Premier, Progress and Prospects in Renewable Hydrogen Generation: A Review
Abstract
1. Introduction
2. Progress in Renewable Hydrogen Production
2.1. Electrolysis
2.2. Thermochemical Process
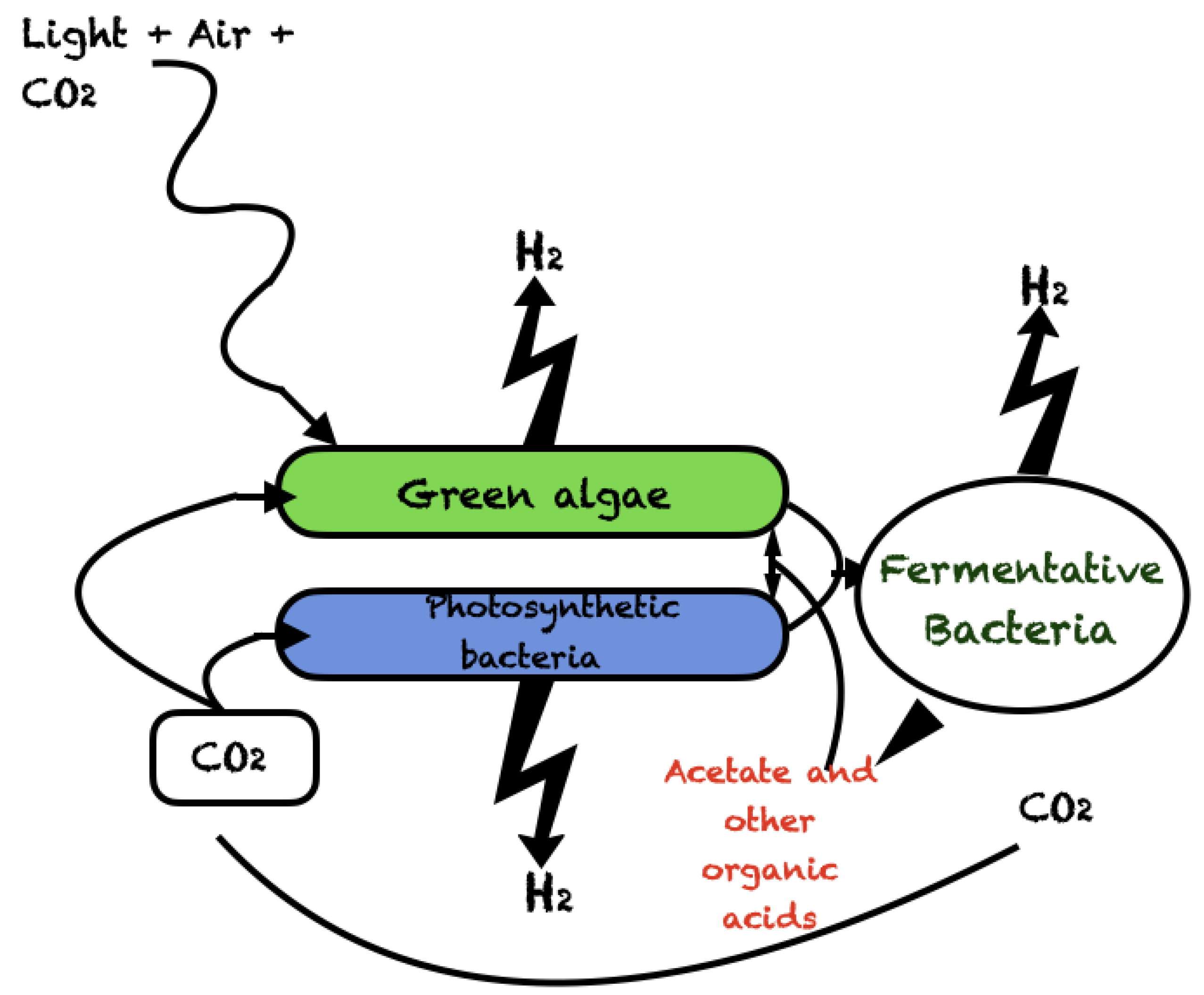
2.3. Photobiological
2.4. Biomass Gasification
2.5. Anaerobic Digestor
2.6. Microbial Electrolysis
2.7. Solar Water Splitting
2.8. Wind-to-Hydrogen
2.9. Dark Fermentation
3. Hydrogen Separation Methods
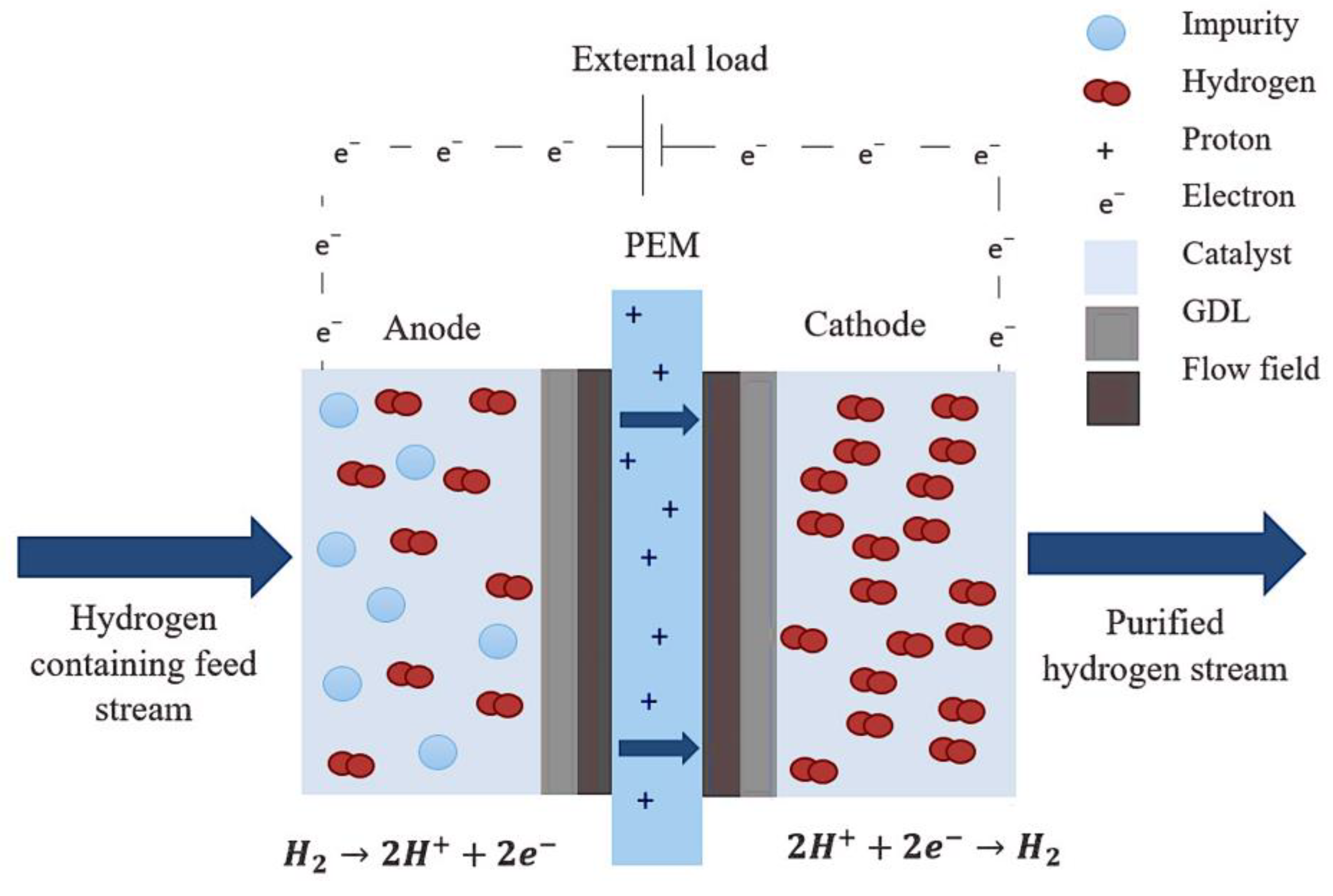
3.1. Membrane-Based Separation Process
3.2. Non-Membrane-Based Separation Process
4. Factors Affecting the Production of Renewable Hydrogen
5. Environmental Impact and Global Scenario of Hydrogen Generation Using Biomass
6. Challenges and Prospects
- Decarbonization and climate change mitigation. Hydrogen is regarded as a clean and adaptable energy source since, when employed in fuel cells or combustion processes, it emits no greenhouse gases. It can significantly contribute to the decarbonization of several industries, including transportation, business, and power generation, assisting in the reduction in greenhouse gas emissions.
- Integration of renewable energy. Hydrogen can be electrolyzed utilizing renewable energy sources as hydroelectric, solar, and wind energy. This makes it possible to produce hydrogen alongside the production of renewable energy, giving a way to store and use extra renewable energy when demand is low. The integration of renewable energy sources and grid flexibility may be significantly aided by hydrogen.
- Hydrogen storage. Hydrogen can be stored and used as a long-term energy storage solution, addressing the erratic nature of renewable energy sources. Hydrogen can also be used to balance the grid. When there is an abundance of energy, it can be converted into hydrogen and stored to be used later when there is a shortage of energy. This can support grid balancing and guarantee a dependable and robust energy system.
- Sector integration and decentralization. By facilitating the use of renewable energy in industries that have historically been challenging to decarbonize, such as heavy industry, shipping, and aviation, hydrogen can help with sector integration. By offering localized energy options, such as off-grid uses and fueling facilities for hydrogen-powered vehicles, it can help assist decentralization.
- Technology improvements and cost savings. Research and development efforts are continually enhancing hydrogen generation technologies, raising their effectiveness, lowering their costs, and boosting system performance. Hydrogen generation is becoming more effective, scalable, and financially viable due to improvements in electrolysis technologies, such as PEM and SOEC.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jefri, M.A.; Zahed, A.H. Energy demands, resources, impact, and technology. Energy Sources 1991, 13, 505–516. [Google Scholar] [CrossRef]
- Rohland, B.; Wendt, H. Hydrogen and fuel cells-the clean energy system. J. Power Sources 1992, 37, 271–277. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Kurz, R.; Winkelmann, B.; Freund, S.; McBain, M.; Keith, M.; Zhang, D.; Cich, S.; Renzi, P.; Schmitt, J. Transport and storage. In Machinery and Energy Systems for the Hydrogen Economy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–249. [Google Scholar]
- Saad Salman, M.; Rambhujun, N.; Pratthana, C.; Lai, Q.; Sapkota, P.; Aguey-Zinsou, K.F. Solid-state hydrogen storage as a future renewable energy technology. In Nano Tools and Devices for Enhanced Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 263–287. [Google Scholar]
- Zhang, L.; Zheng, H.; Wan, T.; Shi, D.; Lyu, L.; Cai, G. An Integrated Control Algorithm of Power Distribution for Islanded Microgrid Based on Improved Virtual Synchronous Generator. IET Renew. Power Gener. 2021, 15, 2674–2685. [Google Scholar] [CrossRef]
- Reigstad, G.A.; Roussanaly, S.; Straus, J.; Anantharaman, R.; de Kler, R.; Akhurst, M.; Sunny, N.; Goldthorpe, W.; Avignon, L.; Pearce, J.; et al. Moving toward the low-carbon hydrogen economy: Experiences and key learnings from national case studies. Adv. Appl. Energy 2022, 8, 100108. [Google Scholar] [CrossRef]
- Katebah, M.; Al-Rawashdeh, M.; Linke, P. Analysis of hydrogen production costs in Steam-Methane Reforming considering integration with electrolysis and CO2 capture. Clean. Eng. Technol. 2022, 10, 100552. [Google Scholar] [CrossRef]
- Massarweh, O.; Al-khuzaei, M.; Al-Shafi, M.; Bicer, Y.; Abushaikha, A.S. Blue hydrogen production from natural gas reservoirs: A review of application and feasibility. J. CO2 Util. 2023, 70, 102438. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Espegren, K.; Damman, S.; Pisciella, P.; Graabak, I.; Tomasgard, A. The role of hydrogen in the transition from a petroleum economy to a low-carbon society. Int. J. Hydrogen Energy 2021, 46, 23125–23138. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. A Review and Evaluation of Photoelectrode Coating Materials and Methods for Photoelectrochemical Hydrogen Production. Int. J. Hydrogen Energy 2016, 41, 7950–7959. [Google Scholar] [CrossRef]
- Goria, K.; Kothari, R.; Singh, A.; Singh, H.M.; Tyagi, V.V. Biohydrogen: Potential applications, approaches, and hurdles to overcome. In Handbook of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 399–418. [Google Scholar]
- Sharma, M.; Mondal, P.; Chakraborty, A.; Kuttippurath, J.; Purkait, M. Effect of Different Molecular Weight Polyethylene Glycol on Flat Sheet Cellulose Acetate Membranes for Evaluating Power Density Performance in Pressure Retarded Osmosis Study. J. Water Process Eng. 2019, 30, 100632. [Google Scholar] [CrossRef]
- Sharma, M.; Chakraborty, A.; Kuttippurath, J.; Yadav, A.K. Potential Power Production from Salinity Gradient at the Hooghly Estuary System. Innov. Energy Res. 2018, 7, 210. [Google Scholar] [CrossRef]
- Sharma, M.; Mondal, P.; Sontakke, A.; Chakraborty, A.; Purkait, M.K. High Performance Graphene-oxide Doped Cellulose Acetate based Ion Exchange Membrane for Environmental Remediation Applications. Int. J. Environ. Anal. Chem. 2021, 1975276. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Chakraborty, A.; Purkait, M.K. Clean energy from salinity gradients using pressure retarded osmosis and reverse electrodialysis: A review. Sustain. Energy Technol. Assess. 2022, 49, 101687. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Sood, T.; Chakraborty, A.; Purkait, M.K. Ameliorated polyvinylidene fluoride based proton exchange membrane impregnated with graphene oxide, and cellulose acetate obtained from sugarcane bagasse for application in microbial fuel cell. J. Environ. Chem. Eng. 2021, 9, 106681. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Sood, T.; Chakraborty, A.; Purkait, M.K. Reduced graphene oxide incorporated polyvinylidene fluoride/cellulose acetate proton exchange membrane for energy extraction using microbial fuel cells. J. Electroanal. Chem. 2022, 907, 115890. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Purkait, M.K. Chapter 16—Energy storage properties of nanomaterials. In Advances in Smart Nanomaterials and Their Applications; Husen, A., Siddiqi, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 337–350. [Google Scholar]
- Harju, H.; Pipitone, G.; Lefferts, L. Influence of the Catalyst Particle Size on the Aqueous Phase Reforming of n-Butanol Over Rh/ZrO2. Front. Chem. 2020, 8, 17. [Google Scholar] [CrossRef]
- Kıpçak, E.; Söğüt, O.Ö.; Akgün, M. Hydrothermal gasification of olive mill wastewater as a biomass source in supercritical water. J. Supercrit. Fluids 2011, 57, 50–57. [Google Scholar] [CrossRef]
- Pipitone, G.; Tosches, D.; Bensaid, S.; Galia, A.; Pirone, R. Valorization of Alginate for the Production of Hydrogen via Catalytic Aqueous Phase Reforming. Catal. Today 2018, 304, 153–164. [Google Scholar] [CrossRef]
- Saleem, M. Possibility of utilizing agriculture biomass as a renewable and sustainable future energy source. Heliyon 2022, 8, E08905. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, T.; Abbasi, S.A. ‘Renewable’ Hydrogen: Prospects and Challenges. Renew. Sustain. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- West, J.B. Henry Cavendish (1731–1810): Hydrogen, carbon dioxide, water, and weighing the world. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L1–L6. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.N.; Jullok, N.; Lau, W.J.; Ong, H.L.; Dong, C.-D. Graphene Oxide Incorporated Polysulfone Substrate for Flat Sheet Thin Film Nanocomposite Pressure Retarded Osmosis Membrane. Membranes 2020, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Sloop, S.E.; Crandon, L.; Allen, M.; Lerner, M.M.; Zhang, H.; Sirisaksoontorn, W.; Gaines, L.; Kim, J.; Lee, M. Cathode Healing Methods for Recycling of Lithium-Ion Batteries. Sustain. Mater. Technol. 2019, 22, e00113. [Google Scholar] [CrossRef]
- Xiang, Q.-Y.; Wu, D.; Bai, Y.; Yan, K.; Yao, W.-Q.; Zhang, L.; Zhang, J.; Cao, J.-L. Atomic Hydrogenation-Induced Paramagnetic-Ferromagnetic Transition in Zinc Ferrite. Ceram. Int. 2016, 42, 16882–16887. [Google Scholar] [CrossRef]
- Raj, K.; Lakhina, P.; Starnger, C. Harnessing Green Hydrogen V21 DIGITAL 29062022; NITI Aayog: New Delhi, India, 2022. [Google Scholar]
- Shiva Kumar, S.; Himabindu, V. Boron-Doped Carbon Nanoparticles Supported Palladium as an Efficient Hydrogen Evolution Electrode in PEM Water Electrolysis. Renew. Energy 2020, 146, 2281–2290. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen Cost Reduction: Scaling Up Electrolysers to Meet the 1.5 °C Climate Goal; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- European Comission. A Hydrogen Strategy for a Climate-Neutral Europe; CELEX_52020DC0301; European Comission: Brussels, Belgium, 2020. [Google Scholar]
- Egeland-Eriksen, T.; Hajizadeh, A.; Sartori, S. Hydrogen-based systems for integration of renewable energy in power systems: Achievements and perspectives. Int. J. Hydrogen Energy 2021, 46, 31963–31983. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and Innovative Solid Oxide Electrolysis Cell Materials: A Short Review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Ruiz, B.; Fuente, E.; Pérez, A.; Taboada-Ruiz, L.; Sanz, J.M.; Calvo, L.F.; Paniagua, S. Employment of Conventional and Flash Pyrolysis for Biomass Wastes from the Textile Industry with Sustainable Prospects. J. Anal. Appl. Pyrolysis 2023, 169, 105864. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A.; Jamil, M.M.; Azli, A.A.M.; Misbah, M.F. A review on biomass-based hydrogen production for renewable energy supply. Int. J. Energy Res. 2015, 39, 1597–1615. [Google Scholar] [CrossRef]
- Gautam, R.; Nayak, J.K.; Ress, N.V.; Steinberger-Wilckens, R.; Ghosh, U.K. Bio-Hydrogen Production through Microbial Electrolysis Cell: Structural Components and Influencing Factors. Chem. Eng. J. 2023, 455, 140535. [Google Scholar] [CrossRef]
- Alvarez, J.; Kumagai, S.; Wu, C.; Yoshioka, T.; Bilbao, J.; Olazar, M.; Williams, P.T. Hydrogen Production from Biomass and Plastic Mixtures by Pyrolysis-Gasification. Int. J. Hydrogen Energy 2014, 39, 10883–10891. [Google Scholar] [CrossRef]
- Touloupakis, E.; Faraloni, C.; Silva Benavides, A.M.; Masojídek, J.; Torzillo, G. Sustained Photobiological Hydrogen Production by Chlorella vulgaris without Nutrient Starvation. Int. J. Hydrogen Energy 2021, 46, 3684–3694. [Google Scholar] [CrossRef]
- Wang, H. Multicriteria sustainability ranking of biohydrogen systems. In Waste to Renewable Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2023; pp. 195–210. [Google Scholar]
- Ni, M.; Leung, M.K.H.; Sumathy, K.; Leung, D.Y.C. Potential of Renewable Hydrogen Production for Energy Supply in Hong Kong. Int. J. Hydrogen Energy 2006, 31, 1401–1412. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-Hydrogen: A Review of Main Routes Production, Processes Evaluation and Techno-Economical Assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Haberl, H.; Sprinz, D.; Bonazountas, M.; Cocco, P.; Desaubies, Y.; Henze, M.; Hertel, O.; Johnson, R.K.; Kastrup, U.; Laconte, P.; et al. Correcting a Fundamental Error in Greenhouse Gas Accounting Related to Bioenergy. Energy Policy 2012, 45, 18–23. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of Hydrogen Production from Biogas Reforming: Technological Advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Kaya, S.; Ozturk, B.; Aykac, H. Hydrogen production from renewable source: Biogas. In Proceedings of the 2013 International Conference on Renewable Energy Research and Applications 2013—ICRERA 2013, Madrid, Spain, 20–23 October 2013; pp. 633–637. [Google Scholar]
- Swartbooi, A.; Kapanji-Kakoma, K.K.; Musyoka, N.M. From Biogas to Hydrogen: A Techno-Economic Study on the Production of Turquoise Hydrogen and Solid Carbons. Sustainability 2022, 14, 11050. [Google Scholar] [CrossRef]
- Da Rosa, A.V.; Ordóñez, J.C. Hydrogen Production. In Fundamentals of Renewable Energy Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 419–470. [Google Scholar]
- Park, M.-J.; Kim, H.-M.; Gu, Y.-J.; Jeong, D.-W. Optimization of Biogas-Reforming Conditions Considering Carbon Formation, Hydrogen Production, and Energy Efficiencies. Energy 2023, 265, 126273. [Google Scholar] [CrossRef]
- Paul, A.; Kurian, M. Catalytic applications of carbon dots. In Carbon Dots in Analytical Chemistry: Detection and Imaging; Elsevier: Amsterdam, The Netherlands, 2022; pp. 337–344. [Google Scholar]
- Tahir, M.B.; Batool, A. Chapter 3—Recent Development in Sustainable Technologies for Clean Hydrogen Evolution: Current Scenario and Future Perspectives. In Sustainable Materials and Green Processing for Energy Conversion; Cheong, K.Y., Apblett, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 97–130. [Google Scholar]
- Ghosh, S.; Hajra, P. Chapter 6—Metal Oxide Catalysts for Photoelectrochemical Water Splitting. In Metal Oxide-Based Nanostructured Electrocatalysts for Fuel Cells, Electrolyzers, and Metal-Air Batteries; Napporn, T.W., Holade, Y., Eds.; Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 105–138. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Passini, L.; Dias, J.A.; Gonçalves, G.F.B.; Ullah, S.; Neto, E.P.F.; Manzani, D. Chapter 16—Halide-Based Perovskites in Photonics: From Photocatalysts to Highly Efficient Optoelectronic Devices. In Perovskite Ceramics; Huamán, J.L.C., Rivera, V.A.G., Eds.; Elsevier Series in Advanced Ceramic Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 547–600. [Google Scholar]
- Sani, Y.M.; Solangi, N.H.; Bello, T.K.; Isa, M.T. Chapter 7—Perovskite-Based Nanomaterials for CO2 Conversion. In Nanomaterials for Carbon Dioxide Capture and Conversion Technologies; Mazari, S.A., Mubarak, N.M., Tripathi, M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 181–209. [Google Scholar]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.H.; Ma, B. Low dimensional metal halide perovskites and hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Goto, Y.; Hisatomi, T.; Wang, Q.; Higashi, T.; Ishikiriyama, K.; Maeda, T.; Sakata, Y.; Okunaka, S.; Tokudome, H.; Katayama, M.; et al. A Particulate Photocatalyst Water-Splitting Panel for Large-Scale Solar Hydrogen Generation. Joule 2018, 2, 509–520. [Google Scholar] [CrossRef]
- Douak, M.; Settou, N. Estimation of Hydrogen Production Using Wind Energy in Algeria. Energy Procedia 2015, 74, 981–990. [Google Scholar] [CrossRef]
- Henry, A.; McCallum, C.; McStay, D.; Rooney, D.; Robertson, P.; Foley, A. Analysis of Wind to Hydrogen Production and Carbon Capture Utilisation and Storage Systems for Novel Production of Chemical Energy Carriers. J. Clean. Prod. 2022, 354, 131695. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of Methane Production in Anaerobic Digestion Process: A Review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Ren, Y.; Si, B.; Liu, Z.; Jiang, W.; Zhang, Y. Promoting Dark Fermentation for Biohydrogen Production: Potential Roles of Iron-Based Additives. Int. J. Hydrogen Energy 2022, 47, 1499–1515. [Google Scholar] [CrossRef]
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts 2021, 11, 591. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen Production, Storage, Utilisation and Environmental Impacts: A Review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. Hydrogen Separation and Purification from Various Gas Mixtures by Means of Electrochemical Membrane Technology in the Temperature Range 100–160 °C. Membranes 2021, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ho, W.S.W. Recent Advances in Polymeric Membranes for CO2 Capture. Chin. J. Chem. Eng. 2018, 26, 2238–2254. [Google Scholar] [CrossRef]
- Lu, H.T.; Li, W.; Miandoab, E.S.; Kanehashi, S.; Hu, G. The Opportunity of Membrane Technology for Hydrogen Purification in the Power to Hydrogen (P2H) Roadmap: A Review. Front. Chem. Sci. Eng. 2021, 15, 464–482. [Google Scholar] [CrossRef]
- Lu, G.Q.; Diniz da Costa, J.C.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic Membranes for Hydrogen Production and Purification: A Critical Review and Perspective. J. Colloid Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef]
- Wang, W.; Olguin, G.; Hotza, D.; Seelro, M.A.; Fu, W.; Gao, Y.; Ji, G. Inorganic Membranes for In-Situ Separation of Hydrogen and Enhancement of Hydrogen Production from Thermochemical Reactions. Renew. Sustain. Energy Rev. 2022, 160, 112124. [Google Scholar] [CrossRef]
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Ahn, S.; You, Y.-W.; Lee, D.-G.; Kim, K.-H.; Oh, M.; Lee, C.-H. Layered Two- and Four-Bed PSA Processes for H2 Recovery from Coal Gas. Chem. Eng. Sci. 2012, 68, 413–423. [Google Scholar] [CrossRef]
- Golmakani, A.; Fatemi, S.; Tamnanloo, J. Investigating PSA, VSA, and TSA Methods in SMR Unit of Refineries for Hydrogen Production with Fuel Cell Specification. Sep. Purif. Technol. 2017, 176, 73–91. [Google Scholar] [CrossRef]
- Aasadnia, M.; Mehrpooya, M.; Ghorbani, B. A Novel Integrated Structure for Hydrogen Purification Using the Cryogenic Method. J. Clean. Prod. 2021, 278, 123872. [Google Scholar] [CrossRef]
- Kumar, K.V.; de Castro, M.C.M.; Martinez-Escandell, M.; Molina-Sabio, M.; Rodriguez-Reinoso, F. Heat of Adsorption and Binding Affinity for Hydrogen on Pitch-Based Activated Carbons. Chem. Eng. J. 2011, 168, 972–978. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Q.; Guo, J.; Hansen, H.A.; Chen, P.; Vegge, T. Balanced Nitrogen and Hydrogen Chemisorption by [RuH6] Catalytic Center Favors Low-Temperature NH3 Synthesis. Cell Rep. Phys. Sci. 2022, 3, 100970. [Google Scholar] [CrossRef]
- Mosquera-Vargas, E.; Tamayo, R.; Morel, M.; Roble, M.; Díaz-Droguett, D.E. Hydrogen Storage in Purified Multi-Walled Carbon Nanotubes: Gas Hydrogenation Cycles Effect on the Adsorption Kinetics and Their Performance. Heliyon 2021, 7, e08494. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Schnitzler, M.C.; da Silva, W.M.; Santos, A.P. Purification of Carbon Nanotubes Produced by the Electric Arc-Discharge Method. Surf. Interfaces 2021, 26, 101389. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future Cost and Performance of Water Electrolysis: An Expert Elicitation Study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Rezazadeh, A. Hydrogen Fuel and Electricity Generation from a New Hybrid Energy System Based on Wind and Solar Energies and Alkaline Fuel Cell. Energy Rep. 2021, 7, 2594–2604. [Google Scholar] [CrossRef]
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen Production, Storage and Transport for Renewable Energy and Chemicals: An Environmental Footprint Assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]

| Hydrogen Generation Technique | Efficiency | Pros | Cons |
|---|---|---|---|
| Steam Methane Reforming (SMR) | 60–70% | Well-established, high production capacity | Reliance on fossil fuels, CO2 emissions |
| Water Electrolysis | 60–80% | Can utilize renewable energy sources | High energy input, capital-intensive |
| Alkaline Electrolysis | 60–70% | Mature technology, relatively low cost | Limited scalability, sensitivity to impurities |
| Proton Exchange Membrane (PEM) Electrolysis | 70–80% | Fast response time, compact design | Expensive materials, sensitivity to impurities |
| Solid Oxide Electrolysis (SOEC) | 70–80% | High efficiency, potential for waste heat utilization | High operating temperature, higher cost |
| High-Temperature PEM Electrolysis | 70–80% | High efficiency, faster operation at elevated temperatures | Higher cost compared to alkaline electrolysis |
| Biological Water Splitting (Photosynthetic and Cyanobacteria) | Varies | Utilizes sunlight and organisms for hydrogen production | Low efficiency, research stage, scalability challenges |
| Thermochemical Water Splitting | Varies | Potential for high efficiency, can use solar or nuclear heat | Complex process, limited commercial viability |
| Process | Description | Efficiency | Cost | Environmental Impact |
|---|---|---|---|---|
| Steam Methane Reforming (SMR) | Reaction between natural gas and steam | High | Low | Moderate CO2 emissions |
| Partial Oxidation (POX) | Combustion of hydrocarbons with limited air | High | Moderate | Moderate CO2 emissions |
| Autothermal Reforming (ATR) | Combination of SMR and POX | High | Moderate | Moderate CO2 emissions |
| Water Electrolysis | Electrochemical splitting of water | Variable | High | None (with renewable energy) |
| Alkaline Electrolysis | Electrolysis using an alkaline solution | Moderate | Moderate | None (with renewable energy) |
| Proton Exchange Membrane (PEM) | Electrolysis using a proton exchange membrane | Moderate | High | None (with renewable energy) |
| Solid Oxide Electrolysis (SOEC) | Electrolysis using a solid oxide electrolyte | Moderate | High | None (with renewable energy) |
| Biomass Gasification | Conversion of biomass into hydrogen gas | Moderate | Moderate | Carbon-neutral (with sustainable biomass) |
| Photobiological Processes | Utilization of photosynthetic organisms | Low | Moderate | None (with renewable energy) |
| Photocatalytic Water Splitting | Use of catalysts and solar energy | Low | High | None (with renewable energy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.; Pramanik, A.; Bhowmick, G.D.; Tripathi, A.; Ghangrekar, M.M.; Pandey, C.; Kim, B.-S. Premier, Progress and Prospects in Renewable Hydrogen Generation: A Review. Fermentation 2023, 9, 537. https://doi.org/10.3390/fermentation9060537
Sharma M, Pramanik A, Bhowmick GD, Tripathi A, Ghangrekar MM, Pandey C, Kim B-S. Premier, Progress and Prospects in Renewable Hydrogen Generation: A Review. Fermentation. 2023; 9(6):537. https://doi.org/10.3390/fermentation9060537
Chicago/Turabian StyleSharma, Mukesh, Arka Pramanik, Gourav Dhar Bhowmick, Akash Tripathi, Makarand Madhao Ghangrekar, Chandan Pandey, and Beom-Soo Kim. 2023. "Premier, Progress and Prospects in Renewable Hydrogen Generation: A Review" Fermentation 9, no. 6: 537. https://doi.org/10.3390/fermentation9060537
APA StyleSharma, M., Pramanik, A., Bhowmick, G. D., Tripathi, A., Ghangrekar, M. M., Pandey, C., & Kim, B.-S. (2023). Premier, Progress and Prospects in Renewable Hydrogen Generation: A Review. Fermentation, 9(6), 537. https://doi.org/10.3390/fermentation9060537









