Sugar Beet Pulp as Raw Material for the Production of Bioplastics
Abstract
1. Introduction
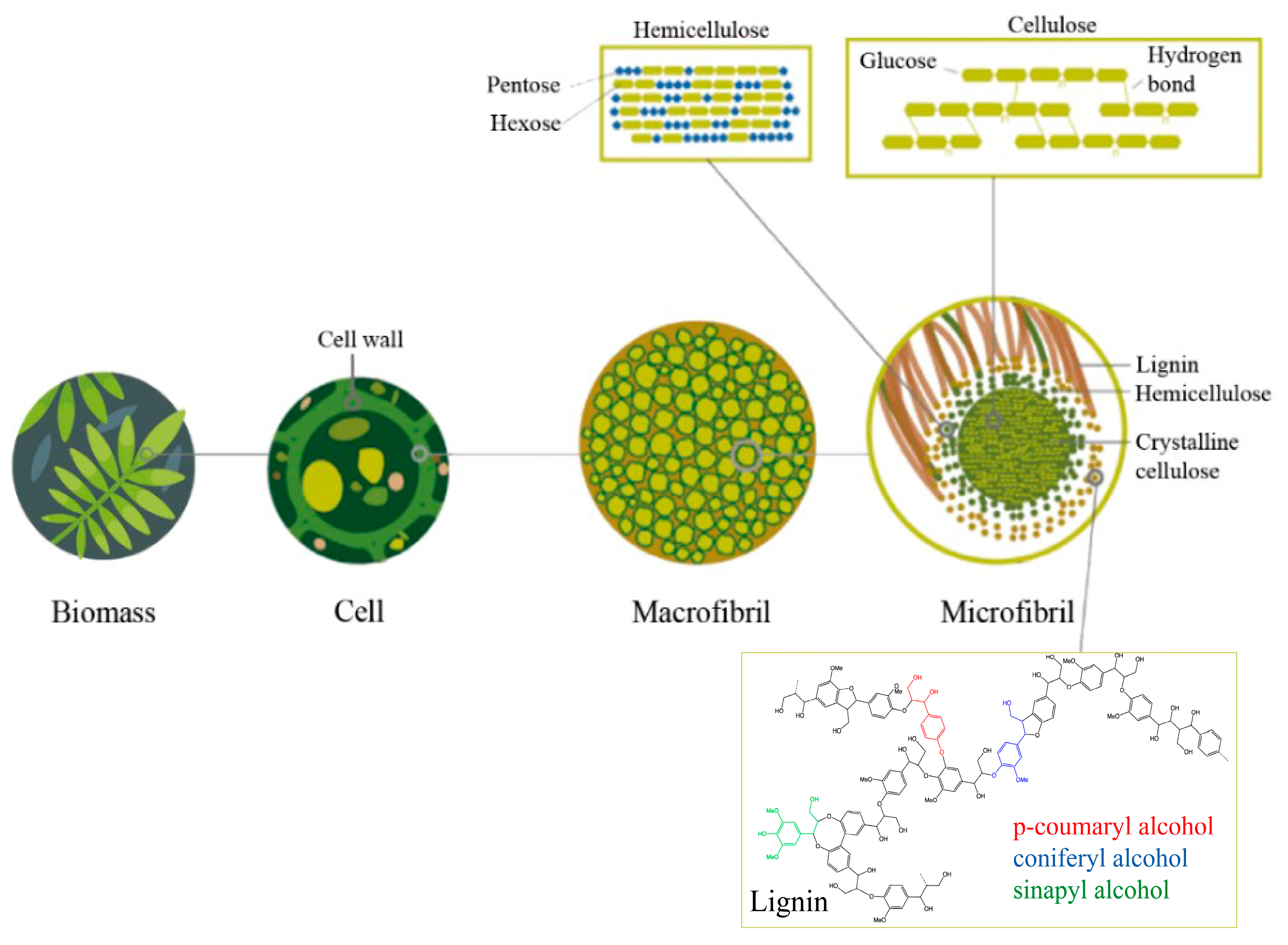
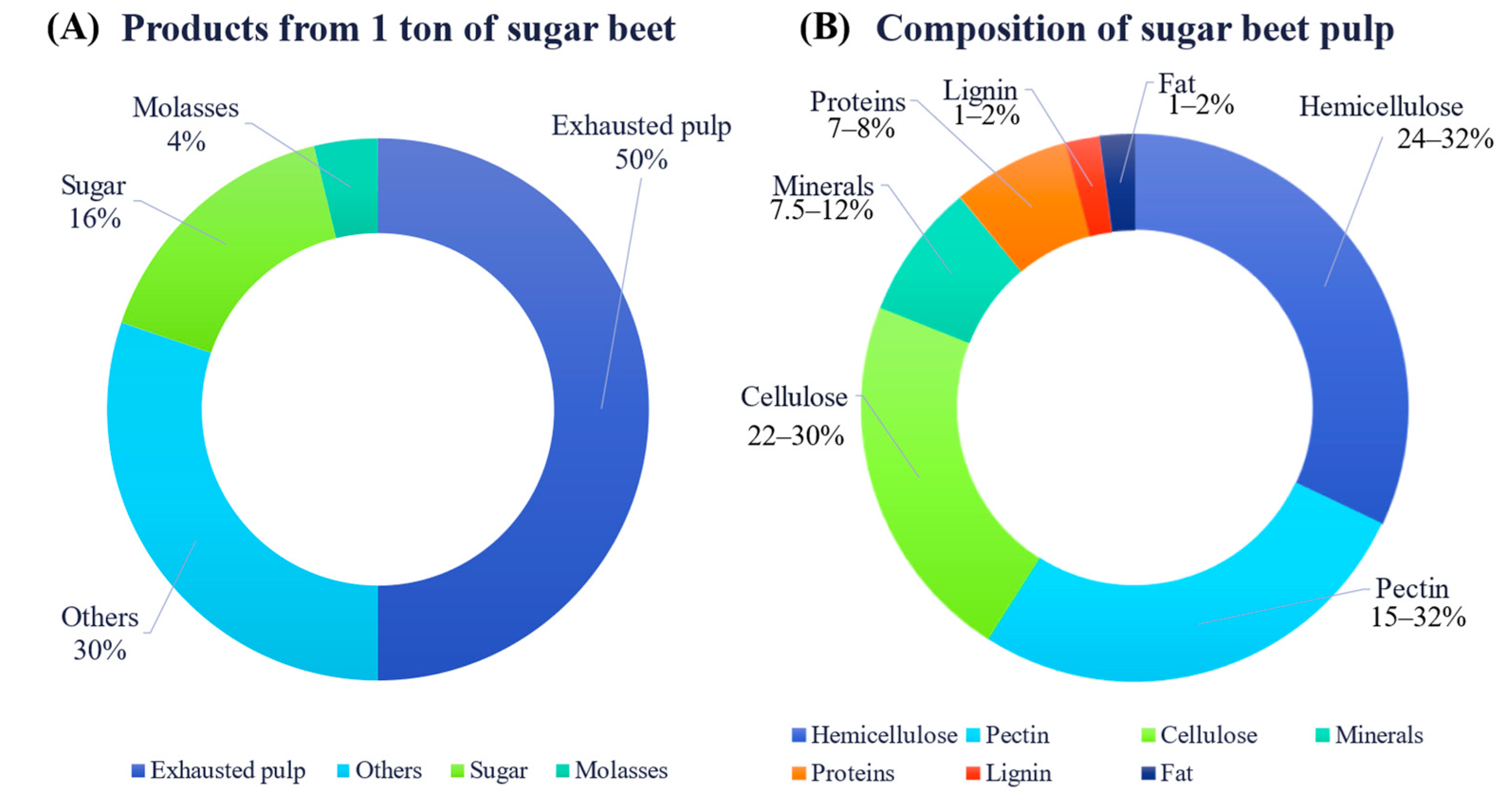
2. Exhausted Sugar Beet Pulp as a Renewable Feedstock
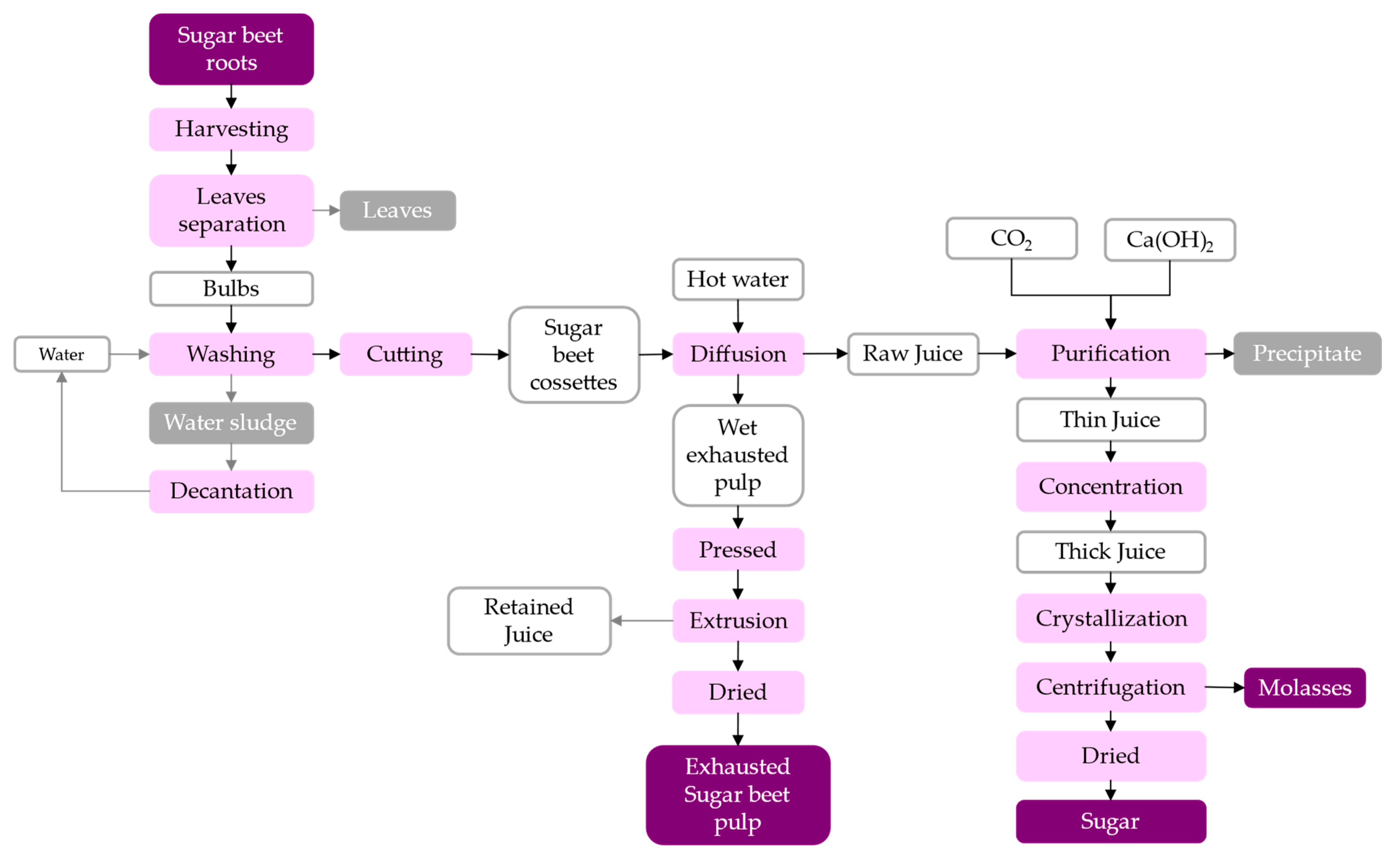
2.1. Processing of Sugar Beet
2.2. Chemical Composition of SBP
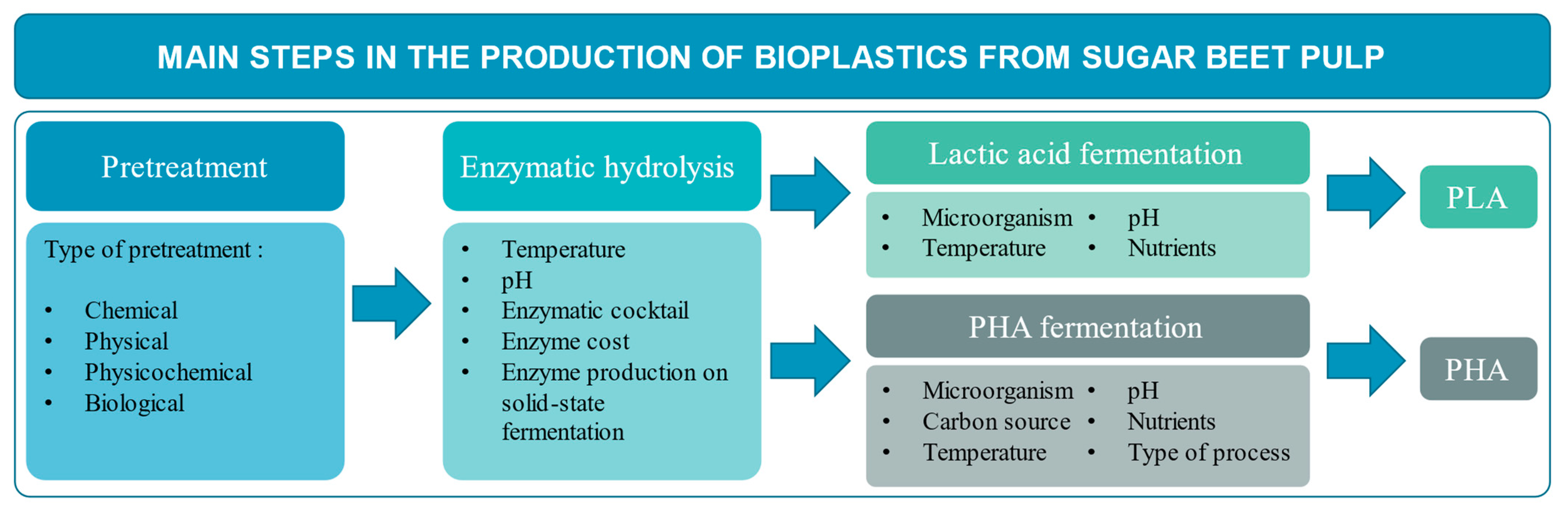
3. Production of Bioplastic Precursors from Sugar Beet Pulp
3.1. Pretreatments Applied to Sugar Beet Pulp
3.2. Enzymatic Hydrolysis of SBP
3.3. Enzyme Production by Solid-State Fermentation
| Reference | T | pH | Agitation | SL | Enzyme Type 1 | EA 2 | Pretreatment | YH |
|---|---|---|---|---|---|---|---|---|
| [58] | 40 °C | 4.8 | - | 5% w/w | Celluclast® 1.5 L Novozyme 431 | 4.2 FPU/g d.m. 28.4 CBU/g d.m. | Ammonia 0.5:1, 85 °C, 5 min | 0.50 g/g |
| 40 °C | 4.8 | - | 5% w/w | Celluclast® 1.5 L Novozyme 431 Viscozyme L | 4.2 FPU/g d.m. 28.4 CBU/g d.m. 0.85 HU/g d.m. 60.2 PGU/g d.m. | Ammonia 0.5:1, 85 °C, 5 min | 0.61 g/g | |
| 40 °C | 4.8 | - | 5% w/w | Celluclast® 1.5 L Novozyme 431 | 4.2 FPU/g d.m. 28.4 CBU/g d.m. | Untreated | 0.15 g/g | |
| 40 °C | 4.8 | - | 5% w/w | Celluclast® 1.5 L Novozyme 431 Viscozyme L | 4.2 FPU/g d.m. 28.4 CBU/g d.m. 0.85 HU/g d.m. 60.2 PGU/g d.m. | Untreated | 0.70 g/g | |
| [53] | 50 °C | 4.8 | 150 rpm | 2% w/w | Celluclast® 1.5 L Novozyme 188 | 15 FPU/g d.m.15 CBU/g d.m. | Ensilage 90 days | 0.19 g/g |
| [57] | 40 °C | 4.8 | 150 rpm | 2.5% w/w | Celluclast® 1.5 L | 5 FPU/g d.m. | HCl pH 1.5, 85 °C, 4 h | 0.27 g/g |
| 40 °C | 4.8 | 150 rpm | 2.5% w/w | Celluclast® 1.5 L | 30 FPU/g d.m. | HCl pH 1.5, 85 °C, 4 h | 0.49 g/g | |
| [72] | 50 °C | 5 | - | 10% w/w | Celustar XL Agropect | 0.75 FPU/g d.m. | Untreated | 0.3 g/g |
| [51] | 50 °C | 4.8 | 150 rpm | 2% w/w | Celluclast® 1.5 L Novozyme 188 Pectinex® | 15 FPU/g d.m. 15 CBU/g d.m. 60 PGU/g d.m. | Diluted acid H2SO4, 0.66%, 120 °C, 2% solid loading | 0.63 g/g |
| [59] | 45 °C | 4.8 | - | 2% w/w | Celluclast® 1.5 L | 20 FPU/g d.m. | Untreated | 0.20 g/g |
| 45 °C | 4.8 | - | 2% w/w | Celluclast® 1.5 L | 20 FPU/g d.m. | HCl pH 1.5, 85 °C, 4 h | 0.38 g/g | |
| 45 °C | 4.8 | - | 2% w/w | Celluclast® 1.5 L | 20 FPU/g d.m. | Autoclave (2.1 bars, 30 min), water 1:20 (w/v) | 0.44 g/g | |
| [60] | 50 °C | 4.8 | - | 5% w/w | Cellulase | 20 FPU/g d.m. | Untreated | 0.18 g/g |
| 50 °C | 4.8 | - | 5% w/w | Cellulase | 20 FPU/g d.m. | HCl 1% w/w, 80 °C, 6 h | 0.38 g/g | |
| 50 °C | 4.8 | - | 5% w/w | Cellulase | 20 FPU/g d.m. | Ammonia 10% w/w, 80 °C, 6 h | 0.49 g/g | |
| 50 °C | 4.8 | - | 5% w/w | Cellulase | 20 FPU/g d.m. | Pectinase 30 U/g, 50 °C, 6 h | 0.23 g/g | |
| 50 °C | 4.8 | - | 5% w/w | Cellulase | 20 FPU/g d.m. | Ammonium oxalate 5% w/w, 80 °C, 6 h | 0.25 g/g | |
| [73] | 50 °C | 5.0 | 150 rpm | 10% w/w | Celluclast® 1.5 L β-glucosidase xylanase exo-polygalacturonase | 2200 FPU/g d.m. 6 CBU/g d.m. 300 HU/g d.m. 110 PGU/g d.m. | Autoclave 120 °C, 20 min | 0.71 g/g |
3.4. Lactic Acid Fermentation
3.4.1. Metabolic Pathways to Produce LA via Fermentation
3.4.2. Production of LA from SBP Hydrolysates
3.5. Polyhydroxyalkanoates Fermentation
3.5.1. Metabolic Pathway to Produce PHAs via Fermentation
3.5.2. Production of PHAs from SBP
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBU | Cellobiase activity units |
| PGU | Polygalacturonase activity units |
| COD | Chemical oxygen demand |
| d.m. | Dry matter |
| FPU | Filter paper unit |
| HU | Hemicellulase activity units |
| LA | Lactic acid |
| LAB | Lactic acid bacteria |
| LCB | Lignocellulosic biomass |
| MRS | Man, Rogosa and Sharpe broth |
| PA | Polyamide |
| PBAT | Polybutylene adipate terephthalate |
| PBS | Polybutylene succinate |
| PCL | Polycaprolactone |
| PE | Polyethene |
| PET | Polyethene terephthalate |
| PGU | Polygalacturonase activity units |
| PHA | Polyhydroxyalkanoate |
| PLA | Poly-lactic acid |
| POS | Pectin-derived oligosaccharides |
| PP | Polypropylene |
| PTT | Polytrimethylene terephthalate |
| RS | Reducing sugars |
| SBP | Sugar beet pulp |
| SLR | Solid-liquid ratio |
| SSF | Solid-state fermentation |
| TS | Total solids |
| U | Unit of enzyme |
| VAP | Value-added product |
| VFAs | Volatile fatty acids |
| VS | Volatile solid |
| YH | Hydrolysis yield |
References
- Narancic, T.; O’Connor, K.E. Plastic Waste as a Global Challenge: Are Biodegradable Plastics the Answer to the Plastic Waste Problem? Microbiology 2019, 165, 129–137. [Google Scholar] [CrossRef]
- Plasctics Europe Plastics-the Facts 2021 An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 24 March 2022).
- Rivero, C.P.; Hu, Y.; Kwan, T.H.; Webb, C.; Theodoropoulos, C.; Daoud, W.; Lin, C.S.K. Bioplastics From Solid Waste. In Current Developments in Biotechnology and Bioengineering: Solid Waste Management; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. ISBN 9780444636751. [Google Scholar]
- European Bioplastic European Bioplastic. Available online: http://www.european-bioplastics.org (accessed on 3 February 2023).
- Martin, O.; Avérous, L. Poly(Lactic Acid): Plasticization and Properties of Biodegradable Multiphase Systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichnikov, M.P.; Shaitan, K.V. Application of Polyhydroxyalkanoates in Medicine and the Biological Activity of Natural Poly(3-Hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A Review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Saniei, H.; Mousavi, S. Surface Modification of PLA 3D-Printed Implants by Electrospinning with Enhanced Bioactivity and Cell Affinity. Polymer 2020, 196, 122467. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sean, T.C.; Kudaiyappan, T.; Bisyarah, U.; Mirarmandi, A.; Faradjeva, E.; Abubakar, A.; Ali, H.; Lavanya, J.; Angalene, A.; et al. Production, Characterization and Application of Nanocarriers Made of Polysaccharides, Proteins, Bio-Polyesters and Other Biopolymers: A Review. Int. J. Biol. Macromol. 2020, 165, 3088–3105. [Google Scholar] [CrossRef]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate ( PHA ): Properties and Modifications. Polymer 2020, 212, 123161. [Google Scholar] [CrossRef]
- Reis, M.; Albuquerque, M.; Villano, M.; Majone, M. Mixed Culture Processes for Polyhydroxyalkanoate Production from Agro-Industrial Surplus/Wastes as Feedstocks. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 6, pp. 669–683. ISBN 9780080885049. [Google Scholar]
- Sen, K.Y.; Baidurah, S. Renewable Biomass Feedstocks for Production of Sustainable Biodegradable Polymer. Curr. Opin. Green Sustain. Chem. 2021, 27, 100412. [Google Scholar] [CrossRef]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From Production to Nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent Advances in Lactic Acid Production by Microbial Fermentation Processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost Competitiveness of Sustainable Bioplastic Feedstocks – A Monte Carlo Analysis for Polylactic Acid. Clean. Eng. Technol. 2022, 6, 100411. [Google Scholar] [CrossRef]
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of Lignocellulosic Biomass. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–15. ISBN 9780128159361. [Google Scholar]
- Sani, R.K. Biorefining of Biomass to Biofuels: Opportunities and Perception; Springer: Berlin/Heidelberg, Germany, 2018; Volume 4, ISBN 978-3-319-67678-4. [Google Scholar]
- Obruca, S.; Benesova, P.; Marsalek, L.; Marova, I. Use of Lignocellulosic Materials for PHA Production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Al-Battashi, H.S.; Annamalai, N.; Sivakumar, N.; Al-Bahry, S.; Tripathi, B.N.; Nguyen, Q.D.; Gupta, V.K. Lignocellulosic Biomass (LCB): A Potential Alternative Biorefinery Feedstock for Polyhydroxyalkanoates Production. Rev. Environ. Sci. Biotechnol. 2019, 18, 183–205. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Streffer, F. Lignocellulose to Biogas and Other Products. JSM Biotechnol. Biomed. Eng. 2014, 2, 1023. [Google Scholar]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Status and Perspectives in Bioethanol Production From Sugar Beet. In Bioethanol Production from Food Crops; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–79. [Google Scholar]
- OECD; FAO. OECD-FAO Agricultural Outlook 2022-2031; OECD: Paris, France, 2022. [Google Scholar]
- Berlowska, J.; Pielech-przybylska, K.; Balcerek, M.; Dziekonska-kubczak, U.; Patelski, P.; Dziugan, P.; Kregiel, D. Simultaneous Saccharification and Fermentation of Sugar Beet Pulp for Efficient Bioethanol Production. Biomed Res. Int. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Panella, L. Sugar Beet as an Energy Crop. Sugar Tech. 2010, 12, 288–293. [Google Scholar] [CrossRef]
- Schonhoff, A.; Ihling, N.; Schreiber, A.; Zapp, P. Environmental Impacts of Biosurfactant Production Based on Substrates from the Sugar Industry. Sustain. Chem. Eng. 2022, 10, 9345–9358. [Google Scholar] [CrossRef]
- Pezzi, G. Considerations of the Technological Quality Assessment of Sugarbeet. Sugar Ind. 2011, 136, 85–89. [Google Scholar] [CrossRef]
- Cubero, M.T.G.; Sanz, M.C.; Benito, G.G.; Puerta, M.G.D. Distribution of Colorants in the Evaporation and First Crystallization Stages. Sugar Ind. 2004, 129, 660–666. [Google Scholar]
- Vaccari, G.; Wawro, P.; Tamburini, E.; Sgualdino, G.; Bernardi, T. Cooling Crystallization of Raw Juice: Laboratory Investigations of Sucrose Crystal Growth Kinetics. Sugar Ind. 1996, 121, 111–117. [Google Scholar]
- Vaccari, G.; Wawro, P.; Tamburini, E.; Sgualdino, G.; Bernardi, T. Cooling Crystallization of Microfiltered Raw Juice and of Traditional Thick Juice: A Comparison. Sugar Ind. 2002, 127, 22–28. [Google Scholar]
- Merkes, R.; Kröhl, M.; Mugele, H.; Sauer, M. Sugarbeet Production Technology in the Year 2000: Cost Reduction, Environmental Protection, Sustainability. Sugar Ind. 2001, 126, 804–811. [Google Scholar]
- Baryga, A.; Połeć, B.; Klasa, A.; Olejnik, T.P. Application of Sugar Beet Pulp Digestate as a Soil Amendment in the Production of Energy Maize. Processes 2021, 9, 765. [Google Scholar] [CrossRef]
- Baryga, A.; Połeć, B.; Klasa, A. Quality of Sugar Beets under the Effects of Digestate Application to the Soil. Processes 2020, 8, 1402. [Google Scholar] [CrossRef]
- Baryga, A.; Polec, B.; Skibniewska, K.A.; Seciu, E.; Grabara, J. Utilisation of Residual Waste From Sugar Beet Pulp Fermentation As Fertiliser in Sustainable Agriculture. J. Environ. Prot. Ecol. 2016, 17, 1048–1057. [Google Scholar]
- Rana, A.K.; Kumar Gupta, V.; Newbold, J.; Roberts, D.; Rees, R.M.; Krishnamurthy, S.; Kumar Thakur, V. Sugar Beet Pulp: Resurgence and Trailblazing Journey towards a Circular Bioeconomy. Fuel 2022, 312, 122953. [Google Scholar] [CrossRef]
- Dinand, E.; Chanzy, H.; Vignon, M.R. Parenchymal Cell Cellulose from Sugar Beet Pulp: Preparation and Properties. Cellulose 1996, 3, 183–188. [Google Scholar] [CrossRef]
- Dinand, E.; Chanzy, H.; Vignon, R.M. Suspensions of Cellulose Microfibrils from Sugar Beet Pulp. Food Hydrocoll. 1999, 13, 275–283. [Google Scholar] [CrossRef]
- Michel, F.; Thibault, J.-F.; Barry, J.-L.; de Baynast, R. Preparation and Characterisation of Dietary Fibre from Sugar Beet Pulp. J. Sci. Food Agric. 1988, 42, 77–85. [Google Scholar] [CrossRef]
- Grahovac, J.; Rončević, Z. Environmental Impacts of the Confectionary Industry. In Environmental Impact of Agro-Food Industry and Food Consumption; Elsevier: Amsterdam, The Netherlands, 2021; pp. 189–216. ISBN 9780128213636. [Google Scholar]
- Ward, D.P.; Cárdenas-Fernández, M.; Hewitson, P.; Ignatova, S.; Lye, G.J. Centrifugal Partition Chromatography in a Biorefinery Context: Separation of Monosaccharides from Hydrolysed Sugar Beet Pulp. J. Chromatogr. A 2015, 1411, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bolaji, I.; Nejad, B.; Billham, M.; Mehta, N.; Smyth, B.; Cunningham, E. Multi-Criteria Decision Analysis of Agri-Food Waste as a Feedstock for Biopolymer Production. Resour. Conserv. Recycl. 2021, 172, 105671. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic Biomass for Bioethanol: An Overview on Pretreatment, Hydrolysis and Fermentation Processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid Pretreatment of Lignocellulosic Biomass for Energy Vectors Production: A Review Focused on Operational Conditions and Techno-Economic Assessment for Bioethanol Production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A Review on the Environment-Friendly Emerging Techniques for Pretreatment of Lignocellulosic Biomass: Mechanistic Insight and Advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment Technologies for an Efficient Bioethanol Production Process Based on Enzymatic Hydrolysis: A Review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Xu, J.K.; Sun, R.C. Recent Advances in Alkaline Pretreatment of Lignocellulosic Biomass. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–459. ISBN 9780128025611. [Google Scholar]
- Rahmati, S.; Doherty, W.; Dubal, D.; Atanda, L.; Moghaddam, L.; Sonar, P.; Hessel, V.; Ostrikov, K.K. Pretreatment and Fermentation of Lignocellulosic Biomass: Reaction Mechanisms and Process Engineering. React. Chem. Eng. 2020, 5, 2017–2047. [Google Scholar] [CrossRef]
- Rezicó, T.; Oros, D.; Markovicó, I.; Kracher, D.; Ludwig, R.; Šantek, B.; Rezic, T.; Oros, D.; Markovicó, I.; Kracher, D.; et al. Integrated Hydrolyzation and Fermentation of Sugar Beet Pulp to Bioethanol. J. Microbiol. Biotechnol. 2013, 23, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Donkoh, E.; Degenstein, J.; Tucker, M.; Ji, Y. Optimization of Enzymatic Hydrolysis of Dilute Acid Pretreated Sugar Beet Pulp Using Response Surface Design. J. Sugarbeet Res. 2012, 49, 26–38. [Google Scholar] [CrossRef]
- El-gendy, N.S.; Madian, H.R.; Nassar, H.N. Response Surface Optimization of the Thermal Acid Pretreatment of Sugar Beet Pulp for Bioethanol Production Using Trichoderma Viride and Saccharomyces Cerevisiae. Recent Pat. Biotechnol. 2015, 9, 50–62. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.S.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Dilute Acid Pretreatment and Fermentation of Sugar Beet Pulp to Ethanol. Appl. Energy 2013, 105, 1–7. [Google Scholar] [CrossRef]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Effect of Several Pretreatments on the Lactic Acid Production from Exhausted Sugar Beet Pulp. Foods 2021, 10, 2414. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, C.; Cheng, Y.S.; Zhang, R.; Jenkins, B.; VanderGheynst, J.S. Effects of Ensilage on Storage and Enzymatic Degradability of Sugar Beet Pulp. Bioresour. Technol. 2011, 102, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Fernández, M.; Bawn, M.; Hamley-Bennett, C.; Bharat, P.K.V.; Subrizi, F.; Suhaili, N.; Ward, D.P.; Bourdin, S.; Dalby, P.A.; Hailes, H.C.; et al. An Integrated Biorefinery Concept for Conversion of Sugar Beet Pulp into Value-Added Chemicals and Pharmaceutical Intermediates. Faraday Discuss. 2017, 202, 415–431. [Google Scholar] [CrossRef]
- Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review. Appl. Sci. 2020, 10, 7698. [Google Scholar] [CrossRef]
- Canilha, L.; Chandel, A.K.; dos Santos Milessi, T.S.; Fernandes Antunes, F.A.; Luiz Da Costa Freitas, W.; Almeida Felipe, M.D.G.; Silva, S.S. Bioconversion of Sugarcane Biomass into Ethanol: An Overview about Composition, Pretreatment Methods, Detoxification of Hydrolysates, Enzymatic Saccharification, and Ethanol Fermentation. J. Biomed. Biotechnol. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Ivetic, D.Z.; Marina, B.S.; Antov, M.G.; Ivetić, D.Ž.; Šćiban, M.B.; Antov, M.G. Enzymatic Hydrolysis of Pretreated Sugar Beet Shreds: Statistical Modeling of the Experimental Results. Biomass Bioenergy 2012, 7, 387–394. [Google Scholar] [CrossRef]
- Foster, B.L.; Dale, B.E.; Doran-Peterson, J.B. Enzymatic Hydrolysis of Ammonia-Treated Sugar Beet Pulp. Appl. Biochem. Biotechnol. 2001, 91, 91–93. [Google Scholar] [CrossRef]
- Ivetic, D.T.; Antov, M.G. The Impact of Pretreatments on Cellulose from Sugar Beet Shreds and Its Susceptibility to Enzymatic Hydrolysis. Cellul. Chem. Technol. 2016, 50, 139–146. [Google Scholar]
- Li, G.; Sun, Y.; Guo, W.; Yuan, L. Comparison of Various Pretreatment Strategies and Their Effect on Chemistry and Structure of Sugar Beet Pulp. J. Clean. Prod. 2018, 181, 217–223. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic Hydrolysis of Lignocellulosic Biomass: Converting Food Waste in Valuable Products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Anand, G.; Yadav, S.; Yadav, D. Production, Purification and Biochemical Characterization of an Exo-Polygalacturonase from Aspergillus Niger MTCC 478 Suitable for Clarification of Orange Juice. 3 Biotech 2017, 7, 122. [Google Scholar] [CrossRef]
- Díaz, A.B.; De Ory, I.; Caro, I.; Blandino, A. Enhance Hydrolytic Enzymes Production by Aspergillus Awamori on Supplemented Grape Pomace. Food Bioprod. Process. 2012, 90, 72–78. [Google Scholar] [CrossRef]
- Brijwani, K.; Oberoi, S.; Vadlani, P.V.; Oberoi, H.S.; Vadlani, P.V. Production of a Cellulolytic Enzyme System in Mixed-Culture Solid-State Fermentation of Soybean Hulls Supplemented with Wheat Bran. Process Biochem. 2010, 45, 120–128. [Google Scholar] [CrossRef]
- Diaz, A.B.; Blandino, A.; Webb, C.; Caro, I. Modelling of Different Enzyme Productions by Solid-State Fermentation on Several Agro-Industrial Residues. Appl. Microbiol. Biotechnol. 2016, 100, 9555–9566. [Google Scholar] [CrossRef] [PubMed]
- Verduzco-Oliva, R.; Gutierrez-Uribe, J.A. Beyond Enzyme Production: Solid State Fermentation (SSF) as an Alternative Approach to Produce Antioxidant Polysaccharides. Sustainability 2020, 12, 495. [Google Scholar] [CrossRef]
- Mansour, A.A.; Arnaud, T.; Lu-Chau, T.A.; Fdz-Polanco, M.; Moreira, M.T.; Rivero, J.A.C. Review of Solid State Fermentation for Lignocellulolytic Enzyme Production: Challenges for Environmental Applications. Rev. Environ. Sci. Biotechnol. 2016, 15, 31–46. [Google Scholar] [CrossRef]
- Yazid, N.A.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Bhargav, S.; Panda, B.P.; Ali, M.; Javed, S. Solid-State Fermentation - An Overview. Chem. Biochem. Eng 2008, 22, 49–70. [Google Scholar]
- Marques, N.P.; de Cassia Pereira, J.; Gomes, E.; da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and Xylanases Production by Endophytic Fungi by Solid State Fermentation Using Lignocellulosic Substrates and Enzymatic Saccharification of Pretreated Sugarcane Bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Cerda, A.; Gea, T.; Vargas-García, M.C.; Sánchez, A. Towards a Competitive Solid State Fermentation: Cellulases Production from Coffee Husk by Sequential Batch Operation and Role of Microbial Diversity. Sci. Total Environ. 2017, 589, 56–65. [Google Scholar] [CrossRef]
- Zieminski, K.; Romanowska, I.; Kowalska, M.; Ziemiński, K.; Romanowska, I.; Kowalska, M. Enzymatic Pretreatment of Lignocellulosic Wastes to Improve Biogas Production. Waste Manag. 2012, 32, 1131–1137. [Google Scholar] [CrossRef]
- Díaz, A.B.; Marzo, C.; Caro, I.; de Ory, I.; Blandino, A. Valorization of Exhausted Sugar Beet Cossettes by Successive Hydrolysis and Two Fermentations for the Production of Bio-Products. Bioresour. Technol. 2017, 225, 225–233. [Google Scholar] [CrossRef]
- Rodriguez Couto, S. Exploitation of Biological Wastes for the Production of Value-Added Products Uncler Solid-State Fermentation Conditions. Biotechnol. J. 2008, 3, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Valorization of Agro-Industrial Wastes to Produce Hydrolytic Enzymes by Fungal Solid-State Fermentation. Waste Manag. Res. 2018, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mouafi, F.E.; Karam, E.A.; Hassan, H.M. Production of Dextranase from Agro-Industrial Wastes by Aspergillus Awamori F-234 under Solid State Fermentation. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1451–1459. [Google Scholar]
- Heerd, D.; Diercks-Horn, S.; Fernández-Lahore, M. Efficient Polygalacturonase Production from Agricultural and Agro-Industrial Residues by Solid-State Culture of Aspergillus Sojae under Optimized Conditions. Springerplus 2014, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.H.; Zhang, H.X.; Qi, H.Y.; Peng, X.W.; Li, B.J. Pectinase Production by Aspergillus Niger Using Wastewater in Solid State Fermentation for Eliciting Plant Disease Resistance. Bioresour. Technol. 2004, 95, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Berna, P.; Desgranges, C.; Durand, A. Substrate Use and Production of α-l-Arabinofuranosidase during Solid-State Culture of Trichoderma Reesei on Sugar Beet Pulp. Enzyme Microb. Technol. 1995, 17, 935–941. [Google Scholar] [CrossRef]
- Leung, C.C.J.; Cheung, A.S.Y.; Zhang, A.Y.Z.; Lam, K.F.; Lin, C.S.K.; Chark Joe Leung, C.; Siu Yeung Cheung, A.; Yan-Zhu Zhang, A.; Fung Lam, K.; Sze Ki Lin, C. Utilisation of Waste Bread for Fermentative Succinic Acid Production. Biochem. Eng. J. 2012, 65, 10–15. [Google Scholar] [CrossRef]
- Pleissner, D.; Kwan, T.H.; Lin, C.S.K. Fungal Hydrolysis in Submerged Fermentation for Food Waste Treatment and Fermentation Feedstock Preparation. Bioresour. Technol. 2014, 158, 48–54. [Google Scholar] [CrossRef]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Valorisation of Food Waste via Fungal Hydrolysis and Lactic Acid Fermentation with Lactobacillus Casei Shirota. Bioresour. Technol. 2016, 217, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Dessie, W.; Zhang, W.; Xin, F.; Dong, W.; Zhang, M.; Ma, J.; Jiang, M. Succinic Acid Production from Fruit and Vegetable Wastes Hydrolyzed by On-Site Enzyme Mixtures through Solid State Fermentation. Bioresour. Technol. 2018, 247, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Conversion of Exhausted Sugar Beet Pulp into Fermentable Sugars from a Biorefinery Approach. Foods 2020, 9, 1351. [Google Scholar] [CrossRef]
- García, C.; Bautista, L.; Rendueles, M.; Díaz, M. A New Synbiotic Dairy Food Containing Lactobionic Acid and Lactobacillus Casei. Int. J. Dairy Technol. 2019, 72, 47–56. [Google Scholar] [CrossRef]
- Shafi, A.; Naeem Raja, H.; Farooq, U.; Akram, K.; Hayat, Z.; Naz, A.; Nadeem, H.R. Antimicrobial and Antidiabetic Potential of Synbiotic Fermented Milk: A Functional Dairy Product. Int. J. Dairy Technol. 2019, 72, 15–22. [Google Scholar] [CrossRef]
- Silva, H.L.A.; Balthazar, C.F.; Silva, R.; Vieira, A.H.; Costa, R.G.B.; Esmerino, E.A.; Freitas, M.Q.; Cruz, A.G. Sodium Reduction and Flavor Enhancer Addition in Probiotic Prato Cheese: Contributions of Quantitative Descriptive Analysis and Temporal Dominance of Sensations for Sensory Profiling. J. Dairy Sci. 2018, 101, 8837–8846. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological Advances in Lactic Acid Production by Lactic Acid Bacteria: Lignocellulose as Novel Subtrate. Biofuels Bioprod. Biorefining 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of Plastics: Current Scenario and Future Prospects for Environmental Safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Pérez-Rivero, C.; Venus, J. Valorisation of Solid Biowastes: The Lactic Acid Alternative. Process Biochem. 2020, 99, 222–235. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Synthesis, Structure and Properties of Poly (Lactic Acid); Springer: Berlin/Heidelberg, Germany, 2018; Volume 279, ISBN 978-3-319-64229-1. [Google Scholar]
- Tan, J.; Abdel-Rahman, M.A.; Sonomoto, K. Biorefinery-Based Lactic Acid Fermentation: Microbial Production of Pure Monomer Product. In Advances in Polymer Science; Springer: New York, NY, USA, 2018; Volume 279, pp. 27–66. [Google Scholar]
- Nancib, A.; Nancib, N.; Meziane-Cherif, D.; Boubendir, A.; Fick, M.; Boudrant, J. Joint Effect of Nitrogen Sources and B Vitamin Supplementation of Date Juice on Lactic Acid Production by Lactobacillus Casei Subsp. Rhamnosus. Bioresour. Technol. 2005, 96, 63–67. [Google Scholar] [CrossRef]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Valorisation of Fungal Hydrolysates of Exhausted Sugar Beet Pulp for Lactic Acid Production. J. Sci. Food Agric. 2021, 101, 4108–4117. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.B.; González, C.; Marzo, C.; Caro, I.; Blandino, A. Feasibility of Exhausted Sugar Beet Pulp as Raw Material for Lactic Acid Production. J. Sci. Food Agric. 2020, 100, 3036–3045. [Google Scholar] [CrossRef]
- Alexandri, M.; Hübner, D.; Schneider, R.; Fröhling, A.; Venus, J. Towards Efficient Production of Highly Optically Pure D-Lactic Acid from Lignocellulosic Hydrolysates Using Newly Isolated Lactic Acid Bacteria. New Biotechnol. 2022, 72, 1–10. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Schneider, R.; Lunelli, B.H.; Rossell, C.E.V.; Filho, R.M.; Venus, J. A Simple Biorefinery Concept to Produce 2G-Lactic Acid from Sugar Beet Pulp (SBP): A High-Value Target Approach to Valorize a Waste Stream. Molecules 2020, 25, 2113. [Google Scholar] [CrossRef]
- Santosh, I.; Ashtavinayak, P.; Amol, D.; Sanjay, P. Enhanced Bioethanol Production from Different Sugarcane Bagasse Cultivars Using Co-Culture of Saccharomyces Cerevisiae and Scheffersomyces (Pichia) Stipitis. J. Environ. Chem. Eng. 2017, 5, 2861–2868. [Google Scholar] [CrossRef]
- Cui, F.; Li, Y.; Wan, C. Lactic Acid Production from Corn Stover Using Mixed Cultures of Lactobacillus Rhamnosus and Lactobacillus Brevis. Bioresour. Technol. 2011, 102, 1831–1836. [Google Scholar] [CrossRef]
- Berlowska, J.; Cieciura, W.; Borowski, S.; Dudkiewicz, M.; Binczarski, M.; Witonska, I.; Otlewska, A.; Kregiel, D. Simultaneous Saccharification and Fermentation of Sugar Beet Pulp with Mixed Bacterial Cultures for Lactic Acid and Propylene Glycol Production. Molecules 2016, 21, 1380. [Google Scholar] [CrossRef]
- Lemoigne, M. Produits de Deshydration et de Polymerisation de l’acide Β= Oxybutyrique. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef]
- Bertrand, J.L.; Ramsay, B.A.; Chavarie, C. Biosynthesis of Poly-β-Hydroxyalkanoates from Pentoses by Pseudomonas Pseudoflava. Appl. Environ. Microbiol. 1990, 56, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Pakalapati, H.; Chang, C.K.; Show, P.L.; Arumugasamy, S.K.; Lan, J.C.W. Development of Polyhydroxyalkanoates Production from Waste Feedstocks and Applications. J. Biosci. Bioeng. 2018, 126, 282–292. [Google Scholar] [CrossRef]
- Kurt-Kızıldoğan, A.; Türe, E.; Okay, S.; Otur, Ç. Improved Production of Poly(3-Hydroxybutyrate) by Extremely Halophilic Archaeon Haloarcula Sp. TG1 by Utilization of RCKT3eng-Treated Sugar Beet Pulp. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Jeffries, T.W. Utilization of Xylose by Bacteria, Yeasts, and Fungi. Adv. Biochem. Eng. Biotechnol. 1983, 27, 1–32. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.J.; Del Rio, L.F.; Orsat, V. Sustainable PHA Production in Integrated Lignocellulose Biorefineries. New Biotechnol. 2019, 49, 161–168. [Google Scholar] [CrossRef]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome Sequence of the Bioplastic-Producing “Knallgas” Bacterium Ralstonia Eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef]
- Cesário, M.T.F.; de Almeida, M.C.M.D. Lignocellulosic Hydrolysates for the Production of Polyhydroxyalkanoates. In Microorganisms in Biorefineries; Springer: Berlin/Heidelberg, Germany, 2015; pp. 79–104. [Google Scholar]
- Lu, X.; Liu, G.; Wang, Y.; Ding, J.; Weng, W. Engineering of an L-Arabinose Metabolic Pathway in Ralstonia Eutropha W50. Wei Sheng Wu Xue Bao 2013, 53, 1267–1275. [Google Scholar]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark Fermentative Hydrogen Production from Hydrolyzed Sugar Beet Pulp Improved by Nitrogen and Phosphorus Supplementation. Bioresour. Technol. 2021, 340, 125622. [Google Scholar] [CrossRef]
- Carvalheira, M.; Marreiros, B.C.; Reis, M. Acids (VFAs) and Bioplastic (PHA) Recovery. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 245–254. ISBN 9780323901789. [Google Scholar]

| Pretreatment Type | Conditions | YH | Reference | |
|---|---|---|---|---|
| Physical | Milling | 0.8–1.0 mm | 0.71 g/g | [24] |
| Milling + Ultrasound | 0.8–1.0 mm 50% or 100% amplitude, 20 min, water or 2% w/w H2SO4 | 0.70–0.76 g/g | [24] | |
| Chemical | Dilute acid | Autoclave, 150 °C, 10 min, 1.1% w/w H2SO4 | 0.82 g/g | [49] |
| Dilute acid | 120 °C, 6 min, 0.1 N HCl | 0.86 g/g | [50] | |
| Dilute acid | 120 °C, 0.66% H2SO4 | 0.63 g/g | [51] | |
| Dilute acid | 1% H2SO4 | 0.49 g/g | [52] | |
| Physicochemical | Milling + Thermal | Autoclave, 121 °C, 30 or 60 min, 2% w/w H2SO4 | 0.85 g/g | [24] |
| Milling + Thermal | Autoclave, 120 °C, 30 or 60 min, water | 0.75 g/g | [24] | |
| Thermal | Autoclave, 121 °C, 20 min | 0.60 g/g | [52] | |
| Biological | Ensiling | Lactobacillus species | 0.95 g/g | [53] |
| Solid-state fermentation | Aspergillus awamori, 70% moisture, 5 days | 0.34 g/g | [52] | |
| Reference | Strain | Conditions | Concentration | YLA |
|---|---|---|---|---|
| [24] | Lactobacillus plantarum HII & Lactobacillus brevis PCM 488 | SSF with co-culture | 60 g/L | 0.55 g/g |
| [96] | L. coryniformis subsp. torquens DSM 20005 & L. preudomesenteroides | SHF with co-culture | 22 g/L | 0.78 g/g |
| [94] | Lactobacillus plantarum | SHF | 30 g/L | 0.12 g/g |
| [52] | Lactobacillus plantarum | SHF with pretreated SBP | 50 g/L | 0.5 g/g |
| [95] | Lactobacillus casei | Fed-fach SSF | 27 g/L | 0.13 g/g |
| [97] | Bacillus coagulans | Continuous fermentation | 35 g/L | 0.71 g/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzo-Gago, C.; Díaz, A.B.; Blandino, A. Sugar Beet Pulp as Raw Material for the Production of Bioplastics. Fermentation 2023, 9, 655. https://doi.org/10.3390/fermentation9070655
Marzo-Gago C, Díaz AB, Blandino A. Sugar Beet Pulp as Raw Material for the Production of Bioplastics. Fermentation. 2023; 9(7):655. https://doi.org/10.3390/fermentation9070655
Chicago/Turabian StyleMarzo-Gago, Cristina, Ana Belén Díaz, and Ana Blandino. 2023. "Sugar Beet Pulp as Raw Material for the Production of Bioplastics" Fermentation 9, no. 7: 655. https://doi.org/10.3390/fermentation9070655
APA StyleMarzo-Gago, C., Díaz, A. B., & Blandino, A. (2023). Sugar Beet Pulp as Raw Material for the Production of Bioplastics. Fermentation, 9(7), 655. https://doi.org/10.3390/fermentation9070655









