Antioxidant Capacity of Lactic Acid Bacteria and Yeasts from Xinjiang Traditional Fermented Dairy Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Microorganisms
2.3. Instruments and Equipment
2.4. Methods
2.4.1. Sample Preparation
2.4.2. Hydroxyl Radical Scavenging Activity
2.4.3. O2− Radical Scavenging Activity
2.4.4. DPPH Radical Scavenging Activity
2.4.5. ABTS+ Radical Scavenging Activity Assays
2.4.6. Lipid Peroxidation Inhibition Activity
2.4.7. Fe2+ Chelating Ability
2.4.8. Antioxidant Enzyme Activity
2.5. Statistical Analysis
3. Results
3.1. Scavenging of Hydroxyl Radicals
3.2. O2− Radical Scavenging Activity
3.3. DPPH Radical Scavenging Activity
3.4. ABTS+ Radical Scavenging Activity
3.5. Lipid Peroxidation Inhibition Activity
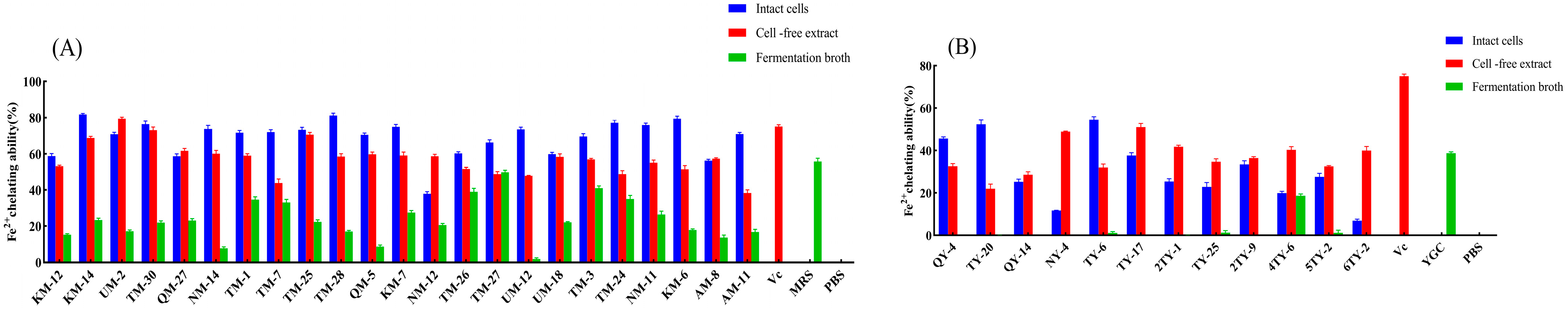
3.6. Fe2+ Chelating Ability
3.7. Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Number | Genus | Species | GenBank | Source |
|---|---|---|---|---|
| KM-12 | Lactobacillus | Lactobacillus gallinarum | MW931798.1 | Uruqmi yogurt pimple |
| KM-14 | Lactobacillus | Lactobacillus gallinarum | MW931799.1 | Uruqmi yogurt pimple |
| UM-2 | Lactobacillus | Lactobacillus gallinarum | MW931811.1 | Uruqmi cheese |
| TM-30 | Lactobacillus | Lactobacillus gallinarum | MW931793.1 | Tashkurgan County cheese |
| QM-27 | Enterococcus | Enterococcus durans | MW931809.1 | Tacheng cheese |
| NM-14 | Enterococcus | Enterococcus durans | MW931841.1 | Habahe County cheese |
| TM-1 | Enterococcus | Enterococcus durans | MW931780.1 | Tashkurgan County cheese |
| TM-7 | Enterococcus | Enterococcus durans | MW931782.1 | Tashkurgan County cheese |
| TM-25 | Enterococcus | Enterococcus durans | MW931789.1 | Tashkurgan County cheese |
| TM-28 | Enterococcus | Enterococcus durans | MW931792.1 | Tashkurgan County cheese |
| QM-5 | Lacticaseibacillus | Lacticaseibacillus paracasei | MW931815.1 | Tacheng cheese |
| KM-7 | Lacticaseibacillus | Lacticaseibacillus paracasei | MW931821.1 | Uruqmi yogurt pimple |
| NM-12 | Lacticaseibacillus | Lacticaseibacillus paracasei | MW931840.1 | Habahe County cheese |
| TM-26 | Lacticaseibacillus | Lacticaseibacillus paracasei | MW931790.1 | Tashkurgan County cheese |
| TM-27 | Lacticaseibacillus | Lacticaseibacillus paracasei | MW931791.1 | Tashkurgan County cheese |
| UM-12 | Enterococcus | Enterococcus faecium | MW931812.1 | Uruqmi cheese |
| UM-18 | Enterococcus | Enterococcus faecium | MW931813.1 | Uruqmi cheese |
| TM-3 | Enterococcus | Enterococcus faecium | MW931781.1 | Tashkurgan County cheese |
| TM-24 | Enterococcus | Enterococcus faecium | MW931788.1 | Tashkurgan County cheese |
| NM-11 | Enterococcus | Enterococcus faecium | MW931839.1 | Habahe County cheese |
| KM-6 | Levilactobacillus | Levilactobacillus brevis | MW931796.1 | Uruqmi yogurt pimple |
| AM-8 | Lacticaseibacillus | Lacticaseibacillus rhamnosus | MW931827.1 | Fuyun County cheese |
| AM-11 | Lacticaseibacillus | Lacticaseibacillus rhamnosus | MW931830.1 | Fuyun County cheese |
| QY-4 | Pichia | Pichia fermentans | GU373759.1 | Tacheng cheese |
| TY-20 | Pichia | Pichia fermentans | MZ314865 | Tashkurgan County cheese |
| QY-14 | Geotrichum | Geotrichum candidum | MN736502.1 | Tacheng cheese |
| NY-4 | Yarrowia | Yarrowia lipolytica | KY110196.1 | Habahe County cheese |
| TY-6 | Yarrowia | Yarrowia lipolytica | MZ314862 | Tashkurgan County cheese |
| TY-17 | zeylanoides | Candida zeylanoides | MZ314864 | Tashkurgan County cheese |
| 2TY-1 | Pichia | Pichia kudriavzevii | OM995975 | Tashkurgan County cheese |
| TY-25 | Guehomyces | Guehomyces pullulans | MZ314866 | Tashkurgan County cheese |
| 2TY-9 | Wickerhamomyces | Wickerhamomyces anomalus | OM995983 | Tashkurgan County cheese |
| 4TY-6 | Kluyveromyces | Kluyveromyces marxianus | OM995998 | Tashkurgan County cheese |
| 5TY-2 | Issatchenkia | Issatchenkia orientalis | OM996002 | Tashkurgan County cheese |
| 6TY-2 | Saccharomyces | Saccharomyces cerevisiae | OM996013 | Tashkurgan County cheese |
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Yadav, M.; Mandeep; Shukla, P. Probiotics of Diverse Origin and Their Therapeutic Applications: A Review. J. Am. Coll. Nutr. 2020, 39, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. Biofactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Paulino Do Nascimento, L.C.; Lacerda, D.C.; Ferreira, D.J.S.; De Souza, E.L.; De Brito Alves, J.L. Limosilactobacillus fermentum, Current Evidence on the Antioxidant Properties and Opportunities to be Exploited as a Probiotic Microorganism. Probiotics Antimicrob. Proteins 2022, 14, 960–979. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martinez-Larranaga, M.R.; Wang, X.; Martinez, M.; Anadon, A.; Martinez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Kim, M.R. Antioxidants of Natural Products. Antioxidants 2021, 10, 612. [Google Scholar] [CrossRef]
- El-Sayed, M.I.; Awad, S.; Abou-Soliman, N.H.I. Improving the antioxidant properties of fermented camel milk using some strains of Lactobacillus. Food Nutr. Sci. 2021, 12, 352–371. [Google Scholar]
- Kahar, G.; Rahman, N.; Yusan, D.; Yalkun, M. Molecular identification andantioxidant activity of yeasts isolated from traditional fermented milk in southern Xinjiang. Food Mach. 2018, 34, 12–19. [Google Scholar]
- Abreu, I.; Albuquerque, R.; Brandao, A.B.P.; Barssotti, L.; De Souza, L.B.; Ferreira, F.G.; Oliveira, L.C.G.; Yokota, R.; Sparvoli, L.G.; Dias, D.D.S.; et al. Saccharomyces boulardii exerts renoprotection by modulating oxidative stress, renin angiotensin system and uropathogenic microbiota in a murine model of diabetes. Life Sci. 2022, 301, 120616. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Li, X.; Zhang, T.; Liang, M.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota. Nutrients 2022, 14, 1272. [Google Scholar] [CrossRef]
- Brandao, L.R.; De Brito Alves, J.L.; Da Costa, W.K.A.; Ferreira, G.A.H.; De Oliveira, M.P.; Gomes Da Cruz, A.; Braga, V.A.; Aquino, J.S.; Vidal, H.; Noronha, M.F.; et al. Live and ultrasound-inactivated Lacticaseibacillus casei modulate the intestinal microbiota and improve biochemical and cardiovascular parameters in male rats fed a high-fat diet. Food Funct. 2021, 12, 5287–5300. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, H.Q.; Zhang, X.; Zhang, H.; Xia, J.; Ding, K.; Fang, Z.Y. Probiotic administration of lactobacillus rhamnosus GR-1 attenuates atherosclerotic plaque formation in ApoE-/- mice fed with a high-fat diet. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3533–3541. [Google Scholar] [PubMed]
- Yasheng, M.; Rixat, E.; Rahman, N. Tolerance and probiotic characteristics of lactic acid bacteria in traditional fermented raw cheese in northern Xinjiang. Microbiol. China 2023, 50, 2044–2062. [Google Scholar]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant Activity and Probiotic Properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, Y.; Li, X.; Mu, G.; Tuo, Y. Screening Human-derived Lactobacillus Strains with Antioxidant Activity. Food Res. Dev. 2019, 40, 199–205. [Google Scholar]
- Yanhong, L.; Bo, J.; Tao, Z.; Wanmeng, M.; Jian, L. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar]
- Song, S.; Liu, X.; Zhao, B.; Abubaker, M.A.; Huang, Y.; Zhang, J. Effects of Lactobacillus plantarum Fermentation on the Chemical Structure and Antioxidant Activity of Polysaccharides from Bulbs of Lanzhou Lily. ACS Omega 2021, 6, 29839–29851. [Google Scholar] [CrossRef]
- Goto, M.; Kuda, T.; Shikano, A.; Charrouf, Z.; Yamauchi, K.; Yokozawa, M.; Takahashi, H.; Kimura, B. Induction of superoxide anion radical-scavenging capacity in an argan press cake-suspension by fermentation using Lactobacillus plantarum Argan-L1. LWT-Food Sci. Technol. 2019, 100, 56–61. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, P.; Lou, L.; Zhan, J.; Fan, M.; Li, D.; Liao, Q. Antioxidant Activities of Lactic Acid Bacteria for Quality Improvement of Fermented Sausage. J. Food Sci. 2017, 82, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Hao, X.Y.; Zhang, X.X.; Zhang, G.X.; Li, X.D.; Liu, L.; Sun, Y.; Pan, Y. Identification of antioxidant peptides from cheddar cheese made with Lactobacillus helveticus. LWT-Food Sci. Technol. 2021, 141, 110866. [Google Scholar] [CrossRef]
- Wang, C.F.; Huang, C.R.; Lu, Y.C. Changes in Bio-Functional Compounds, ACE Inhibition, and Antioxidant Capacity after Mixed Fermentation of Eight Whole Grains. Fermentation 2023, 9, 209. [Google Scholar] [CrossRef]
- Lin, M.Y.; Yen, C.L. Reactive oxygen species and lipid peroxidation product-scavenging ability of yogurt organisms. J. Dairy Sci. 1999, 82, 1629–1634. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Sun, Q.; Dong, F.; Liu, Q. Antioxidant potential of a unique LAB culture isolated from Harbin dry sausage: In vitro and in a sausage model. Meat Sci. 2015, 110, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Amanatidou, A.; Smid, E.J.; Bennik, M.H.J.; Gorris, L.G.M. Antioxidative properties of Lactobacillus sake upon exposure to elevated oxygen concentrations. FEMS Microbiol. Lett. 2001, 203, 87–94. [Google Scholar] [CrossRef]
- Chen, L.-S.; Ma, Y.; Chen, L.-J.; Zhao, C.-H.; Maubois, J.-L.; Jiang, T.-M.; Li, H.-M.; He, S.-H. Antioxidant activity of two yeasts and their attenuation effect on 4-nitroquinoline 1-oxide inducedin vitrolipid peroxidation. Int. J. Food Sci. Technol. 2010, 45, 555–561. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Chen, M.J.; Lin, C.W. Antimutagenic and antioxidant properties of milk-kefir and soymilk-kefir. J. Agric. Food Chem. 2005, 53, 2467–2474. [Google Scholar] [CrossRef]
- Mendonca, J.D.S.; Guimaraes, R.C.A.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.P.; Marcelino, G.; Bogo, D.; Freitas, K.C.; Hiane, P.A.; De Padua Melo, E.S.; Vilela, M.L.B.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef]
- Martinez-Cayuela, M. Oxygen free radicals and human disease. Biochimie 1995, 77, 147–161. [Google Scholar] [CrossRef]
- Ren, D.; Li, C.; Qin, Y.; Yin, R.; Du, S.; Ye, F.; Liu, C.; Liu, H.; Wang, M.; Li, Y.; et al. In vitro evaluation of the probiotic and functional potential of Lactobacillus strains isolated from fermented food and human intestine. Anaerobe 2014, 30, 1–10. [Google Scholar] [CrossRef]
- Ding, W.; Wang, L.; Zhang, J.; Ke, W.; Zhou, J.; Zhu, J.; Guo, X.; Long, R. Characterization of antioxidant properties of lactic acid bacteria isolated from spontaneously fermented yak milk in the Tibetan Plateau. J. Funct. Foods 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, D.; Zhang, S.; Qi, Y.; Wu, Y.; Gao, B.; Liu, Z. Screening and Identification of Lactic ACID Bacteria in Traditional Pickles and Analysis of the Antioxidant Properties. China Condiment 2022, 47, 5–9. [Google Scholar]
- Huang, Y.; Jiang, J.; Zhang, K.; Yao, P. Screening of High Antioxidant Lactic Acid Bacteria from Preserved Vegetables in Northern Anhui Province. Farm Prod. Process. 2020, 2, 19–21, 28. [Google Scholar]
- Siesto, G.; Pietrafesa, R.; Infantino, V.; Thanh, C.; Pappalardo, I.; Romano, P.; Capece, A. In Vitro Study of Probiotic, Antioxidant and Anti-Inflammatory Activities among Indigenous Saccharomyces cerevisiae Strains. Foods 2022, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cai, Y.; Chen, Z.; Shi, H.; Zhou, Y.; Yang, Y.; Tu, R.; Chen, G.; Wang, S. Screening and Characterization of Potential Antioxidant Probiotics Isolated from the Gut of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Front. Mar. Sci. 2022, 9, 1175. [Google Scholar] [CrossRef]
- Pieniz, S.; Andreazza, R.; Okeke, B.C.; Camargo, F.A.; Brandelli, A. Antimicrobial and antioxidant activities of Enterococcus species isolated from meat and dairy products. Braz. J. Biol. 2015, 75, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Lili, D.; Xinran, L.; Yongyue, G.; Xiaoling, C.; Xiaomi, W.; Fengling, B.; Shumin, Y.; Xiaohua, G. Screening for and Identification of Lactic Acid Bacteria with Antioxidant Activity from the Intestinal Tract of Fish. Food Sci. 2021, 42, 127–132. [Google Scholar]
- Han, Q.; Kong, B.; Chen, Q.; Sun, F.; Zhang, H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Foods 2017, 32, 391–400. [Google Scholar] [CrossRef]
- Jie, W.; Wu, Y.; Bai, X.; Zhou, L. Isolation, Identification and Antioxidant Evaluation of Lactic Acid Bacteria from Sichuan Traditional Pickles. Food Ferment. Sci. Technol. 2022, 58, 35–41. [Google Scholar]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef]
- Wang, H.; Li, L. Comprehensive Evaluation of Probiotic Property, Hypoglycemic Ability and Antioxidant Activity of Lactic Acid Bacteria. Foods 2022, 11, 1363. [Google Scholar] [CrossRef]
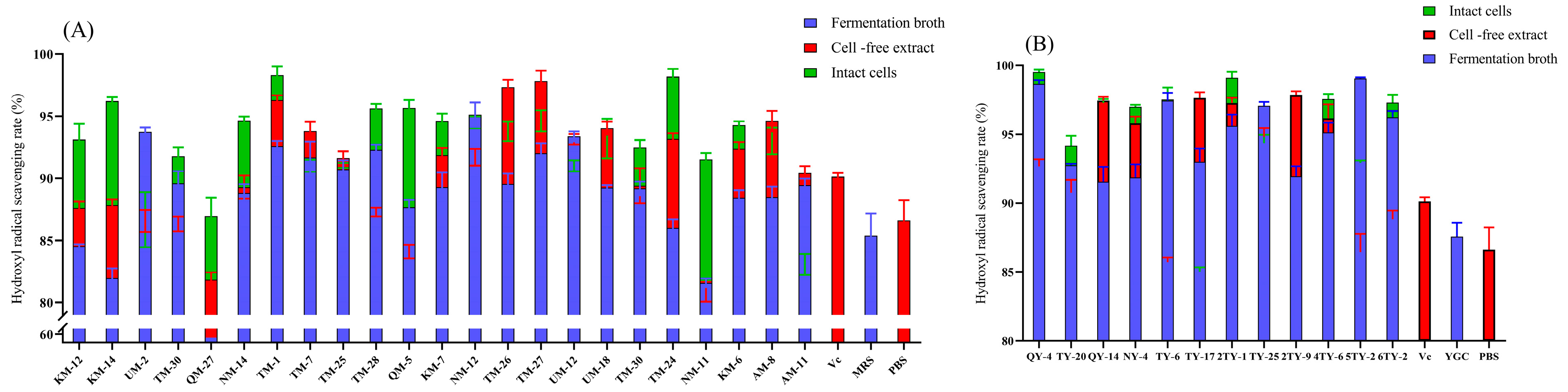
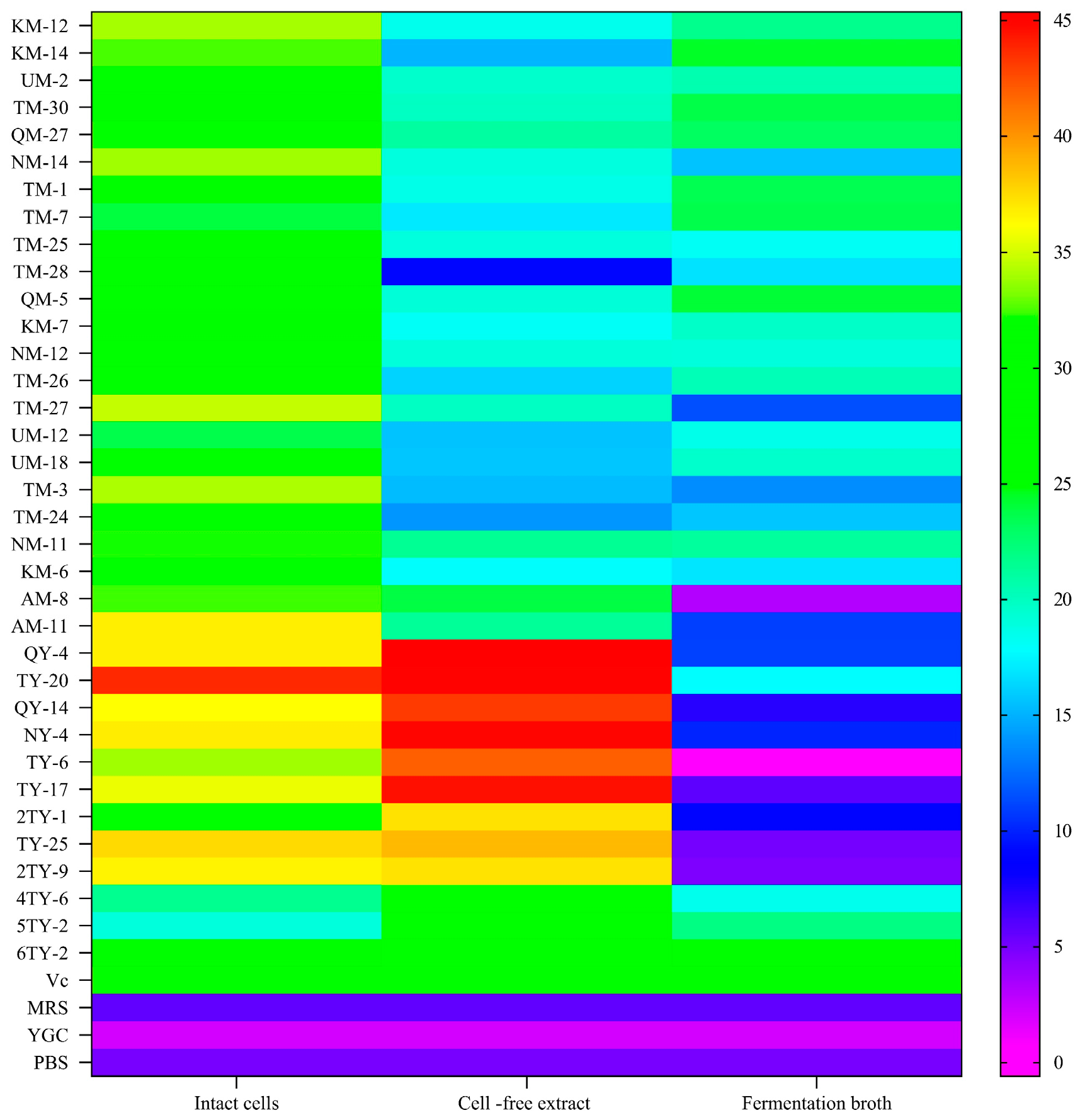
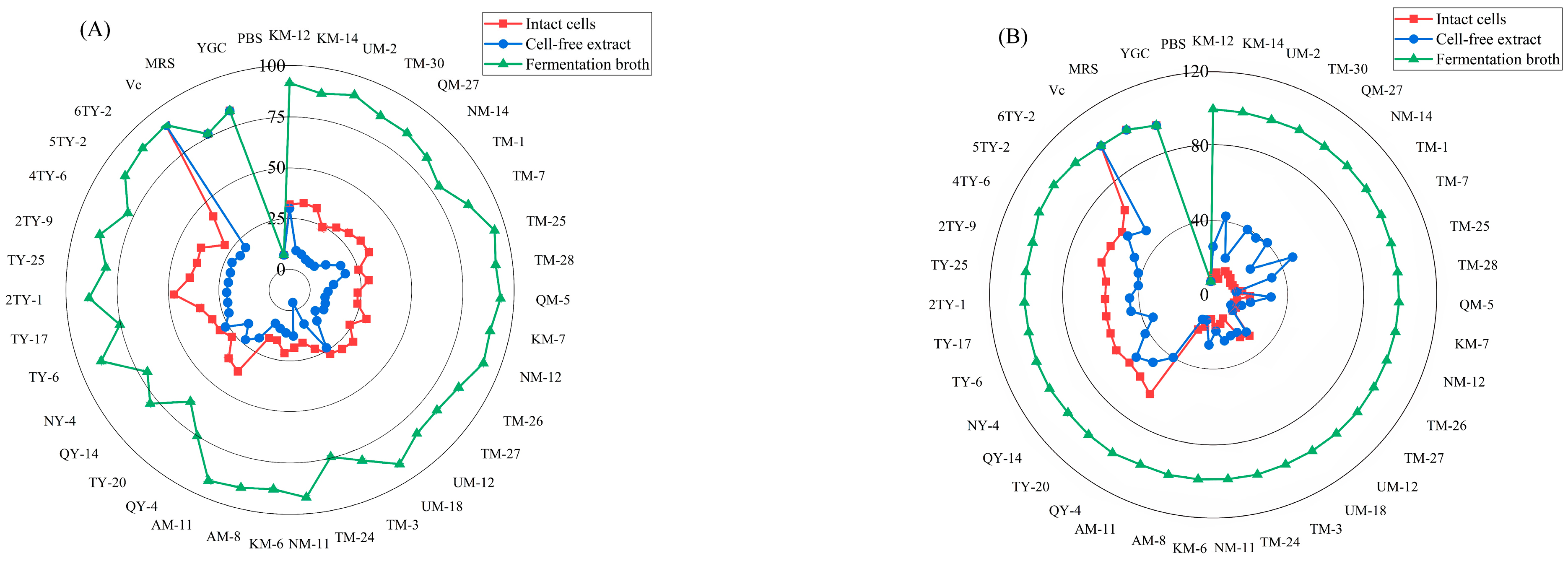
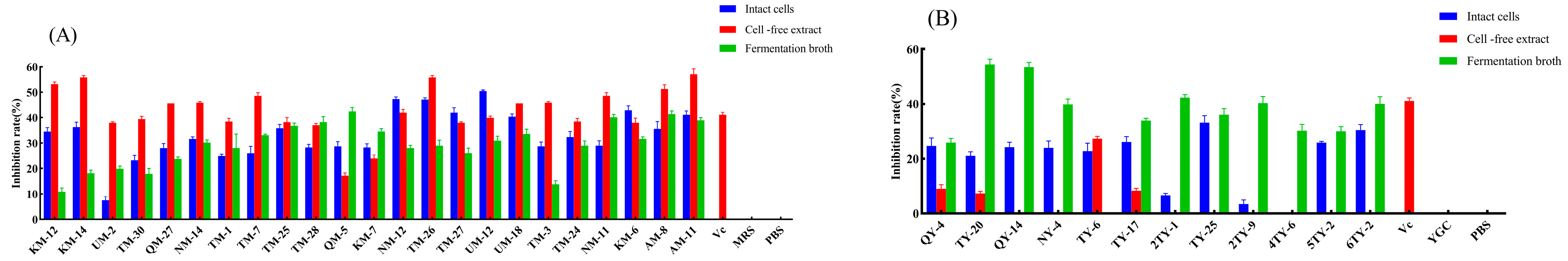
| Strain Number | SOD | GPX | CAT Vitality | ||
|---|---|---|---|---|---|
| Inhibition Rate (%) | Vitality (U/mL) | Inhibition Rate (%) | Vitality (U/mL) | (U/mL) | |
| KM-14 | 29.50 ± 0.90 cd | 4.6545 ± 0.1995 cd | 9.90 ± 0.99 efg | 0.0340 ± 0.0034 efg | - |
| NM-14 | 39.55 ± 2.32 ab | 7.3254 ± 07368 ab | - | - | 19.7980 ±0.4009 d |
| TM-28 | 15.15 ± 0.60 e | 1.9854 ± 0.0927 e | 23.94 ± 2.82 de | 0.0579 ± 0.0068 de | 49.5629 ±2.5470 c |
| NM-12 | 30.35 ± 0.90 cd | 4.8464 ± 0.2069 cd | 34.45 ± 2.23 b | 0.1396 ± 0.0090 b | 19.9336 ± 3.0720 d |
| TM-27 | 40.88 ± 0.94 ab | 7.6904 ± 0.2942 ab | - | - | 16.3052 ± 1.8400 de |
| UM-12 | 44.99 ± 1.18 a | 9.1054 ± 0.4284 a | 31.73 ± 1.67 bc | 0.1123 ± 0.0059 bc | 3.7488 ± 0.9260 ef |
| UM-18 | 33.91 ± 0.86 bcd | 5.7053 ± 0.2181 bcd | 24.83 ± 1.34 bc | 0.1260 ± 0.0068 bc | 27.1753 ± 2.0760 d |
| TM-24 | 44.84 ± 1.34 a | 9.0542 ± 0.4795 a | 10.08 ± 1.46 ef | 0.0409 ± 0.0059 ef | - |
| NM-11 | 43.95 ± 1.03 a | 8.7241 ± 0.3646 a | 4.08 ± 1.02 fg | 0.0136 ± 0.0068 fg | 92.0948 ± 1.0170 a |
| AM-11 | 28.21 ± 0.79 d | 4.3687 ± 0.1717 d | 58.82 ± 1.68 a | 0.2383 ± 0.0068 a | - |
| QY-4 | 36.95 ± 0.79 bc | 6.5178 ± 0.2234 bc | 29.35 ± 1.88 cd | 0.0919 ± 0.0059 cd | 97.2890 ± 0.5346 a |
| TY-20 | 33.38 ± 3.84 bcd | 5.6766 ± 0.9557 bcd | 8.75 ± 2.50 fg | 0.0238 ± 0.0068 fg | 3.3724 ± 1.7850 ef |
| TY-6 | 35.10 ± 0.47 bcd | 6.0116 ± 0.1247 bcd | 22.73 ± 0.91 cd | 0.0851 ± 0.0034 cd | - |
| TY-25 | 29.60 ± 1.44 cd | 4.6842 ± 0.3294 cd | 11.54 ± 1.67 ef | 0.0409 ± 0.0059 ef | 63.9560 ± 2.1760 b |
| 6TY-2 | 27.67 ± 0.59 d | 4.2520 ± 0.1239 d | 27.27 ± 4.16 ef | 0.1021 ± 0.0156 bc | 14.8899 ± 2.1130 de |
| PBS | 5.32 ± 0.32 e | 0.6230 ± 0.0409 e | 3.74 ± 0.94 fg | 0.0255 ± 0.0034 fg | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduxukur, D.; Tursuntay, A.; Zhu, X.; Wang, X.; Rahman, N. Antioxidant Capacity of Lactic Acid Bacteria and Yeasts from Xinjiang Traditional Fermented Dairy Products. Fermentation 2023, 9, 639. https://doi.org/10.3390/fermentation9070639
Abduxukur D, Tursuntay A, Zhu X, Wang X, Rahman N. Antioxidant Capacity of Lactic Acid Bacteria and Yeasts from Xinjiang Traditional Fermented Dairy Products. Fermentation. 2023; 9(7):639. https://doi.org/10.3390/fermentation9070639
Chicago/Turabian StyleAbduxukur, Dilihumar, Adila Tursuntay, Xiaoying Zhu, Xiaoyi Wang, and Nurgvl Rahman. 2023. "Antioxidant Capacity of Lactic Acid Bacteria and Yeasts from Xinjiang Traditional Fermented Dairy Products" Fermentation 9, no. 7: 639. https://doi.org/10.3390/fermentation9070639
APA StyleAbduxukur, D., Tursuntay, A., Zhu, X., Wang, X., & Rahman, N. (2023). Antioxidant Capacity of Lactic Acid Bacteria and Yeasts from Xinjiang Traditional Fermented Dairy Products. Fermentation, 9(7), 639. https://doi.org/10.3390/fermentation9070639






