Molecular Hydrogen Treatment of Sake Yeast and kuratsuki Bacteria Affects Sake Taste
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Microorganisms
2.2. Estimation of Sake Taste
2.3. Statistical Analysis
3. Results and Discussion
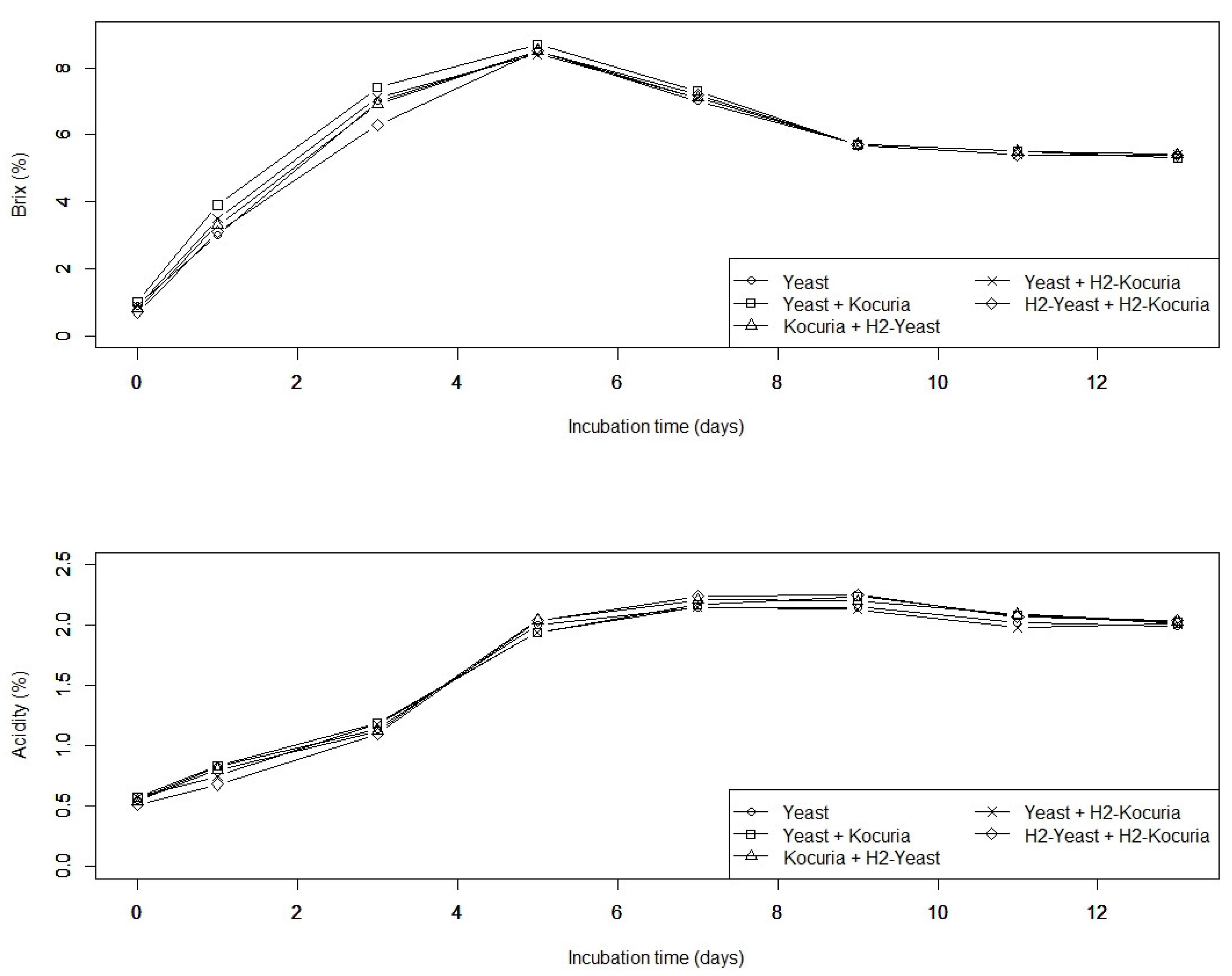
3.1. Effect of H2 Treatment on Ethanol Fermentation of Sake Yeast
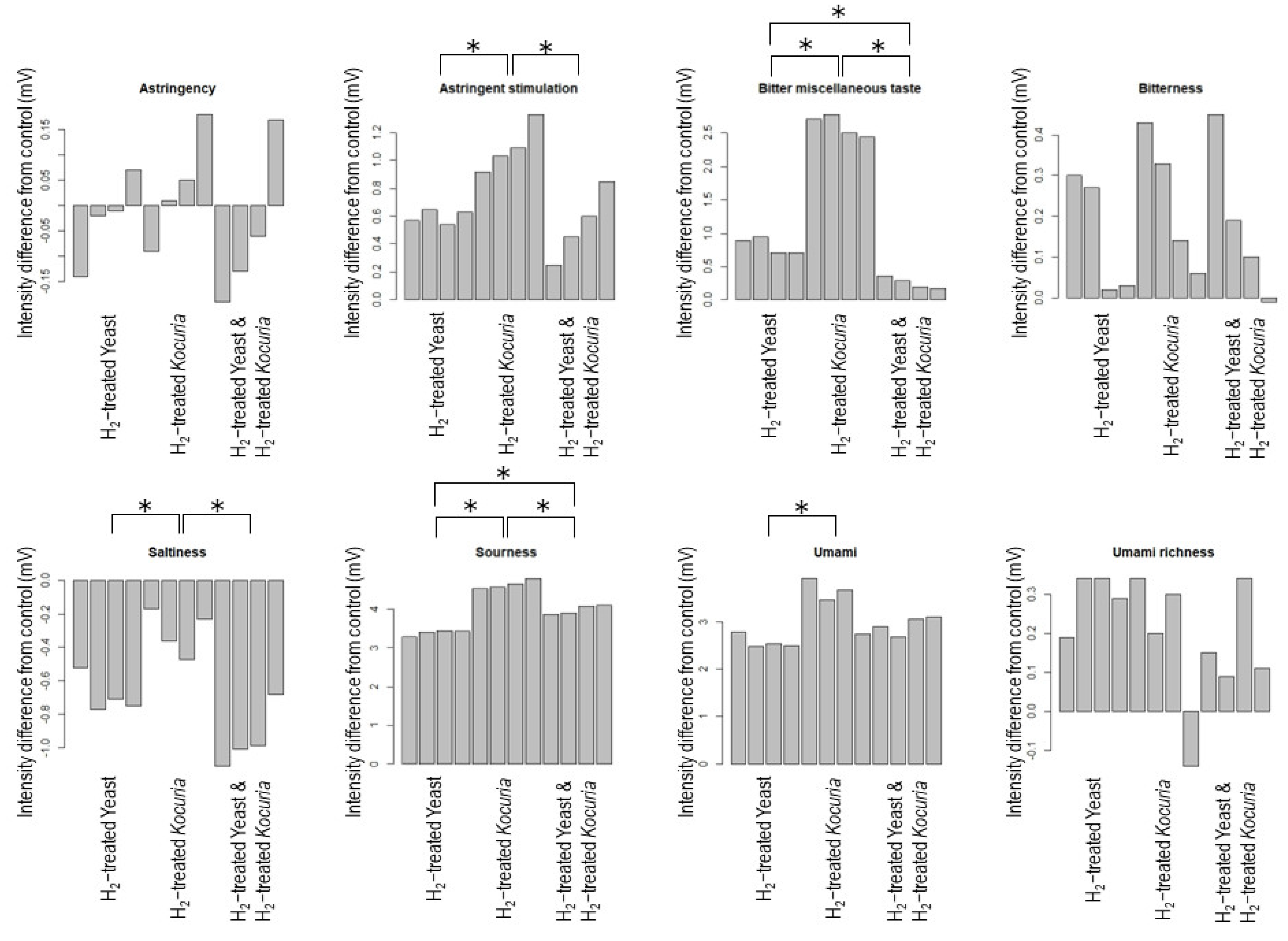
3.2. H2 Treatment Affects Sake Taste
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Sato, B.; Rikitake, M.; Seo, T.; Kurokawa, R.; Hara, Y.; Naritomi, Y.; Hara, H.; Nagao, T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: An open-label pilot study. Med. Gas Res. 2012, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.J.; Tang, J.; Zhang, J.H. The evolution of molecular hydrogen: A noteworthy potential therapy with clinical signature. Med. Gas Res. 2013, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Sato, B.; Shibata, S.; Sakai, T.; Hara, Y.; Naritomi, Y.; Koyanagi, S.; Hara, H.; Nagao, T. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: A randomized, double-blind, placebo-controlled pilot study. Int. Immunopharmacol. 2014, 21, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T. Therapeutic Efficacy of Molecular Hydrogen: A New Mechanistic Insight. Curr. Pharm. Des. 2019, 25, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Schacherer, J.; Shapiro, J.A.; Ruderfer, D.M.; Kruglyak, L. Comprehensive polymorphism survey elucidates population structure of Saccharomyces cerevisiae. Nature 2009, 458, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Yamada, O.; Suzuki, H.; Fujita, M. The relationship between ginjo-shu flavor characteristics and the yeast diversity of the moromi. J. Brew. Soc. Japan 1999, 94, 733–736. (In Japanese) [Google Scholar] [CrossRef]
- Katou, T.; Namise, M.; Kitagaki, H.; Akao, T.; Shimoi, H. QTL mapping of sake brewing characteristics of yeast. J. Biosci. Bioeng. 2009, 107, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Mimura, N.; Isogai, A.; Iwashita, K.; Bamba, T.; Fukusaki, E. Gas chromatography/mass spectrometry based component profiling and quality prediction for Japanese sake. J. Biosci. Bioeng. 2014, 118, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Kashima, M. History, lineage and phenotypic differentiation of sake yeast. Biosci. Biotechnol. Biochem. 2019, 83, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H. Sake brewing and bacteria inhabiting sake breweries. Front. Microbiol. 2021, 12, 602380. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Hiroshima, T.; Yoshida, K. Sake brewing with the addition of kuratsuki bacteria of a different sake brewery. Seibutsu Kogaku Kaishi 2022, 100, 390–391. (In Japanese) [Google Scholar]
- Yazaki, A.; Nishida, H. Effect of kuratsuki Kocuria on sake brewing in different koji conditions. FEMS Microbiol. Lett. 2023, 370, fnad020. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, E.; Terashima, K.; Shiraki, Y.; Nishida, H. Diversity of Bacillus isolates from the sake brewing process at a sake brewery. Microorganisms 2021, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Ohta, M.; Lee, M.; Mills, D.A. Indigenous bacteria and fungi drive traditional kimoto sake fermentations. Appl. Environ. Microbiol. 2014, 80, 5522–5529. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, T.; Nakagawa, A.; Kiyohara, M.; Matsui, H.; Tsuji, A.; Barla, F.; Take, H.; Katsuyama, Y.; Tokuda, K.; Nakamura, S.; et al. Tracing microbiota changes in yamahai-moto, the traditional Japanese sake starter. Biosci. Biotechnol. Biochem. 2016, 80, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Kumano, M.; Sugimoto, Y.; Ito, M.; Ohashi, M.; Sunada, K.; Takahashi, T.; Yamada, T.; Takagi, H. Metabolic switching of sake yeast by kimoto lactic acid bacteria through the [GAR+] non-genetic element. J. Biosci. Bioeng. 2018, 126, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Inoue, A.; Kanamoto, E.; Yoshida, S.; Yamada, M.; Toda, H.; Nishida, H. Co-cultivation of sake yeast and Kocuria isolates from the sake brewing process. FEMS Microbiol. Lett. 2021, 368, fnab053. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Kimura, Y.; Yamada, M.; Nishida, H. Genomic information of Kocuria isolates from sake brewing process. AIMS Microbiol. 2021, 7, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Toko, K. Taste sensor. Sens. Actuators B Chem. 2000, 64, 205–215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, M.; Nishida, H. Molecular Hydrogen Treatment of Sake Yeast and kuratsuki Bacteria Affects Sake Taste. Fermentation 2023, 9, 516. https://doi.org/10.3390/fermentation9060516
Saito M, Nishida H. Molecular Hydrogen Treatment of Sake Yeast and kuratsuki Bacteria Affects Sake Taste. Fermentation. 2023; 9(6):516. https://doi.org/10.3390/fermentation9060516
Chicago/Turabian StyleSaito, Misaki, and Hiromi Nishida. 2023. "Molecular Hydrogen Treatment of Sake Yeast and kuratsuki Bacteria Affects Sake Taste" Fermentation 9, no. 6: 516. https://doi.org/10.3390/fermentation9060516
APA StyleSaito, M., & Nishida, H. (2023). Molecular Hydrogen Treatment of Sake Yeast and kuratsuki Bacteria Affects Sake Taste. Fermentation, 9(6), 516. https://doi.org/10.3390/fermentation9060516




