The Effect of pH on the Production and Composition of Short- and Medium-Chain Fatty Acids from Food Waste in a Leachate Bed Reactor at Room Temperature
Abstract
1. Introduction
2. Material and Methods
2.1. Characteristics of Food Waste and Inoculum
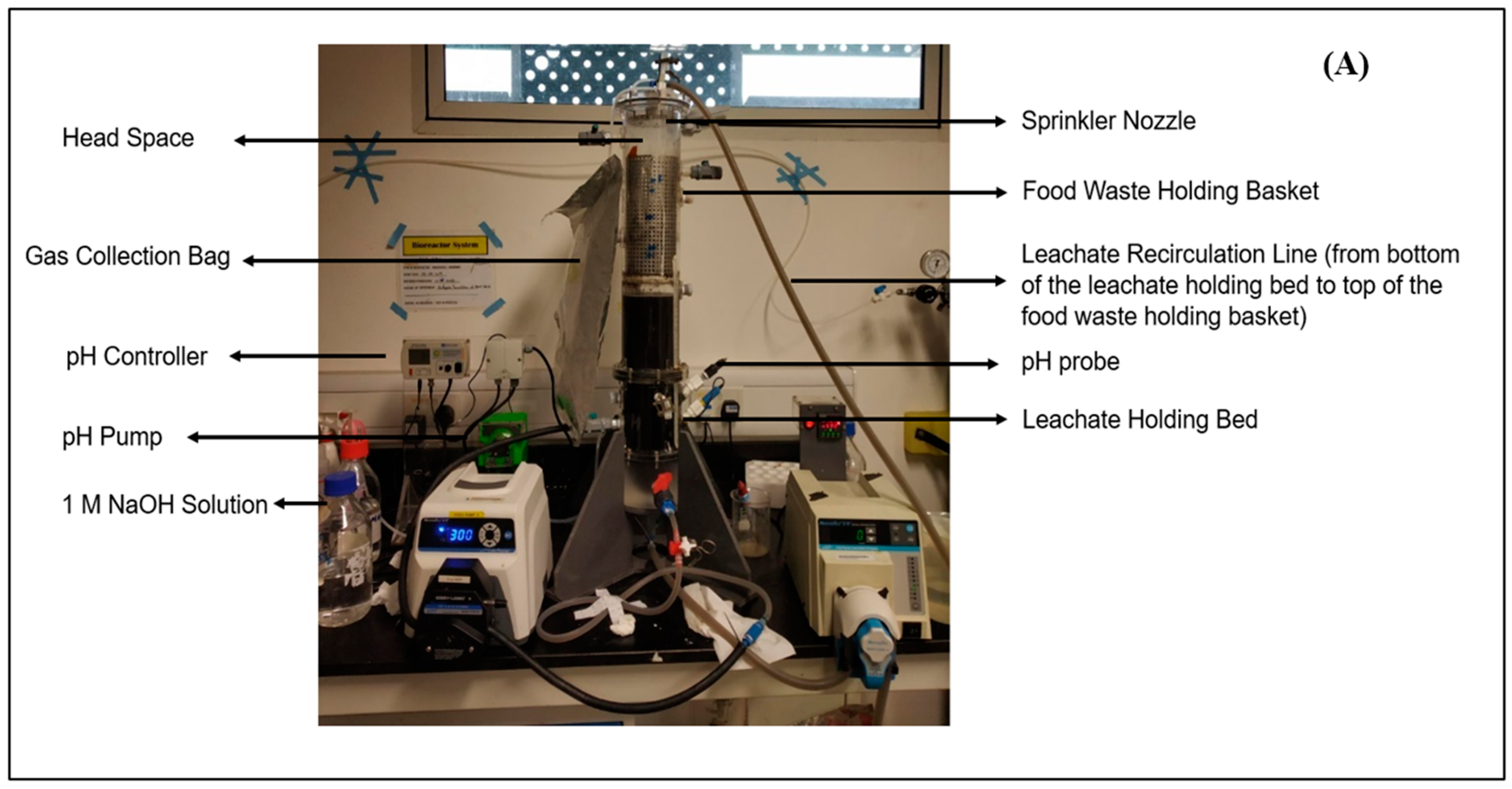
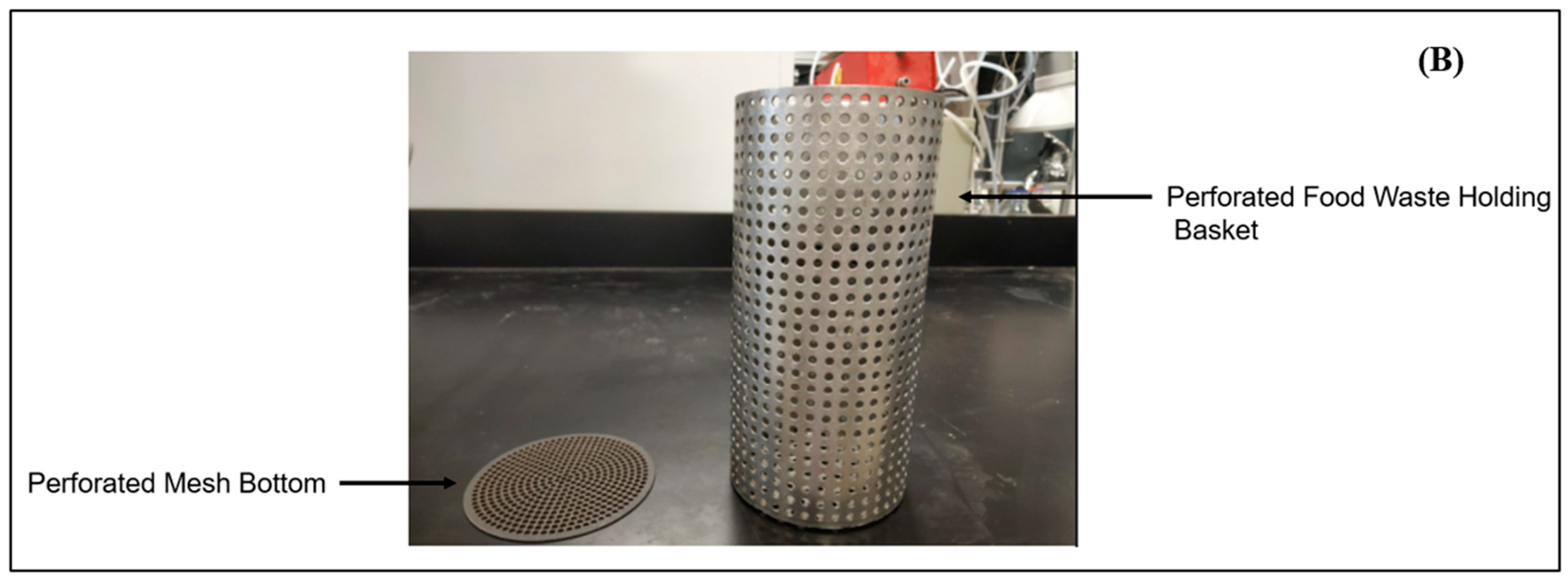
2.2. LBR Design
2.3. LBR Experimental Procedure
2.4. Sampling and Analytical Methods
2.5. Calculations
2.6. Microbial Community and Statistical Analysis
3. Results and Discussion
3.1. Hydrolysis of Food Waste at Different pHs
3.2. TFA Production at Different pH
3.3. TFA Composition at Different pH
3.4. Microbial Community Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arras, W.; Hussain, A.; Hausler, R.; Guiot, S. Mesophilic, thermophilic and hyperthermophilic acidogenic fermentation of food waste in batch: Effect of inoculum source. Waste Manag. 2019, 87, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Silpa, K.; Lisa, Y.; Perinaz, B.T.; van Woerden, F. What a Waste 2.0 A Global Snapshot of Solid Waste Management to 2050; World Bank Group: Washington, DC, USA, 2018. [Google Scholar]
- Xiong, Z.; Hussain, A.; Lee, H.S. Food waste treatment with a leachate bed reactor: Effects of inoculum to substrate ratio and reactor design. Bioresour. Technol. 2019, 285, 121350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yan, B.; Wong, J.W.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Wu, H.; Weimer, P.J.; Dalke, R.; Granda, C.B.; Mai, J.; Urgun-Demirtas, M. Microbial communities for valorizing biomass using the carboxylate platform to produce volatile fatty acids: A review. Bioresour. Technol. 2022, 344, 126253. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev. Environ. Sci. Biotechnol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Coma, M.; Martinez-Hernandez, E.; Abeln, F.; Raikova, S.; Donnelly, J.; Arnot, T.C.; Allen, M.J.; Honge, D.D.; Chuck, C.J. Organic waste as a sustainable feedstock for platform chemicals. Faraday Discuss. 2017, 202, 175–195. [Google Scholar] [CrossRef]
- Chang, H.N.; Kim, N.-J.; Kang, J.; Jeong, C.M. Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol. Bioprocess Eng. 2010, 15, 1–10. [Google Scholar] [CrossRef]
- Bhatt, A.H.; Ren, Z.; Tao, L. Value Proposition of Untapped Wet Wastes: Carboxylic Acid Production through Anaerobic Digestion. iScience 2020, 23, 101221. [Google Scholar] [CrossRef]
- Moretto, G.; Valentino, F.; Pavan, P.; Majone, M.; Bolzonella, D. Optimization of urban waste fermentation for volatile fatty acids production. Waste Manag. 2019, 92, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Radadiya, P.; Lee, J.; Venkateshwaran, K.; Benn, N.; Lee, H.-S.; Hussain, A. Acidogenic fermentation of food waste in a leachate bed reactor (LBR) at high volumetric organic Loading: Effect of granular activated carbon (GAC) and sequential enrichment of inoculum. Bioresour. Technol. 2022, 361, 127705. [Google Scholar] [CrossRef] [PubMed]
- Talan, A.; Pokhrel, S.; Tyagi, R.; Drogui, P. Biorefinery strategies for microbial bioplastics production: Sustainable pathway towards Circular Bioeconomy. Bioresour. Technol. Rep. 2022, 17, 100875. [Google Scholar] [CrossRef]
- Uçkun Kiran, E.; Trzcinski, A.P.; Liu, Y. Platform chemical production from food wastes using a biorefinery concept. J. Chem. Technol. Biotechnol. 2015, 90, 1364–1379. [Google Scholar] [CrossRef]
- Wu, S.-L.; Luo, G.; Sun, J.; Wei, W.; Song, L.; Ni, B.-J. Medium chain fatty acids production from anaerobic fermentation of waste activated sludge. J. Clean. Prod. 2021, 279, 123482. [Google Scholar] [CrossRef]
- Hussain, A.; Filiatrault, M.; Guiot, S.R. Acidogenic digestion of food waste in a thermophilic leach bed reactor: Effect of pH and leachate recirculation rate on hydrolysis and volatile fatty acid production. Bioresour. Technol. 2017, 245, 1–9. [Google Scholar] [CrossRef]
- Xiong, Z.; Hussain, A.; Lee, J. Food waste fermentation in a leach bed reactor: Reactor performance, and microbial ecology and dynamics. Bioresour. Technol. 2019, 274, 153–161. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Browne, J.D.; Allen, E.; Murphy, J.D. Improving hydrolysis of food waste in a leach bed reactor. Waste Manag. 2013, 33, 2470–2477. [Google Scholar] [CrossRef]
- Chakraborty, D.; Karthikeyan, O.P.; Selvam, A.; Palani, S.G.; Ghangrekar, M.M.; Wong, J.W. Two-phase anaerobic digestion of food waste: Effect of semi-continuous feeding on acidogenesis and methane production. Bioresour. Technol. 2021, 346, 126396. [Google Scholar] [CrossRef]
- Hussain, A.; Lee, J.; Xiong, Z.; Wang, Y.; Lee, H.-S. Butyrate production and purification by combining dry fermentation of food waste with a microbial fuel cell. J. Environ. Manag. 2021, 300, 113827. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, G.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Karthikeyan, O.P.; Selvam, A.; Wong, J.W. Effect of inoculum to substrate ratio on the hydrolysis and acidification of food waste in leach bed reactor. Bioresour. Technol. 2012, 126, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-J.; Kim, B.J.; Jeong, C.-M.; Choi, J.-D.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef]
- Saha, S.; Lee, H.S. High-rate carboxylate production in dry fermentation of food waste at room temperature. Sci. Total Environ. 2020, 714, 136695. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Cysneiros, D.; Banks, C.J.; Heaven, S.; Karatzas, K.-A.G. The effect of pH control and “hydraulic flush” on hydrolysis and Volatile Fatty Acids (VFA) production and profile in anaerobic leach bed reactors digesting a high solids content substrate. Bioresour. Technol. 2012, 123, 263–271. [Google Scholar] [CrossRef]
- Xu, S.Y.; Lam, H.P.; Karthikeyan, O.P.; Wong, J.W. Optimization of food waste hydrolysis in leach bed coupled with methanogenic reactor: Effect of pH and bulking agent. Bioresour. Technol. 2011, 102, 3702–3708. [Google Scholar] [CrossRef]
- Yu, P.; Tu, W.; Wu, M.; Zhang, Z.; Wang, H. Pilot-scale fermentation of urban food waste for volatile fatty acids production: The importance of pH. Bioresour. Technol. 2021, 332, 125116. [Google Scholar] [CrossRef] [PubMed]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Y.; Liu, J.Z.; Wong, J.W.C. Control of lactic acid production during hydrolysis and acidogenesis of food waste. Bioresouce Technol. 2018, 247, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Han, G.H.; Oh, B.R.; Chun, Y.N.; Eom, C.-Y.; Kim, S.W. Volumetric scale-up of a three stage fermentation system for food waste treatment. Bioresour. Technol. 2008, 99, 4394–4399. [Google Scholar] [CrossRef]
- Ye, N.F.; Lü, F.; Shao, L.M.; Godon, J.J.; He, P.J. Bacterial community dynamics and product distribution during pH-adjusted fermentation of vegetable wastes. J. Appled Microbiol. 2007, 103, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Maru, B.T.; Lopez, F.; Medina, F.; Constantí, M. Improvement of biohydrogen and usable chemical products from glycerol by co-culture of Enterobacter spH1 and Citrobacter Freundii H3 using different supports as surface immobilization. Fermentation 2021, 7, 154. [Google Scholar] [CrossRef]
- Liu, C.; Du, Y.; Zheng, J.; Qiao, Z.; Luo, H.; Zou, W. Production of caproic acid by Rummeliibacillus suwonensis 3B-1 isolated from the pit mud of strong-flavor baijiu. J. Biotechnol. 2022, 35, 33–40. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Optimization of fermentative hydrogen production by Enterococcus faecium INET2 using response surface methodology. Int. J. Hydrogen Energy 2019, 44, 1483–1491. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Peña, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. MSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef]
- Yamada, H.; Watabe, Y.; Suzuki, Y.; Koike, S.; Shimamoto, S.; Kobayashi, Y. Chemical and microbial characterization for fermentation of water-soluble cellulose acetate in human stool cultures. J. Sci. Food Agric. 2021, 101, 2950–2960. [Google Scholar] [CrossRef]
- Brame, J.E.; Liddicoat, C.; Abbott, C.A.; Breed, M.F. The potential of outdoor environments to supply beneficial butyrate-producing bacteria to humans. Sci. Total Environ. 2021, 777, 146063. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Y.; Zhang, N.; Shen, Y.; Ni, J. Draft Genome Sequence of Dysogonomonas Macrotermitis Strain JCM 19375T, Isolated from the Gut of a Termite. Genome Annoucements 2015, 3, e00963-15. [Google Scholar]
- Slováková, L.; Dušková, D.; Marounek, M. Fermentation of pectin and glucose, and activity of pectin-degrading enzymes in the rabbit caecal bacterium Bifidobacterium pseudolongum. Lett. Appled Microbiol. 2001, 35, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Opdahl, L. Identification of Candidate Cellulose Utilizing Bacteria from the Rumen of Beef Cattle, Using Bacterial Community Profiling and Metagenomics. 2017. Available online: https://openprairie.sdstate.edu/cgi/viewcontent.cgi?article=2688&context=etd (accessed on 24 January 2023).
- Andersen, S.J.; De Groof, V.; Khor, W.C.; Roume, H.; Props, R.; Coma, M.; Rabaey, K. A Clostridium group IV species dominates and suppresses a mixed culture fermentation by tolerance to medium chain fatty acids products. Front. Bioeng. Biotechnol. 2017, 5, 8. [Google Scholar] [CrossRef] [PubMed]



| Parameters | LBR-UC | LBR-5.5 | LBR-6.5 | LBR-7.5 | LBR-8.5 |
|---|---|---|---|---|---|
| Cumulative SCOD production (g SCOD) | 71.3 ± 0.1 | 89.0 ± 3.4 | 110.8 ± 1.7 | 112.5 ± 2.6 | 111.7 ± 4.1 |
| Hydrolysis yield (g SCOD/kg VSadded) | 462 ± 6.0 | 577 ± 22.1 | 718 ± 11.2 | 729 ± 17.1 | 724 ± 26.6 |
| Acetate (g CODFA/L) | 3.85 ± 0.0 | 4.5 ± 0.42 | 6.15 ± 0.52 | 7.43 ± 0.3 | 10.7 ± 0.75 |
| Propionate (g CODFA/L) | 0.57 ± 0.05 | 1.76 ± 0.08 | 2.57 ± 0.22 | 2.6 ± 0.2 | 4.1 ± 0.2 |
| iso-Butyrate (g CODFA/L) | - | 0.08 ± 0.0 | 0.09 ± 0.00 | 0.24 ± 0.01 | 0.23 ± 0.05 |
| n-Butyrate (g CODFA/L) | 5.72 ± 0.0 | 9.72 ± 0.13 | 16.3 ± 1.4 | 5.9 ± 0.3 | 3.63 ± 0.1 |
| iso-Valerate (g CODFA/L) | - | 0.13 ± | 0.15 ± 0.01 | 0.32 ± 0.02 | 0.31 ± 0.00 |
| n-Valerate (g CODFA/L) | - | 0.76 ± 0.00 | 1.2 ± 0.09 | 1.3 ± 0.0 | 0.32 ± 0.01 |
| iso-Caproate (g CODFA/L) | - | 0.09 ± 0.01 | 0.08 ± 0.00 | 0.1 ± 0.0 | 0.09 ± 0.0 |
| n-Caproate (g CODFA/L) | - | 2.54 ± 0.01 | 2.83 ± 0.23 | 0.24 ± 0.1 | 0.11 ± 0.0 |
| Heptanoate (g CODFA/L) | - | 0.29 ± 0.00 | 0 | 0.28 ± 0.0 | 0 |
| TVFA production (C2-C7) (g CODFA) | 25.5 ± 0.2 | 71.4 ± 0.6 | 99.2 ± 3.0 | 67.4 ± 3.4 | 65.3 ± 4.2 |
| TVFA yield (g CODFA/kg VSadded) | 165 ± 1.1 | 463 ± 4.3 | 643 ± 19.2 | 437 ± 22.1 | 423 ± 27.4 |
| TVFA (C2-C7):SCOD (%) | 36 | 80 | 90 | 60 | 58 |
| Lactate (g CODLactate) | 39.3 ± 0.3 | - | - | - | - |
| Lactate yield (g CODLactate/kg VSadded) | 255 ± 2.1 | - | - | - | - |
| Lactate: SCOD (%) | 55 | - | - | - | - |
| Acidification yield (g COD/kg VSadded) | 420 ± 5.8 | 463 ± 4.8 | 643 ± 19.2 | 437 ± 22.1 | 423 ± 27.4 |
| Acidification (%) | 91 | 80 | 90 | 60 | 58 |
| Time | Cumulative SCOD Production (g SCOD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | LBR-UC | LBR-5.5 | LBR-6.5 | LBR-7.5 | LBR-8.5 | |||||
| g SCOD | % | g SCOD | % | g SCOD | % | g SCOD | % | g SCOD | % | |
| 0 | 0.0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| 2 | 61.5 | 86% | 31.8 | 36% | 47.9 | 43% | 28.1 | 25% | 39.3 | 35% |
| 4 | 63.8 | 89% | 46.2 | 52% | 74.6 | 67% | 45.1 | 40% | 55.5 | 50% |
| 6 | 69.8 | 98% | 57.3 | 64% | 83.9 | 76% | 48.5 | 43% | 58.6 | 52% |
| 8 | 71.1 | 100% | 62.7 | 70% | 91.1 | 82% | 59.3 | 53% | 66.9 | 60% |
| 10 | 80.6 | 113% | 72.8 | 82% | 102.3 | 92% | 80.3 | 71% | 87.2 | 78% |
| 12 | 68.6 | 96% | 81.3 | 91% | 108.6 | 98% | 98.8 | 88% | 100.1 | 90% |
| 14 | 71.3 | 100% | 89.0 | 100% | 110.8 | 100% | 112.5 | 100% | 111.7 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radadiya, P.; Latika, A.; Fei, X.; Lee, J.; Mishra, S.; Hussain, A. The Effect of pH on the Production and Composition of Short- and Medium-Chain Fatty Acids from Food Waste in a Leachate Bed Reactor at Room Temperature. Fermentation 2023, 9, 518. https://doi.org/10.3390/fermentation9060518
Radadiya P, Latika A, Fei X, Lee J, Mishra S, Hussain A. The Effect of pH on the Production and Composition of Short- and Medium-Chain Fatty Acids from Food Waste in a Leachate Bed Reactor at Room Temperature. Fermentation. 2023; 9(6):518. https://doi.org/10.3390/fermentation9060518
Chicago/Turabian StyleRadadiya, Pooja, Ashika Latika, Xunchang Fei, Jangho Lee, Saurabh Mishra, and Abid Hussain. 2023. "The Effect of pH on the Production and Composition of Short- and Medium-Chain Fatty Acids from Food Waste in a Leachate Bed Reactor at Room Temperature" Fermentation 9, no. 6: 518. https://doi.org/10.3390/fermentation9060518
APA StyleRadadiya, P., Latika, A., Fei, X., Lee, J., Mishra, S., & Hussain, A. (2023). The Effect of pH on the Production and Composition of Short- and Medium-Chain Fatty Acids from Food Waste in a Leachate Bed Reactor at Room Temperature. Fermentation, 9(6), 518. https://doi.org/10.3390/fermentation9060518








