Isolation of γ-Aminobutyric Acid (GABA)-Producing Lactic Acid Bacteria with Anti-Inflammatory Effects from Fermented Foods in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of LAB
2.2. Screening of GABA-Producing LAB
2.3. Quantitative Analysis of GABA Using an Amino Acid Analyzer
2.4. Anti-Inflammatory Effect of Selected Strains on Murine Macrophages (RAW 246.7 Cells)
2.4.1. Preparation of Cell-Free Supernatant (CFS)
2.4.2. Culture of RAW 264.7 Cells
2.4.3. Cell Viability Assay
2.4.4. Nitric Oxide (NO) Production
2.4.5. Protein Extraction
2.4.6. Western Blotting
2.5. Statistical Analysis
3. Results and Discussion
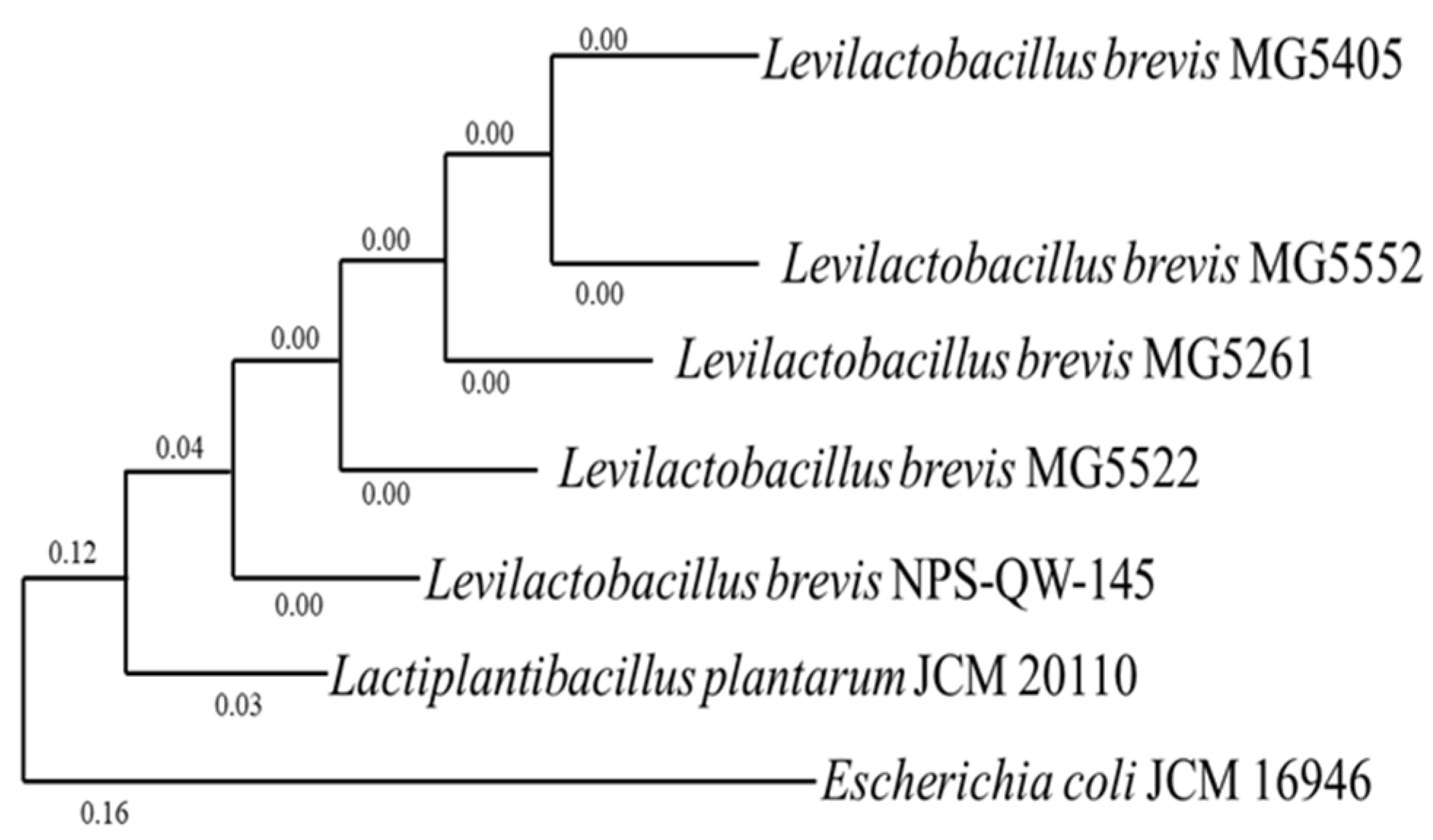
3.1. Isolation of LAB from Different Food Sources
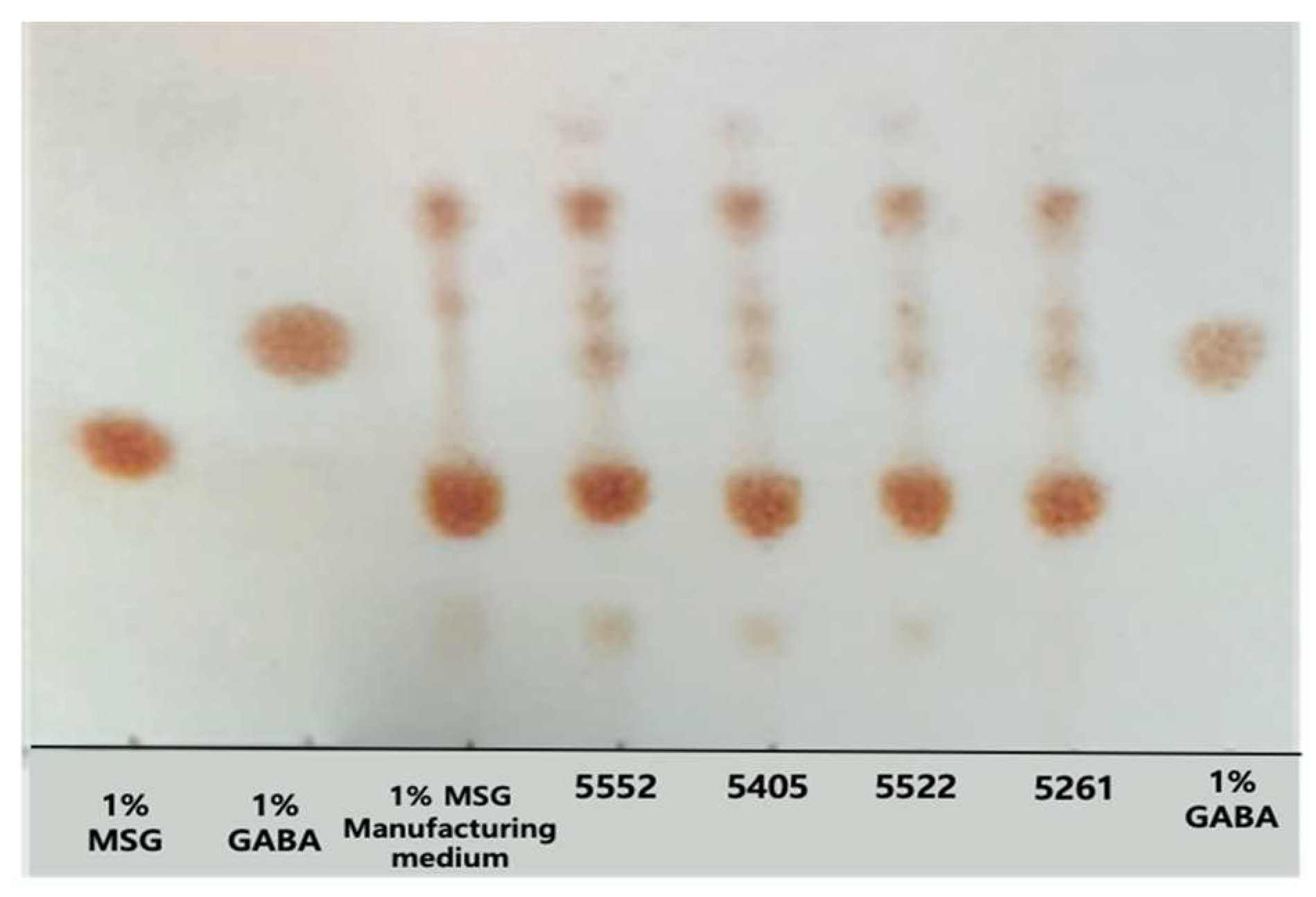
3.2. Screening of GABA-Producing LAB Strains
3.3. Quantitative Analysis of GABA Produced by the Selected LAB Strains
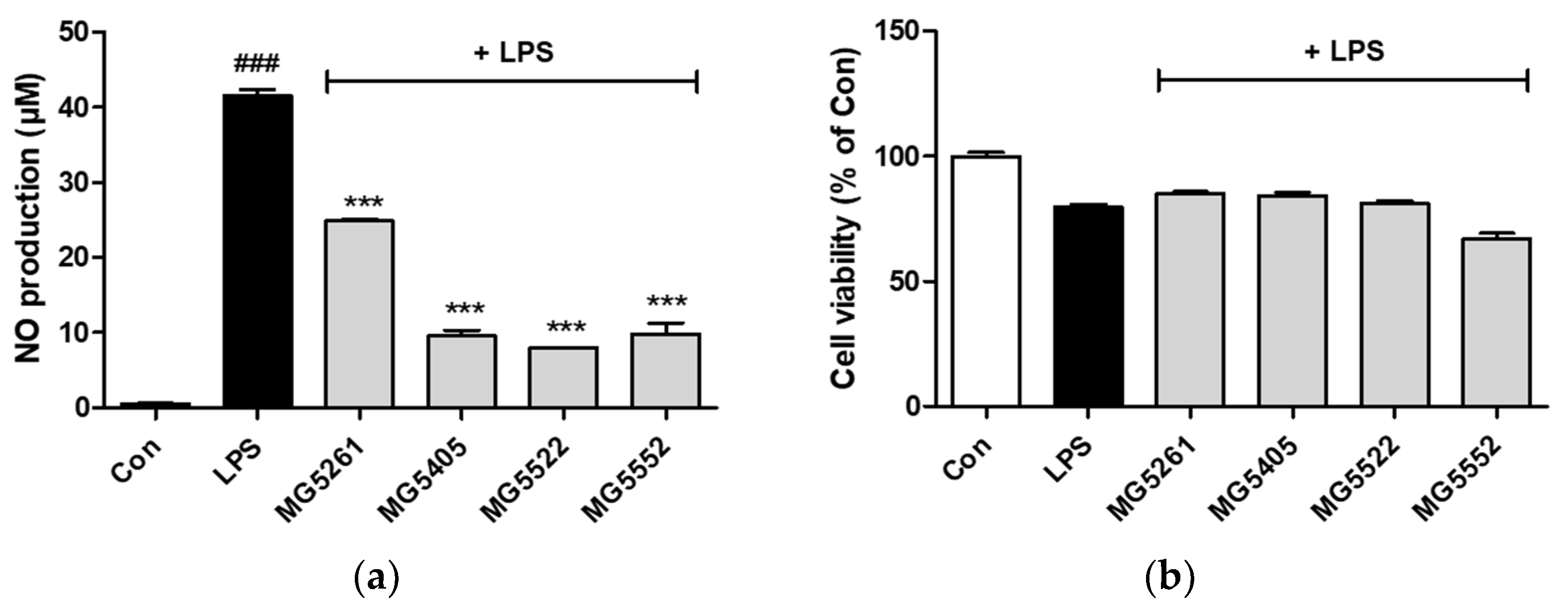
3.4. Effect of CFS of GABA-Producing Strains on Viability of and NO Production in RAW 264.7 Cells
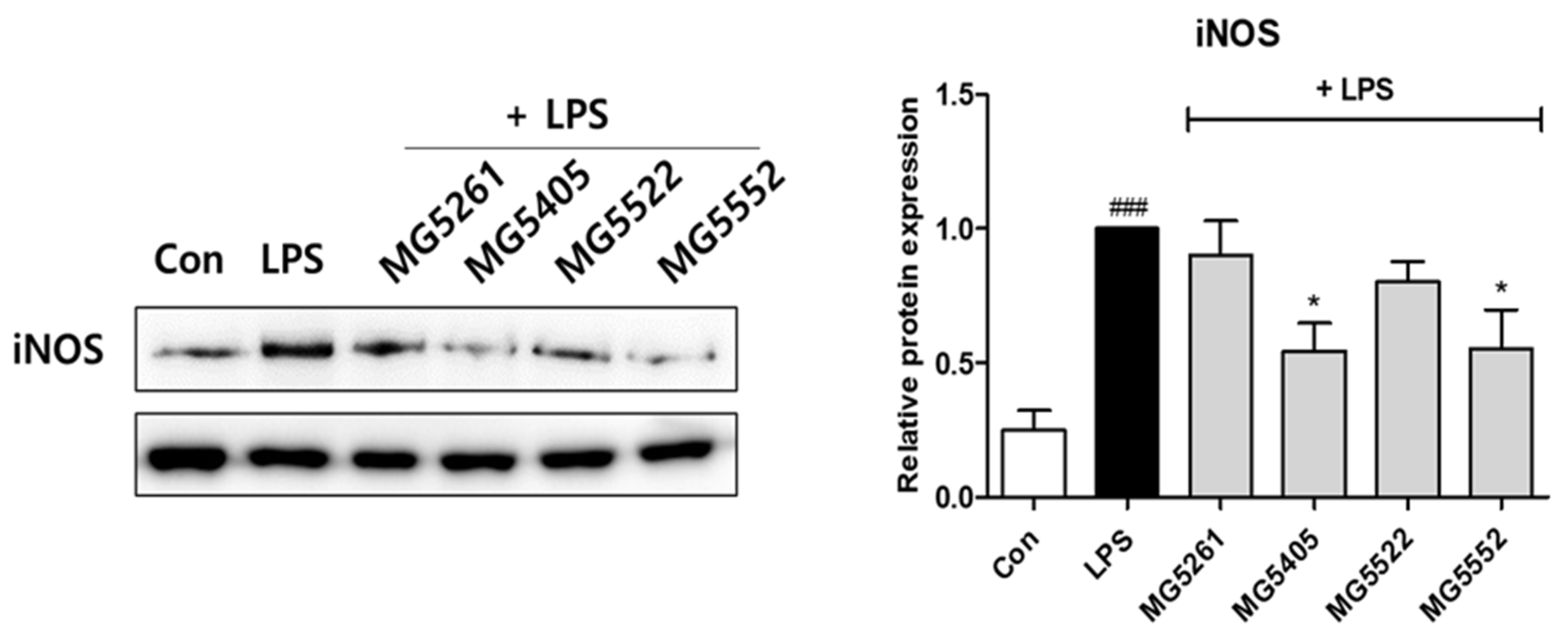
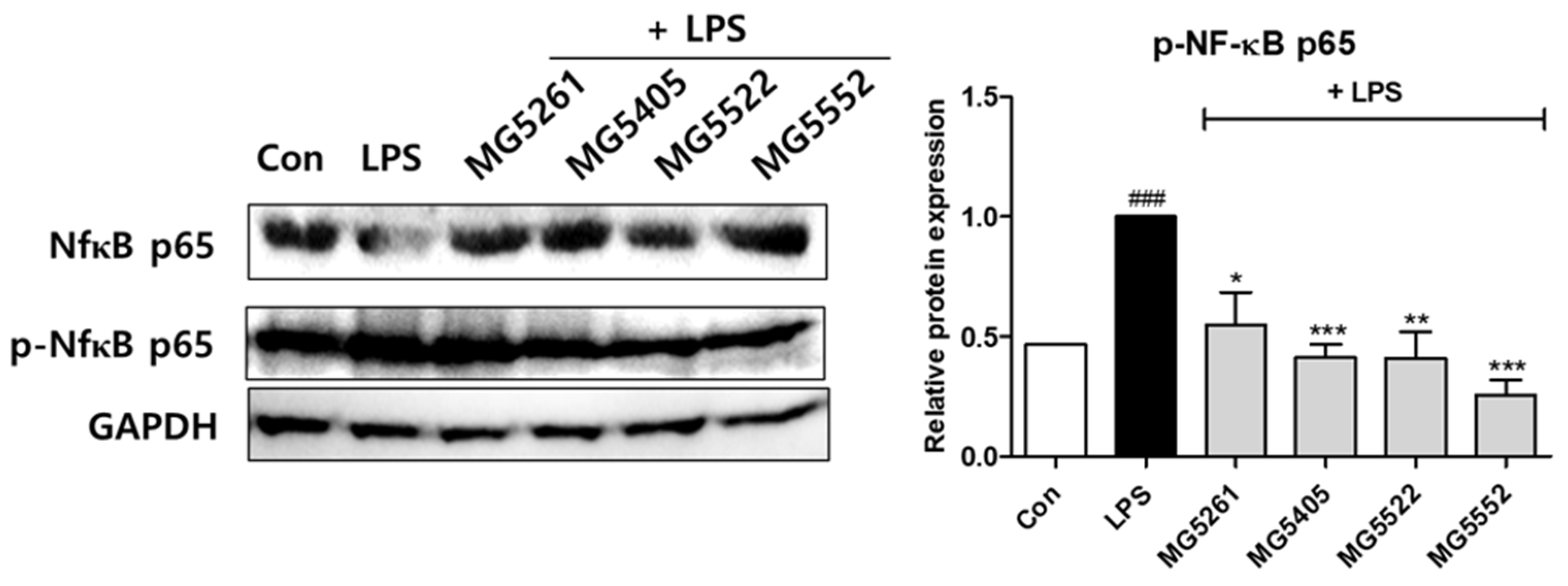
3.5. Effect of CFS of GABA-Producing Strains on NF-κB Activation in RAW 264.7 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crittenden, D.L.; Chebib, M.; Jordan, M.J.T. Stabilization of Zwitterions in Solution: GABA Analogues. J. Phys. Chem. A 2005, 109, 4195–4201. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, D.L.; Chebib, M.; Jordan, M.J.T. Stabilization of Zwitterions in Solution: γ-Aminobutyric Acid (GABA). J. Phys. Chem. A 2004, 108, 203–211. [Google Scholar] [CrossRef]
- Strain, G.W. Nutrition, Brain Function and Behavior. Psychiatr. Clin. N. Am. 1981, 4, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Mitsushima, D.; Shwe, T.T.W.; Funabashi, T.; Shinohara, K.; Kimura, F. GABA Release in the Medial Preoptic Area of Cyclic Female Rats. Neuroscience 2002, 113, 109–114. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of gaba (γ-Aminobutyric acid) by microorganisms: A review. Braz J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef]
- Masuda, K.; Guo, X.-F.; Uryu, N.; Hagiwara, T.; Watabe, S. Isolation of Marine Yeasts Collected from the Pacific Ocean Showing a High Production of-Aminobutyric Acid. Biosci. Biotechnol. Biochem. 2008, 72, 3265–3272. [Google Scholar] [CrossRef]
- Seo, M.-J.; Lee, J.-Y.; Nam, Y.-D.; Lee, S.-Y.; Park, S.-L.; Yi, S.-H.; Lee, M.-H.; Roh, S.W.; Choi, H.-J.; Lim, S.-I. Production of γ-Aminobutyric Acid by Lactobacillus Brevis 340G Isolated from Kimchi and Its Application to Skim Milk. Food Eng. Prog. 2013, 17, 418–423. [Google Scholar] [CrossRef]
- Li, H.; Qiu, T.; Huang, G.; Cao, Y. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb Cell Fact. 2010, 9, 85. [Google Scholar] [CrossRef]
- Wallace, T.C.; Guarner, F.; Madsen, K.; Cabana, M.D.; Gibson, G.; Hentges, E.; Sanders, M.E. Human Gut Microbiota and Its Relationship to Health and Disease. Nutr. Rev. 2011, 69, 392–403. [Google Scholar] [CrossRef]
- Hwanhlem, N.; Watthanasakphuban, N.; Riebroy, S.; Benjakul, S.; H-Kittikun, A.; Maneerat, S. Probiotic Lactic Acid Bacteria from Kung-Som: Isolation, Screening, Inhibition of Pathogenic Bacteria. Int. J. Food Sci. Technol. 2010, 45, 594–601. [Google Scholar] [CrossRef]
- Delcenserie, V.; Martel, D.; Lamoureux, M.; Amiot, J.; Boutin, Y.; Roy, D. Immunomodulatory Effects of Probiotics in the Intestinal Tract. Curr. Issues Mol. Biol. 2008, 10, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA Exerts Protective and Regenerative Effects on Islet Beta Cells and Reverses Diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef]
- Kook, M.; Cheol, C.S. Production of GABA (Gamma Amino Butyric Acid) by Lactic Acid Bacteria. Food Sci. Anim. Resour. 2013, 33, 377–389. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, K.S. Isolation and Identification of γ-Aminobutyric Acid (GABA)-Producing Lactic Acid Bacteria from Kimchi. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 777–785. [Google Scholar] [CrossRef]
- Kanklai, J.; Somwong, T.C.; Rungsirivanich, P.; Thongwai, N. Screening of GABA-Producing Lactic Acid Bacteria from Thai Fermented Foods and Probiotic Potential of Levilactobacillus Brevis F064A for GABA-Fermented Mulberry Juice Production. Microorganisms 2020, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, X.; Chen, Y.; Jiang, W.; Chen, H. The Effect of Five Probiotic Lactobacilli Strains on the Growth and Biofilm Formation of Streptococcus Mutans. Oral Dis 2015, 21, e128–e134. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, M.Y.; Ji, G.E.; Lee, Y.S.; Hwang, K.T. Production of Gamma-Aminobutyric Acid in Black Raspberry Juice during Fermentation by Lactobacillus Brevis GABA100. Int. J. Food Microbiol. 2009, 130, 12–16. [Google Scholar] [CrossRef]
- Park, K.-B.; Oh, S.-H. Isolation and Characterization of Lactobacillus Buchneri Strains with High γ-Aminobutyric Acid Producing Capacity from Naturally Aged Cheese. Food Sci. Biotechnol. 2006, 15, 86–90. [Google Scholar]
- Veno, Y.; Hayakawa, K.; Takahashi, S.; Oda, K. Purification and Characterization of Glutamate Decarboxylase from Lactobacillus Brevis IFO 12005. Biosci. Biotechnol. Biochem. 1997, 61, 1168–1171. [Google Scholar] [CrossRef]
- Mancini, A.; Carafa, I.; Franciosi, E.; Nardin, T.; Bottari, B.; Larcher, R.; Tuohy, K.M. In Vitro Probiotic Characterization of High GABA Producing Strain Lactobacilluas Brevis DSM 32386 Isolated from Traditional “Wild” Alpine Cheese. Ann. Microbiol. 2019, 69, 1435–1443. [Google Scholar] [CrossRef]
- Shreshtha, S.; Sharma, P.; Kumar, P.; Sharma, R.; Singh, S.P. Nitric Oxide: It’s Role in Immunity. J. Clin. Diagn. Res. 2018, 12, 1–5. [Google Scholar] [CrossRef]
- Aktan, F. INOS-Mediated Nitric Oxide Production and Its Regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Bhandage, A.K.; Jin, Z.; Korol, S.V.; Shen, Q.; Pei, Y.; Deng, Q.; Espes, D.; Carlsson, P.O.; Kamali-Moghaddam, M.; Birnir, B. GABA Regulates Release of Inflammatory Cytokines From Peripheral Blood Mononuclear Cells and CD4+ T Cells and Is Immunosuppressive in Type 1 Diabetes. EBioMedicine 2018, 30, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lu, X.; Yang, S.; Zou, Y.; Zeng, F.; Xiong, S.; Cao, Y.; Zhou, W. The Anti-Inflammatory Activity of GABA-Enriched Moringa Oleifera Leaves Produced by Fermentation with Lactobacillus Plantarum LK-1. Front. Nutr. 2023, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Lee, Y.J.; Yoon, J.J.; Choi, E.S.; Namgung, S.; Jin, X.J.; Jeong, D.H.; Kang, D.G.; Lee, H.S. Hwangryunhaedoktang Exerts Anti-Inflammation on LPS-Induced NO Production by Suppressing MAPK and NF-ΚB Activation in RAW264.7 Macrophages. J. Integr. Med. 2017, 15, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Van Thanh, N.; Jang, H.J.; Vinh, L.B.; Linh, K.T.P.; Huong, P.T.T.; Cuong, N.X.; Nam, N.H.; Van Minh, C.; Kim, Y.H.; Yang, S.Y. Chemical Constituents from Vietnamese Mangrove Calophyllum Inophyllum and Their Anti-Inflammatory Effects. Bioorg. Chem. 2019, 88, 102921. [Google Scholar] [CrossRef]
- Chalmers, S.A.; Garcia, S.J.; Reynolds, J.A.; Herlitz, L.; Putterman, C. NF-KB Signaling in Myeloid Cells Mediates the Pathogenesis of Immune-Mediated Nephritis. J. Autoimmun. 2019, 98, 33–43. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, C.H.; Jayasooriya, R.G.P.T.; Dilshara, M.G.; Lee, S.; Choi, Y.H.; Seo, Y.T.; Kim, G.Y. Hydrangenol Inhibits Lipopolysaccharide-Induced Nitric Oxide Production in BV2 Microglial Cells by Suppressing the NF-ΚB Pathway and Activating the Nrf2-Mediated HO-1 Pathway. Int. Immunopharmacol. 2016, 35, 61–69. [Google Scholar] [CrossRef]
- Niu, X.; Zang, L.; Li, W.; Xiao, X.; Yu, J.J.; Yao, Q.; Zhao, J.; Ye, Z.; Hu, Z.; Li, W. Anti-Inflammatory Effect of Yam Glycoprotein on Lipopolysaccharide-Induced Acute Lung Injury via the NLRP3 and NF-ΚB/TLR4 Signaling Pathway. Int. Immunopharmacol. 2020, 81, 106024. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.J.; Lee, G.H.; Huang, J.J.; Whang, W.K.; Zhang, X.D.; Yook, C.S.; Liu, X.Q. Anti-Inflammatory Effects of Two Lupane-Type Triterpenes from Leaves of Acanthopanax Gracilistylus on LPS-Induced RAW264.7 Macrophages. Food Sci. Technol. 2021, 42, e89721. [Google Scholar] [CrossRef]
| Species | Strain | Isolation Source | GABA Concentration (mg/mL) | |
|---|---|---|---|---|
| MRS | MRS + 1%MSG | |||
| Control | - | - | 0.00 | 0.00 |
| Levilactobacillus brevis | MG5261 | Fermented food | 0.304 | 0.624 |
| Levilactobacillus brevis | MG5405 | Fermented food | 0.260 | 0.585 |
| Levilactobacillus brevis | MG5522 | Fermented food | 0.281 | 0.591 |
| Levilactobacillus brevis | MG5552 | Fermented food | 0.322 | 0.976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-L.; Nguyen, T.H.; Kim, J.-S.; Park, J.-Y.; Kang, C.-H. Isolation of γ-Aminobutyric Acid (GABA)-Producing Lactic Acid Bacteria with Anti-Inflammatory Effects from Fermented Foods in Korea. Fermentation 2023, 9, 612. https://doi.org/10.3390/fermentation9070612
Kim Y-L, Nguyen TH, Kim J-S, Park J-Y, Kang C-H. Isolation of γ-Aminobutyric Acid (GABA)-Producing Lactic Acid Bacteria with Anti-Inflammatory Effects from Fermented Foods in Korea. Fermentation. 2023; 9(7):612. https://doi.org/10.3390/fermentation9070612
Chicago/Turabian StyleKim, Ye-Lim, Trung Hau Nguyen, Jin-Seong Kim, Jeong-Yong Park, and Chang-Ho Kang. 2023. "Isolation of γ-Aminobutyric Acid (GABA)-Producing Lactic Acid Bacteria with Anti-Inflammatory Effects from Fermented Foods in Korea" Fermentation 9, no. 7: 612. https://doi.org/10.3390/fermentation9070612
APA StyleKim, Y.-L., Nguyen, T. H., Kim, J.-S., Park, J.-Y., & Kang, C.-H. (2023). Isolation of γ-Aminobutyric Acid (GABA)-Producing Lactic Acid Bacteria with Anti-Inflammatory Effects from Fermented Foods in Korea. Fermentation, 9(7), 612. https://doi.org/10.3390/fermentation9070612






