of ITS1 and ITS4 were compared with other sequences deposited in the National Center for Biotechnology Information (NCBI) database using the basic local alignment tool (BLAST, http://www.ncbi.nlm.nih.gov, accessed on 29 May 2023).
2.6. Radial Growth Rate Determination of P. crustosum ABQ1
P. crustosum ABQ1 was inoculated on potato dextrose agar plates at the central location of the plate using a toothpick containing fungal spores, which was pierced into the PDA medium. The radial growth rate (expressed in µm/h) was determined by measuring the diameter of the fungal colony as a function of time at different incubation temperatures (5, 10, 15, 20, 25, 30, 33, and 35 °C). The experiments were performed in triplicate.
2.7. Solid-State Fermentation for Cellulase and Xylanase Production Using Penicillium crustosum ABQ1 and a Non-Pretreated Support
The culture medium for solid-state fermentation (SSF), using non-pretreated water hyacinth biomass as a support (dried, ground, and sieved through a 20 mesh) had the following compositions (g/L): (A) glucose or xylose, 5; (NH4)2SO4, 4.73; KH2PO4, 1.98; MgSO4, 0.25; CaCl2, 0.39; NaCl, 0.13; and FeSO4∙7H2O, 0.091. (B) Glucose or xylose, 30; (NH4)2SO4, 9.46; KH2PO4, 3.96; MgSO4, 0.50; CaCl2, 0.78; NaCl, 0.26; and FeSO4∙7H2O, 0.182.
Two grams of dried water hyacinth (DWH), ranging in size from 0.420 to 0.841 mm, were placed into 250 mL Erlenmeyer flasks and sterilized at 121 °C for 15 min. Then, 150 mL of the sterilized culture medium was inoculated with Penicillium crustosum ABQ1 spores to reach 7.5 × 106 spores per milliliter (for a final concentration of 3 × 107 spores per gram of DWH) and distributed at a rate of 8 mL per flask. This mixture was thoroughly homogenized using a spatula. SSF was performed at 80% w/w of humidity and 30 °C. Two flasks were taken every 24 h. The fermented solids were placed into plastic bags and stored at −20 °C for further analysis.
2.8. Solid-State Fermentation for Cellulase and Xylanase Production Using Penicillium crustosum ABQ1 and a Pretreated Support
The concentrated culture medium for SSF, using pretreated support (DWH), had the following composition (g/L): (NH4)2SO4 30.96, KH2PO4, 12.92; and MgCl2, 1.23.
The dried water hyacinth (DWH) was thermochemically pretreated as follows: 40 g of DWH (with a particle sizes ranging from 0.420 to 0.841 mm) was placed into a plastic bag and 80 mL of H2SO4 0.6 M (w/v) was added and homogenized, applying manual pressure to fully incorporate the acid solution into the DWH. The mixture was placed into a beaker and autoclaved at 121 °C (15 psi) for 20 min following a sudden decompression. After cooling, 40 mL of 2.4 M NaOH solution was added, mixing thoroughly to neutralize the pH. This mixture was referred to as the pretreated support. To investigate the effect of the H2SO4 concentration in the DWH pretreatment on CMCase and xylanase production by SSF, DWH was pretreated using different H2SO4 concentrations (0.2, 0.6, and 1 M) and different NaOH concentrations (0.4, 2.4, and 4 M), respectively, to neutralize them.
For a final concentration of 7.5 × 106 spores per gram of DWH, 40 mL of sterilized concentrated culture medium was inoculated with 3 × 108 spores and added to the pretreated support (160 g). After manual homogenization of the solid mixture, 10 g samples were distributed in 250 mL Erlenmeyer flasks and incubated at 30 °C. Two flasks were removed every 24 h. The fermented solids were placed into plastic bags and stored at −20 °C for further analysis.
Considering the pretreatment that resulted in maximum production of CMCase and xylanase, the effect of the DWH particle size (ranging from 0.841 to 2 mm) and the inoculum size (3 × 107 spores/g DWH) on the CMCase and xylanase production were also studied.
2.9. Assay Techniques
The humidity and dry matter were determined by drying 5 g of fermented solid matter at 100 °C to a constant weight (24 h).
pH was measured by adding 1 g of fermented solid and 10 mL of distilled water into a 50 mL centrifuge tube. The mixture was vigorously stirred for 10 min, and then the pH was measured with a potentiometer (LAQUA, HORIBA Scientific, Kyoto, Japan).
The protein concentration in the enzyme extracts was determined using the Bradford method [46].
2.10. Analysis of Sugars by HPLC
Monosaccharides released by acid-thermal pretreatment of the water hyacinth (after NaOH neutralization) were identified and quantified by high-performance liquid chromatography (HPLC, Waters®, Milford, MA, USA) (Waters 600 system) with refractive index detector model 2414. An Aminex HPX-87P column (BioRad, Dubai, United Arab Emirates) was operated at 80 °C and 0.6 mL/min flux, with HPLC water as the mobile phase. The injection volume was 20 µL. Samples were diluted 1:10 and filtered through a 0.22 µm membrane before being injected. Glucose, xylose, arabinose, galactose, and mannose standards were prepared at 1 g/L.
2.11. CMCase and Xylanases Assays
For the enzymatic assays, a crude extract was prepared as follows: 1 mL of citrate buffer (0.1 M, pH 5.3) was added to 0.3 g of the fermented solid in 2 mL microtubes. The microtubes were shaken vigorously in a vortex for 1 h and then centrifuged at 4500 rpm for 10 min. The supernatants were recovered (enzymatic extracts).
CMCase and xylanase activities were determined by measuring the release of reducing sugars (RS) from carboxymethyl cellulose (CMC) and xylan using the 3,5-dinitrosalicylic acid (DNS) method [47], with glucose and xylose, respectively, as standards. Enzyme activity was determined by mixing 20 μL of appropriately diluted enzymatic extract and 180 μL of a substrate solution (1% w/v CMC or 1% w/v beechwood xylan, dissolved in citrate buffer 0.1 M, pH 5.3) into a glass tube and incubating at 50 °C for 10 min. The reactions were stopped by placing tubes in an ice water bath, adding 50 μL of NaOH 1.6 N and 250 μL of the DNS reagent, and mixing with a vortex after each addition. The tubes were then heated in a boiling water bath for 5 min and cooled down to room temperature; then, 2 mL of distilled water were added to each tube and mixed with a vortex. Optical density was measured at 540 nm using a spectrophotometer (DR/2010, HACH, Loveland, COLO, USA). Controls were prepared with distilled water instead of the enzyme extract. Each reaction was performed in triplicate. One unit (U) of cellulase (CMCase) or xylanase activity was defined as the amount of enzyme that released 1 μmol of RS, corresponding to glucose or xylose, respectively, per minute under the assay conditions.
2.12. Effect of Temperature and pH on the Activity and Stability of CMCase and Xylanases from the Crude Enzymatic Extract of Penicillium crustosum
The activity and stability of CMCase and xylanase as a function of temperature and pH were studied using crude enzymatic extracts obtained from the SSF of Penicillium crustosum ABQ1, with pretreated water hyacinth biomass as the carbon source, support, and inducer. PMSF 1 mM and chloramphenicol 100 ppm were added to each extract. The CMCase and xylanase activities were evaluated at different temperatures (from 5 to 90 °C, with intervals of 5 °C) and pH values (from 3 to 9, with intervals of 0.5). For pH assays, citrate buffer (from 3 to 7) and Tris-HCl (from 7.5 to 9) were prepared at 0.1 mM. Thermostability was tested at 10, 30, 40, 50, 60, and 70 °C, keeping a constant pH at 5.3. The crude enzymatic extract was incubated at the target temperature, taking samples at certain time intervals and assaying residual enzymatic activities (at 50 °C and pH 5.3 for 10 min). Deactivation energies were calculated using the Arrhenius equation, where the slope of the approximately linear part of each curve was considered as the value of k, which represented the rate constant. All assays were performed in triplicate.
3. Results and Discussion
3.1. Isolation and Identification of a Fungal Strain Producing Cellulases and Xylanases
Nine mesophilic fungal strains (at 30 °C) and five thermophilic fungal strains (at 45 °C) were isolated from samples of decaying water hyacinth. Of these, one strain was selected based on its CMCase and xylanase production by solid-state fermentation (results not shown).
Taxonomic identification of Penicillium crustosum ABQ1, isolated from water hyacinth collected from the shore of Lake Chapala, was performed by sequencing the amplified products of ITS1, ITS2, and 5.8S rDNA. A sequence of 610 bp from the ribosomal ITS region of P. crustosum ABQ1 was closely related to other P. crustosum strains (99.8% of similarity), according to the NCBI database. This sequence was deposited in GenBank under the accession number NR_077153.1. In addition, a neighbor-joining phylogenetic tree was created based on the alignment of sequences from the ribosomal genes of several Penicillium species obtained from the GenBank NCBI database, with the assistance of the MEGA v. 6.0 software (Figure 1) [44].
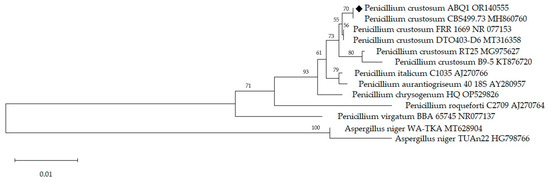
Figure 1.
Phylogenetic tree constructed from sequences of regions ITS1-5.8S-ITS2 of Penicillium crustosum ABQ1, compared to sequences of other fungal strains from the GenBank NCBI database. The Aspergillus niger strain was used as the outgroup. The names of fungal species are supplemented with the GenBank accession number. The scale represents 0.01 substitutions per nucleotide position, and bootstrap values are based on 500 replicates.
3.2. Characterization of the Radial Growth Rate of P. crustosum ABQ1
P. crustosum ABQ1 was grown on Petri dishes containing potato dextrose agar at different incubation temperatures to characterize its growth rate as a function of temperature. In Figure 2, it can be noted that P. crustosum ABQ1 was able to grow at low temperatures. Measurements of hyphal extension rates (radial growth rate) indicated that this fungal strain can be considered as psychrotolerant, since it is capable of growing at 5 °C [48]. The radial growth rate, measured at 5 °C (62 μm/h), corresponded to 35% of the maximum radial growth rate reached at 25 °C (177 μm/h). Additionally, the activation and deactivation energies of 535.63 J/mol and 4887.63 J/mol, respectively, were calculated using the Arrhenius equation.
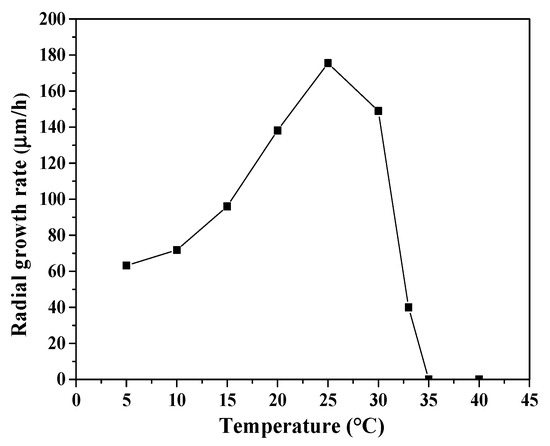
Figure 2.
Effect of incubation temperature on the radial growth rate of Penicillium crustosum ABQ1. The error bars represent the standard deviation (n = 3), but are not shown when they are smaller than the symbols.
3.3. Cellulase and Xylanase Production of P. crustosum ABQ1 by Solid-State Fermentation (SSF), Using Glucose and Xylose as Starters for Fungal Growth
To promote initial fungal growth, P. crustosum ABQ1 was cultured on SSF, using glucose and xylose as carbon sources at two impregnating culture medium concentrations (5 and 30 g/L). It is worth noting that (i) natural water hyacinth biomass (non-pretreated) was used as the main carbon source, support for SSF, and inducer for cellulase and xylanase synthesis; and (ii) the culture medium with a high monosaccharide concentration (30 g/L) contained twice the concentrations of the other components of the culture medium with respect to the culture medium with a low monosaccharide concentration (5 g/L).
Cellulases (CMCase) and xylanases were simultaneously produced by P. crustosum ABQ1 cultured on SSF. Using glucose or xylose as the starter for fungal growth, the maximum level of enzyme production was achieved between 24 and 48 h of culture (Figure 3). Glucose was a suitable carbon source with which to initiate fungal growth and then to promote the synthesis of CMCase and xylanase. Both glucose concentrations were equally convenient to promote enzyme synthesis. On the other hand, xylose at a concentration of 30 g/L more effectively stimulated CMCase and xylanase production, while with xylose at 5 g/L, xylanases were barely produced and CMCase was not produced. After 72 h, xylanase activity decreased gradually over the culture time, while CMCase activity declined more rapidly.
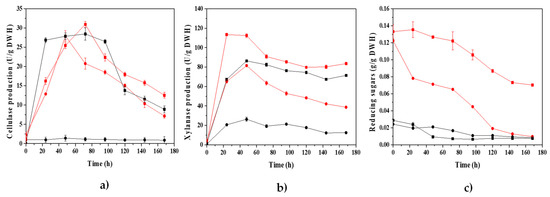
Figure 3.
Kinetics of cellulase (CMCase) (a) and xylanase (b) production, as well as reducing sugars consumption (c) of P. crustosum ABQ1, on solid-state fermentation using culture media containing glucose 5 ( ) and 30 (
) and 30 ( ) g/L, or xylose 5 (
) g/L, or xylose 5 ( ) and 30 (
) and 30 ( ) g/L. Data represent the mean and the standard deviation of three assays.
) g/L. Data represent the mean and the standard deviation of three assays.
 ) and 30 (
) and 30 ( ) g/L, or xylose 5 (
) g/L, or xylose 5 ( ) and 30 (
) and 30 ( ) g/L. Data represent the mean and the standard deviation of three assays.
) g/L. Data represent the mean and the standard deviation of three assays.
For SSF, when xylose at 5 and 30 g/L and glucose at 5 g/L were used, reducing sugars were gradually consumed and, by the end of the cultures, the sugars were almost depleted. On the other hand, with glucose at 30 g/L, the reducing sugars were partially consumed, which revealed that the rate of consumption of sugars by P. crustosum ABQ1 was lower than the rate of release of sugars due to hydrolysis of the water hyacinth biomass by the action of cellulolytic and xylanolytic enzymes synthesized by the fungus during SSF (Figure 3c).
The maximum activity levels of CMCase produced by P. crustosum ABQ1 were 28.43 and 30.93 U/g dry water hyacinth (DWH), obtained at 5 and 30 g/L of glucose, respectively; while the maximum activity levels of xylanases were 86.35 and 113.46 U/g DWH, obtained at 5 and 30 g/L of glucose, respectively. Both enzyme productions were comparable to values reported by other researchers using SSF [49,50,51,52,53].
3.4. Cellulase and Xylanase Production of P. crustosum ABQ1 by Solid-State Fermentation (SSF) Using Chemically Pretreated Water Hyacinth Biomass as the Support, Carbon Source, and Inducer
Pretreatment of lignocellulosic biomass is a strategy to reduce its recalcitrant nature and to make cellulose an inducible feedstock for cellulase synthesis. This pretreatment promotes lignin separation and hemicellulose hydrolysis, and reduces cellulose crystallinity, to expose the maximum cellulose content to microorganisms [54]. In order to make the acid pretreatment more efficient [31], the water hyacinth biomass was ground to reach fine particle sizes (from 0.420 to 0.841 mm).
Three acid-thermal pretreatments were applied to water hyacinth biomass at the following H2SO4 concentrations: 0.2, 0.6, and 1 M. These pretreatments were performed at 121 °C for 20 min. Once the reactions were completed, NaOH was added to the pretreated biomasses at quantities sufficient to raise the pH to 6.5.
P. crustosum ABQ1 was cultured by SSF using an inoculum size of 7.5 × 106 spores/g DWH and a chemically pretreated water hyacinth biomass as the support, carbon source, and inducer for cellulase and xylanase synthesis. For SSF, the culture medium was modified as follows: monosaccharides, trace elements, CaCl2, NaCl, and FeSO4 were eliminated. Thus, the components of the impregnating culture medium were reduced to only three inexpensive chemical compounds: (NH4)2SO4, KH2PO4, and MgCl2.
As shown in Figure 4a,b, the maximum production of cellulases (CMCase) and xylanases (142.89 and 574.86 U/g DWH, respectively) increased with SSF using water hyacinth biomass pretreated at 0.2 M H2SO4 when compared to the enzyme productions obtained for the best SSF performed with non-pretreated water hyacinth biomass (natural WH). Nevertheless, the enzyme production levels were astoundingly augmented in SSF when water hyacinth biomass was pretreated with 0.6 M H2SO4. In fact, CMCase and xylanase production increased to reach maximum values of 647.51 and 4257.35 U/g DWH, respectively, after 96 h of culture. These spectacular values of enzymatic activities are among the highest values reported to date [31,32,55]. Our enzymatic production levels compared to other works using Penicillium species cultured on SSF are even more outstanding. For instance, a mutant strain of P. oxalicum achieved 70 and 850 U/gds of CMCase and xylanase activity, respectively, with alkali-peroxide-pretreated sugarcane bagasse. P. purpurogenum produced 1097 U/gds of xylanase activity with alkali-pretreated corn cobs. P. echinolatum produced 282.36 and 10 U/gds of cellulase and xylanase activity, respectively, with a mixture of pretreated sugar cane bagasse and wheat bran. P. citrinum NCIM 768, when co-cultured with Trichoderma reesei NCIM 1186, produced 6.71 FPU/gds with steam-pretreated wheat bran [40,56,57,58].
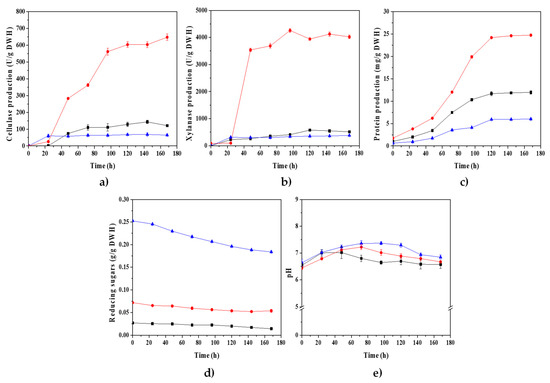
Figure 4.
Kinetics of cellulases (CMCase) (a) and xylanases (b), protein production (c), sugar consumption (d), and pH (e) of P. crustosum ABQ1 cultured on SSF. Water hyacinth biomass was subjected to three acid-thermal pretreatments using H2SO4 solutions at 0.2 M ( ), 0.6 M (
), 0.6 M ( ), and 1 M (
), and 1 M ( ), and heated at 121 °C for 20 min. The dry water hyacinth (DWH)-pretreated biomass was used as the carbon source, support, and inducer for the synthesis of the enzymes. Data represent the means and the standard deviations of three assays.
), and heated at 121 °C for 20 min. The dry water hyacinth (DWH)-pretreated biomass was used as the carbon source, support, and inducer for the synthesis of the enzymes. Data represent the means and the standard deviations of three assays.
 ), 0.6 M (
), 0.6 M ( ), and 1 M (
), and 1 M ( ), and heated at 121 °C for 20 min. The dry water hyacinth (DWH)-pretreated biomass was used as the carbon source, support, and inducer for the synthesis of the enzymes. Data represent the means and the standard deviations of three assays.
), and heated at 121 °C for 20 min. The dry water hyacinth (DWH)-pretreated biomass was used as the carbon source, support, and inducer for the synthesis of the enzymes. Data represent the means and the standard deviations of three assays.
Remarkably, for longer culture times, the enzymes production remained relatively constant, without any apparent loss of enzyme activities. These massive enzyme productions could be related to the availability of monosaccharides (as carbon sources) released during the acid hydrolysis of the water hyacinth biomass, but they could also be related mostly to the release of oligosaccharides, which function as the true inducers of the cellulase and xylanase synthesis [59,60]. On the other hand, for the SSF, using water hyacinth biomass pretreated at 1 M H2SO4, the maximal production of CMCase and xylanase decreased (68.36 and 379.34 U/g DWH, respectively).
Even though high monosaccharide concentrations were released during the acid pretreatment with H2SO4 1 M, the concentration of oligosaccharides was reduced compared to that reached with the 0.6 M concentration (Table 1), which could be the reason for the decreased synthesis of enzymes. Another reason that cannot be ignored is that various microbial growth inhibitors (mainly weak acids, furan derivatives, and phenolic compounds) could be generated, as the acid pretreatment was more severe. Indeed, regarding water hyacinth biomass pretreatment at 1 M H2SO4, less abundant fungal growth was perceived during SSF compared to the fungal growth achieved with 0.6 M H2SO4 pretreatment (results not shown).

Table 1.
Percentages of released sugars assayed by HPLC after subjecting the water hyacinth biomass to acid-thermal pretreatments at three concentrations of sulfuric acid (0.2, 0.6, and 1 M).
As the sulfuric acid concentration increased in the pretreatment to which the water hyacinth biomass was subjected, the hemicellulose was increasingly hydrolyzed. Indeed, the released reducing sugar (Figure 4d, at the beginning of fungal culture), monosaccharide (arabinose, xylose, and galactose), and oligosaccharide concentrations increased. Table 1 shows the percentage of each liberated sugar, as determined by HPLC, related to each H2SO4 concentration used in the pretreatment. The monosaccharides and oligosaccharides released from the WH biomass allowed for an easy assimilation of fungal growth during SSF and a notoriously improved enzyme production level.
In Figure 4d, it can be appreciated, at the beginning of the fungal culture, that, the thermochemical pretreatment applied to WH biomass released more reducing sugars (RS) as the H2SO4 concentration was increased. The RS released after pretreatments at 0.2, 0.6, and 1 M H2SO4 were 37.37, 95.46, and 252.42 mg RS/g DWH, respectively. Since the RS concentrations remained relatively constant during the SSF using the three pretreated biomasses, it could be assumed that the enzymatic hydrolysis rates of polysaccharides (cellulose and hemicellulose) from the WH-pretreated biomass were higher than the fungal consumption rates of RS.
On the other hand, protein production during SSF seemed to be related to enzyme production (Figure 4c). Therefore, it could be considered a good indicator of CMCase and xylanases production during the SSF of P. crustosum ABQ1. It is worth noting that the pH remained relatively constant (7 ± 0.5), suggesting that high concentrations of organic acids were not produced during the SSF (Figure 4e).
3.5. Effects of the Particle Size of Water Hyacinth-Pretreated Biomass and the Inoculum Size on Cellulase and Xylanase Production
Taking into consideration, the best result obtained regarding the enzyme production (pretreatment of water hyacinth biomass with H2SO4 0.6 M and 121 °C for 20 min, a particle size range of WH biomass of 0.42 to 0.841 mm, and an inoculum size of 7.5 × 106 spores/g DWH), the effects of a WH biomass particle size range of 0.841 to 2.00 mm and an inoculum size of 3 × 107 spores/g DWH on cellulose and xylanase production were explored (Figure 5). For the study of the inoculum size, a WH biomass particle size range of 0.420 to 0.841 mm was selected, while for the particle size study, an inoculum size of 7.5 × 106 spores/g DWH was chosen.
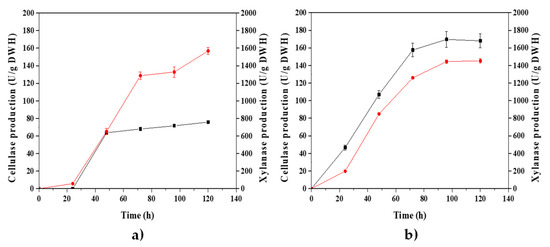
Figure 5.
Kinetics of cellulase (CMCase,  ) and xylanase (
) and xylanase ( ) production of P. crustosum ABQ1 cultured on SSF, using a WH biomass particle size range of 0.841 and 2.00 mm (a) and an inoculum size of 3 × 107 spores/g DWH (b). WH biomass was pretreated (at H2SO4 0.6 M and 121 °C for 20 min) and used as the carbon source, support, and inducer for the synthesis of the enzymes. Data represent the means and the standard deviations of three assays.
) production of P. crustosum ABQ1 cultured on SSF, using a WH biomass particle size range of 0.841 and 2.00 mm (a) and an inoculum size of 3 × 107 spores/g DWH (b). WH biomass was pretreated (at H2SO4 0.6 M and 121 °C for 20 min) and used as the carbon source, support, and inducer for the synthesis of the enzymes. Data represent the means and the standard deviations of three assays.
 ) and xylanase (
) and xylanase ( ) production of P. crustosum ABQ1 cultured on SSF, using a WH biomass particle size range of 0.841 and 2.00 mm (a) and an inoculum size of 3 × 107 spores/g DWH (b). WH biomass was pretreated (at H2SO4 0.6 M and 121 °C for 20 min) and used as the carbon source, support, and inducer for the synthesis of the enzymes. Data represent the means and the standard deviations of three assays.
) production of P. crustosum ABQ1 cultured on SSF, using a WH biomass particle size range of 0.841 and 2.00 mm (a) and an inoculum size of 3 × 107 spores/g DWH (b). WH biomass was pretreated (at H2SO4 0.6 M and 121 °C for 20 min) and used as the carbon source, support, and inducer for the synthesis of the enzymes. Data represent the means and the standard deviations of three assays.
As can be observed in Figure 5, the maximum productions of cellulases (CMCase) and xylanases (at 96 h) decreased for a WH biomass particle size of 0.841 to 2.00 mm (75.69 and 1568.48 U/g DWH, respectively) and for an inoculum size of 3 × 107 spores/g DWH (170.79 and 1515.34 U/g DWH, respectively) when compared to those values obtained for the SSF performed at 7.5 × 106 spores/g DWH and 0.420 to 0.841 mm (Figure 4, WH-pretreated biomass with H2SO4 0.6 M). These results reveal the importance of using a fine particle size to improve heat transport in the WH biomass during pretreatment and, thus, to facilitate the hydrolysis of the lignocellulosic matrix. Additionally, smaller particle sizes provide a larger surface and better support for fungal growth on SSF, as well as superior gas and heat exchange [61].
Furthermore, the reduction in enzyme production, which was obtained at high spore concentration of P. crustosum ABQ1 (3 × 107 spores/g DWH) and used to inoculate the SSF, might be attributed to poor germination due to high spore density. As was previously reported by Gillot et al. [62], the spore germination of P. camemberti is self-regulated by quorum sensing, with 1-octanol as the main volatile compound produced at a high spore density. These researchers found that an inoculum size of 1 × 106 spores/mL led to a germination percentage of around 100%, while at 1 × 108 spores/mL, germination was almost negligible. The inoculum sizes used in this work were 7.5 × 106 and 3 × 107 spores/g DWH, which correspond to 1.87 × 106 and 7.50 × 106 spores/mL of culture medium impregnated in the dry water hyacinth, respectively.
3.6. Effects of Temperature and pH on Cellulase and Xylanase Activity and Stability Using the Crude Enzymatic Extract of P. crustosum ABQ1
The effects of incubation temperature and pH on the activity and thermostability of CMCase and xylanase were studied using the crude enzymatic extract of P. crustosum ABQ1, which was obtained by SSF with chemically pretreated water hyacinth biomass as the support, carbon source, and inducer. Figure 6a shows optimum temperatures of 55 and 50 °C for CMCase and xylanase activities, respectively, while Figure 6b shows a plateau of optimum pH around 5 ± 0.5 for both types of enzyme activities. The calculated values of activation and deactivation energies were 344.82 and 1285.47 J/mol and 533.47 and 1512.06 J/mol for CMCase and xylanase activities, respectively.
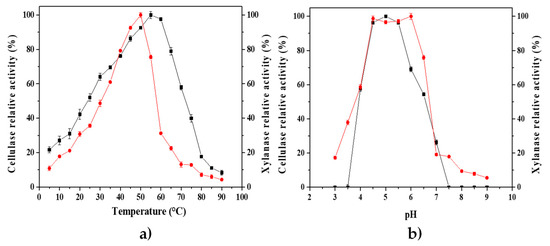
Figure 6.
Effects of temperature (a) and pH (b) on the hydrolytic activity of P. crustosum enzymatic extract, obtained from SSF with WH-pretreated biomass. Cellulase (CMCase,  ) and xylanase (
) and xylanase ( ) activities were measured at different temperatures (from 5 to 90 °C) and pH values (from 3 to 9). Data represent the means and the standard deviations of three assays.
) activities were measured at different temperatures (from 5 to 90 °C) and pH values (from 3 to 9). Data represent the means and the standard deviations of three assays.
 ) and xylanase (
) and xylanase ( ) activities were measured at different temperatures (from 5 to 90 °C) and pH values (from 3 to 9). Data represent the means and the standard deviations of three assays.
) activities were measured at different temperatures (from 5 to 90 °C) and pH values (from 3 to 9). Data represent the means and the standard deviations of three assays.
The thermostability of P. crustosum ABQ1 enzyme extract, obtained during SSF with water hyacinth-pretreated biomass, is shown in Figure 7. At 10 and 30 °C, the xylanase activity was largely stable, while CMCase lost 8 and 62% of its activity after 200 h, respectively. Regarding the thermostabilities at 40, 50, 60, and 70 °C, CMCase and xylanase showed the following half-lives: 23.52, 12.61, 0.71, and 0.04 h; and 6.45, 2.37, 0.27, and 0.02 h, respectively. The deactivation energy values for CMCase and xylanase were 2008.66 and 2736.44 J/mol, respectively (Figure 7).
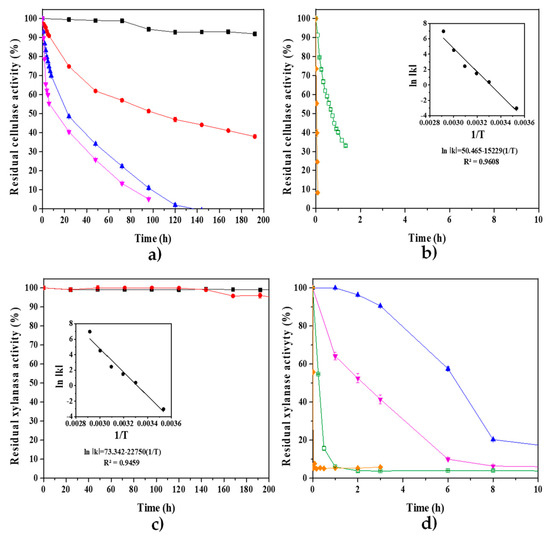
Figure 7.
Thermostability of cellulase (CMCase, (a,b)) and xylanase (c,d) of P. crustosum ABQ1 enzyme extract, obtained during SSF with WH-pretreated biomass. The evaluated incubation temperatures were 10 ( ), 30 (
), 30 ( ), 40 (
), 40 ( ), 50 (
), 50 ( ), 60 (
), 60 ( ), and 70 (
), and 70 ( ) °C. The pH was set at 5.3. The residual enzyme activity was assayed at pH 5.3 and 50 °C. The error bars represent the standard deviation (n = 3), but are not shown when they are smaller than the symbols.
) °C. The pH was set at 5.3. The residual enzyme activity was assayed at pH 5.3 and 50 °C. The error bars represent the standard deviation (n = 3), but are not shown when they are smaller than the symbols.
 ), 30 (
), 30 ( ), 40 (
), 40 ( ), 50 (
), 50 ( ), 60 (
), 60 ( ), and 70 (
), and 70 ( ) °C. The pH was set at 5.3. The residual enzyme activity was assayed at pH 5.3 and 50 °C. The error bars represent the standard deviation (n = 3), but are not shown when they are smaller than the symbols.
) °C. The pH was set at 5.3. The residual enzyme activity was assayed at pH 5.3 and 50 °C. The error bars represent the standard deviation (n = 3), but are not shown when they are smaller than the symbols.
It is worth noting that the crude enzymatic extract is active and thermostable at low temperatures, which coincides with the low incubation temperatures that favor the growth of P. crustosum ABQ1 (Figure 2). Work is ongoing to use the P. crustosum ABQ1 enzyme extract in the simultaneous saccharification of lignocellulosic residues (mostly WH) and alcoholic fermentation with mesophilic yeasts.
4. Conclusions
Penicillium crustosum ABQ1 was newly isolated from Chapala Lake in Jalisco, Mexico, showing to be a promising and efficient CMCase and xylanase producer under solid-state fermentation.
Water hyacinth (WH) is a cost-effective substrate which can also be used as a support for solid-state fermentation, as well as an inducer for cellulase and xylanase synthesis.
The thermochemical pretreatment of the water hyacinth biomass promoted hemicellulose hydrolysis and provided mono- and oligo-saccharides to encourage fungal growth and enzyme production, as well as an enhanced accessible surface area for solid-state fermentation (SSF).
A low-cost solid culture medium was formulated based on WH-pretreated biomass impregnated with a saline solution containing only (NH4)2SO4, KH2PO4, and MgCl2.
The maximum CMCase and xylanase production levels of P. crustosum cultured on SSF were obtained using the WH-pretreated biomass with H2SO4 0.6 M and 121 °C for 20 min. The simultaneous CMCase and xylanase production levels reached in this work after 96 h of culture (647.51 and 4257.35 U/g DWH, respectively) are among the highest values ever reported.
Lower levels of inoculum concentration (7.5 × 106 spores/g DWH) and smaller particle sizes of WH-pretreated biomass (0.42 to 0.841 mm) favored CMCase and xylanase production.
CMCase and xylanase showed maximum activity levels at 55 and 50 °C, respectively, and maximum activity levels at pH values around 5 ± 0.5 for both types of enzyme activities. In regard to the thermostability levels at 40, 50, 60, and 70 °C, CMCase and xylanase showed the following half-lives, respectively: 23.52, 12.61, 0.71, and 0.04 h; and 6.45, 2.37, 0.27, and 0.02 h.
Thus, the results indicated that Penicillium crustosum ABQ1 is an outstanding producer of cellulase and xylanase under SSF conditions when a very cheap and highly available substrate, support, and inducer is employed, i.e., the water hyacinth, which is considered to be a weed that is harmful to the environment.
Author Contributions
Conceptualization, J.C.; methodology, J.C.; validation, C.E.-A.; formal analysis, C.E.-A.; investigation, C.E.-A. and C.S.-S.; resources, J.C.; data curation, C.E.-A.; writing—original draft preparation, E.R.-B.; writing—review and editing, J.C. and E.R.-B.; visualization, E.R.-B.; supervision, J.C.; project administration, J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Council of Science and Technology of Jalisco (COECYTJAL) through the research project grant FODECIJAL 8155-2019 “Sustainable use of water hyacinth that thrives as weeds on the Santiago River”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to the large amount of information that needed to be synthesized to be presented in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Villamagna, A.M.; Murphy, B.R. Ecological and Socio-Economic Impacts of Invasive Water Hyacinth (Eichhornia crassipes): A Review. Freshw. Biol. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Sharma, A.; Aggarwal, N.K.; Saini, A.; Yadav, A. Beyond Biocontrol: Water Hyacinth. Opportunities and Challenges. J. Environ. Sci. Technol. 2016, 9, 26–48. [Google Scholar] [CrossRef]
- Dirar, H.A.; El Amin, H.B. Methane Fermentation of Water Hyacinth: Effect of Solids Concentration and Inoculum Source. Mircen J. Appl. Microbiol. Biotechnol. 1988, 4, 299–312. [Google Scholar] [CrossRef]
- Malik, A. Environmental Challenge Vis a Vis Opportunity: The Case of Water Hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef]
- Thamaga, K.H.; Dube, T. Remote Sensing of Invasive Water Hyacinth (Eichhornia crassipes): A Review on Applications and Challenges. Remote Sens. Appl. Soc. Environ. 2018, 10, 36–46. [Google Scholar] [CrossRef]
- Wu, H.; Ding, J. Abiotic and Biotic Determinants of Plant Diversity in Aquatic Communities Invaded by Water Hyacinth [Eichhornia crassipes (Mart.) Solms]. Front. Plant Sci. 2020, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.K.; Hernandez, J.; Holtzapple, M.T. Effects of Temperature and Pretreatment Conditions on Mixed-Acid Fermentation of Water Hyacinths Using a Mixed Culture of Thermophilic Microorganisms. Bioresour. Technol. 2010, 101, 7510–7515. [Google Scholar] [CrossRef]
- Su, W.; Sun, Q.; Xia, M.; Wen, Z.; Yao, Z. The Resource Utilization of Water Hyacinth (Eichhornia crassipes [Mart.] Solms) and Its Challenges. Resources 2018, 7, 46. [Google Scholar] [CrossRef]
- Mathur, P.; Mathur, S.M. Water Hyacinth: A Useful Plant to Improve Rural Economy. In Energy and Environment: Select Proceedings of ICWEES-2016; Springer: Singapore, 2018; pp. 31–38. [Google Scholar] [CrossRef]
- Gaurav, G.K.; Mehmood, T.; Cheng, L.; Klemeš, J.J.; Shrivastava, D.K. Water Hyacinth as a Biomass: A Review. J. Clean. Prod. 2020, 277, 122214. [Google Scholar] [CrossRef]
- Jafari, N. Ecological and Socio-Economic Utilization of Water Hyacinth (Eichhornia crassipes Mart Solms). J. Appl. Sci. Environ. Manag. 2010, 14, 43–49. [Google Scholar] [CrossRef]
- Li, F.; He, X.; Srishti, A.; Song, S.; Tan, H.T.W.; Sweeney, D.J.; Ghosh, S.; Wang, C.H. Water Hyacinth for Energy and Environmental Applications: A Review. Bioresour. Technol. 2021, 327, 124809. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Madhavan, A.; Alphonsa, J.A.; Vivek, N.; Gnansounou, E.; Castro, E.; Faraco, V. Water Hyacinth a Potential Source for Value Addition: An Overview. Bioresour. Technol. 2017, 230, 152–162. [Google Scholar] [CrossRef]
- Wilkie, A.C.; Evans, J.M. Aquatic Plants: An Opportunity Feedstock in the Age of Bioenergy. Biofuels 2010, 1, 311–321. [Google Scholar] [CrossRef]
- Karouach, F.; Ben Bakrim, W.; Ezzariai, A.; Sobeh, M.; Kibret, M.; Yasri, A.; Hafidi, M.; Kouisni, L. A Comprehensive Evaluation of the Existing Approaches for Controlling and Managing the Proliferation of Water Hyacinth (Eichhornia crassipes): Review. Front. Environ. Sci. 2022, 9, 767871. [Google Scholar] [CrossRef]
- Nigam, J.N. Bioconversion of Water-Hyacinth (Eichhornia crassipes) Hemicellulose Acid Hydrolysate to Motor Fuel Ethanol by Xylose-Fermenting Yeast. J. Biotechnol. 2002, 97, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Cheng, G.; Sathitsuksanoh, N.; Wu, D.; Varanasi, P.; George, A.; Balan, V.; Gao, X.; Kumar, R.; Dale, B.E.; et al. Comparison of Different Biomass Pretreatment Techniques and Their Impact on Chemistry and Structure. Front. Energy Res. 2015, 2, 62. [Google Scholar] [CrossRef]
- Rezania, S.; Din, M.F.M.; Mohamad, S.E.; Sohaili, J.; Taib, S.M.; Yusof, M.B.M.; Kamyab, H.; Darajeh, N.; Ahsan, A. Review on Pretreatment Methods and Ethanol Production from Cellulosic Water Hyacinth. BioResources 2017, 12, 2108–2124. [Google Scholar] [CrossRef]
- Chandel, A.K.; Chandrasekhar, G.; Silva, M.B.; Silvério Da Silva, S. The Realm of Cellulases in Biorefinery Development. Crit. Rev. Biotechnol. 2012, 32, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.-f.; Yang, G.; Jönsson, L.J. Bacterial Cellulose Production from Cotton-Based Waste Textiles: Enzymatic Saccharification Enhanced by Ionic Liquid Pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in Bioconversion of Lignocellulose: Biofuels, Platform Chemicals & Biorefinery Concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Esteghlalian, A.; Hashimoto, A.G.; Fenske, J.J.; Penner, M.H. Modeling and Optimization of the Dilute-Sulfuric-Acid Pretreatment of Corn Stover, Poplar and Switchgrass. Bioresour. Technol. 1997, 59, 129–136. [Google Scholar] [CrossRef]
- Mussatto, S.; Teixeira, J. Lignocellulose as Raw Material in Fermentation Processes. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 897–907. [Google Scholar]
- Das, A.; Ghosh, P.; Paul, T.; Ghosh, U. Production of Bioethanol as Useful Biofuel through the Bioconversion of Water Hyacinth (Eichhornia crassipes). 3 Biotech 2016, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Isarankura-Na-Ayudhya, C.; Tantimongcolwat, T.; Kongpanpee, T.; Prabkate, P.; Prachayasittikul, V. Appropriate Technology for the Bioconversion of Water Hyacinth (Eichhornia crassipes) to Liquid Ethanol: Future Prospects for Community Strengthening and Sustainable Development. EXCLI J. 2007, 6, 167–176. [Google Scholar]
- Satyanagalakshmi, K.; Sindhu, R.; Binod, P.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Bioethanol Production from Acid Pretreated Water Hyacinth by Separate Hydrolysis and Fermentation. J. Sci. Ind. Res. 2011, 70, 156–161. [Google Scholar]
- Romero-Borbón, E.; Oropeza-González, A.E.; González-García, Y.; Córdova, J. Thermochemical and Enzymatic Saccharification of Water Hyacinth Biomass into Fermentable Sugars. Processes 2022, 10, 210. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Verma, P. A Detailed Overview of Xylanases: An Emerging Biomolecule for Current and Future Prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Sajith, S.; Priji, P.; Sreedevi, S.; Benjamin, S. An Overview on Fungal Cellulases with an Industrial Perspective. J. Nutr. Food Sci. 2016, 6, 461. [Google Scholar] [CrossRef]
- Dey, P.; Rangarajan, V.; Singh, J.; Nayak, J.; Dilip, K.J. Current Perspective on Improved Fermentative Production and Purification of Fungal Cellulases for Successful Biorefinery Applications: A Brief Review. Biomass Convers. Biorefinery 2022, 12, 967–995. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current Perspective on Production and Applications of Microbial Cellulases: A Review. Bioresour. Bioprocess. 2021, 8, 95. [Google Scholar] [CrossRef]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.d.S.; Woiciechowski, A.L.; Soccol, C.R. Current Advances in On-Site Cellulase Production and Application on Lignocellulosic Biomass Conversion to Biofuels: A Review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Polizeli, M.L.T.M.; Rizzatti, A.C.S.; Monti, R.; Terenzi, H.F.; Jorge, J.A.; Amorim, D.S. Xylanases from Fungi: Properties and Industrial Applications. Appl. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J. Microbial Xylanases and Their Industrial Application in Pulp and Paper Biobleaching: A Review. 3 Biotech 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Parameswaran, B.; Pandey, A. Hydrolysis of Pretreated Rice Straw by an Enzyme Cocktail Comprising Acidic Xylanase from Aspergillus Sp. for Bioethanol Production. Renew. Energy 2016, 98, 9–15. [Google Scholar] [CrossRef]
- Hansen, G.H.; Lübeck, M.; Frisvad, J.C.; Lübeck, P.S.; Andersen, B. Production of Cellulolytic Enzymes from Ascomycetes: Comparison of Solid State and Submerged Fermentation. Process Biochem. 2015, 50, 1327–1341. [Google Scholar] [CrossRef]
- Marques, G.L.; dos Santos Reis, N.; Silva, T.P.; Ferreira, M.L.O.; Aguiar-Oliveira, E.; de Oliveira, J.R.; Franco, M. Production and Characterisation of Xylanase and Endoglucanases Produced by Penicillium Roqueforti ATCC 10110 Through the Solid-State Fermentation of Rice Husk Residue. Waste Biomass Valorization 2018, 9, 2061–2069. [Google Scholar] [CrossRef]
- Valle-Pérez, A.U.; Flores-Cosío, G.; Amaya-Delgado, L. Bioconversion of Agave Bagasse to Produce Cellulases and Xylanases by Penicillium Citrinum and Aspergillus Fumigatus in Solid-State Fermentation. Waste Biomass Valorization 2021, 12, 5885–5897. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Zhao, S.; Lin, X.; Zhang, T.; Li, C.X.; Luo, X.M.; Feng, J.X. Improvement of Cellulase and Xylanase Production in Penicillium Oxalicum under Solid-State Fermentation by Flippase Recombination Enzyme/Recognition Target-Mediated Genetic Engineering of Transcription Repressors. Bioresour. Technol. 2021, 337, 125366. [Google Scholar] [CrossRef]
- Scholl, A.L.; Menegol, D.; Pitarelo, A.P.; Fontana, R.C.; Filho, A.Z.; Ramos, L.P.; Dillon, A.J.P.; Camassola, M. Elephant Grass Pretreated by Steam Explosion for Inducing Secretion of Cellulases and Xylanases by Penicillium Echinulatum S1M29 Solid-State Cultivation. Ind. Crops Prod. 2015, 77, 97–107. [Google Scholar] [CrossRef]
- Maeda, R.N.; Barcelos, C.A.; Anna, L.M.M.S.; Pereira, N. Cellulase Production by Penicillium Funiculosum and Its Application in the Hydrolysis of Sugar Cane Bagasse for Second Generation Ethanol Production by Fed Batch Operation. J. Biotechnol. 2013, 163, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qin, X.; Luo, X.-M.; Nong, Q.; Yang, Q.; Zhang, Z.; Gao, Y.; Lv, F.; Chen, Y.; Yu, Z.; et al. Efficient Enzymatic Hydrolysis and Simultaneous Saccharification and Fermentation of Sugarcane Bagasse Pulp for Ethanol Production by Cellulase from Penicillium oxalicum EU2106 and Thermotolerant Saccharomyces cerevisiae ZM1-5. Biomass Bioenergy 2015, 77, 53–63. [Google Scholar] [CrossRef]
- Silva, N.F.D.S.; Simões, M.R.; Knob, A.; De Moraes, S.S.; Henn, C.; Da ConceiçãO Silva, J.L.; Simão, R.D.C.G.; Maller, A.; Kadowaki, M.K. Improvement in the Bleaching of Kraft Pulp with Xylanase from Penicillium Crustosum FP 11 Isolated from the Atlantic Forest. Biocatal. Biotransform. 2016, 34, 119–127. [Google Scholar] [CrossRef]
- Aksenov, A.S.; Tyshkunova, I.V.; Poshina, D.N.; Guryanova, A.A.; Chukhchin, D.G.; Sinelnikov, I.G.; Terentyev, K.Y.; Skorik, Y.A.; Novozhilov, E.V.; Synitsyn, A.P. Biocatalysis of Industrial Kraft Pulps: Similarities and Differences between Hardwood and Softwood Pulps in Hydrolysis by Enzyme Complex of Penicillium Verruculosum. Catalysts 2020, 10, 536. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Dhakar, K.; Sharma, A.; Pandey, A. Cold, PH and Salt Tolerant Penicillium Spp. Inhabit the High Altitude Soils in Himalaya, India. World J. Microbiol. Biotechnol. 2014, 30, 1315–1324. [Google Scholar] [CrossRef]
- Dias, L.M.; dos Santos, B.V.; Albuquerque, C.J.B.; Baeta, B.E.L.; Pasquini, D.; Baffi, M.A. Biomass Sorghum as a Novel Substrate in Solid-State Fermentation for the Production of Hemicellulases and Cellulases by Aspergillus niger and A. Fumigatus. J. Appl. Microbiol. 2018, 124, 708–718. [Google Scholar] [CrossRef]
- Ezeilo, U.R.; Wahab, R.A.; Mahat, N.A. Optimization Studies on Cellulase and Xylanase Production by Rhizopus oryzae UC2 Using Raw Oil Palm Frond Leaves as Substrate under Solid State Fermentation. Renew. Energy 2020, 156, 1301–1312. [Google Scholar] [CrossRef]
- Ezeilo, U.R.; Wahab, R.A.; Tin, L.C.; Zakaria, I.I.; Huyop, F.; Mahat, N.A. Fungal-Assisted Valorization of Raw Oil Palm Leaves for Production of Cellulase and Xylanase in Solid State Fermentation Media. Waste Biomass Valorization 2020, 11, 3133–3149. [Google Scholar] [CrossRef]
- Tian, M.; Wai, A.; Guha, T.K.; Hausner, G.; Yuan, Q. Production of Endoglucanase and Xylanase Using Food Waste by Solid-State Fermentation. Waste Biomass Valorization 2018, 9, 2391–2398. [Google Scholar] [CrossRef]
- Camassola, M.; Dillon, A.J.P. Cellulases and Xylanases Production by Penicillium Echinulatum Grown on Sugar Cane Bagasse in Solid-State Fermentation. Appl. Biochem. Biotechnol. 2010, 162, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Chou, Y.-P.; Wu, S.-Y.; Liu, C.-M. Pretreatment Conditions of Rice Straw for Simultaneous Hydrogen and Ethanol Fermentation by Mixed Culture. Int. J. Hydrogen Energy 2016, 41, 4421–4428. [Google Scholar] [CrossRef]
- Chaudhary, R.; Kuthiala, T.; Singh, G.; Rarotra, S.; Kaur, A.; Arya, S.K.; Kumar, P. Current Status of Xylanase for Biofuel Production: A Review on Classification and Characterization. Biomass Convers. Biorefinery 2021, 13, 8773–8791. [Google Scholar] [CrossRef]
- Sunkar, B.; Kannoju, B.; Bhukya, B. Optimized Production of Xylanase by Penicillium Purpurogenum and Ultrasound Impact on Enzyme Kinetics for the Production of Monomeric Sugars From Pretreated Corn Cobs. Front. Microbiol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Camassola, M.; Dillon, A.J.P. Production of Cellulases and Hemicellulases by Penicillium Echinulatum Grown on Pretreated Sugar Cane Bagasse and Wheat Bran in Solid-State Fermentation. J. Appl. Microbiol. 2007, 103, 2196–2204. [Google Scholar] [CrossRef]
- Lodha, A.; Pawar, S.; Rathod, V. Optimised Cellulase Production from Fungal Co-Culture of Trichoderma Reesei NCIM 1186 and Penicillium Citrinum NCIM 768 under Solid State Fermentation. J. Environ. Chem. Eng. 2020, 8, 103958. [Google Scholar] [CrossRef]
- Rastegari, A.A. Molecular Mechanism of Cellulase Production Systems in Penicillium; Elsevier B.V.: Amsterdam, The Netherlands, 2017; ISBN 9780444635013. [Google Scholar]
- Najjarzadeh, N.; Matsakas, L.; Rova, U.; Christakopoulos, P. Effect of Oligosaccharide Degree of Polymerization on the Induction of Xylan-Degrading Enzymes by Fusarium oxysporum f. Sp. Lycopersici. Molecules 2020, 25, 5849. [Google Scholar] [CrossRef]
- Krishna, C. Solid-State Fermentation Systems-An Overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Gillot, G.; Decourcelle, N.; Dauer, G.; Barbier, G.; Coton, E.; Delmail, D.; Mounier, J. 1-Octanol, a Self-Inhibitor of Spore Germination in Penicillium camemberti. Food Microbiol. 2016, 57, 8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).