A Review on the Interspecies Electron Transfer of Methane Production in Anaerobic Digestion System
Abstract
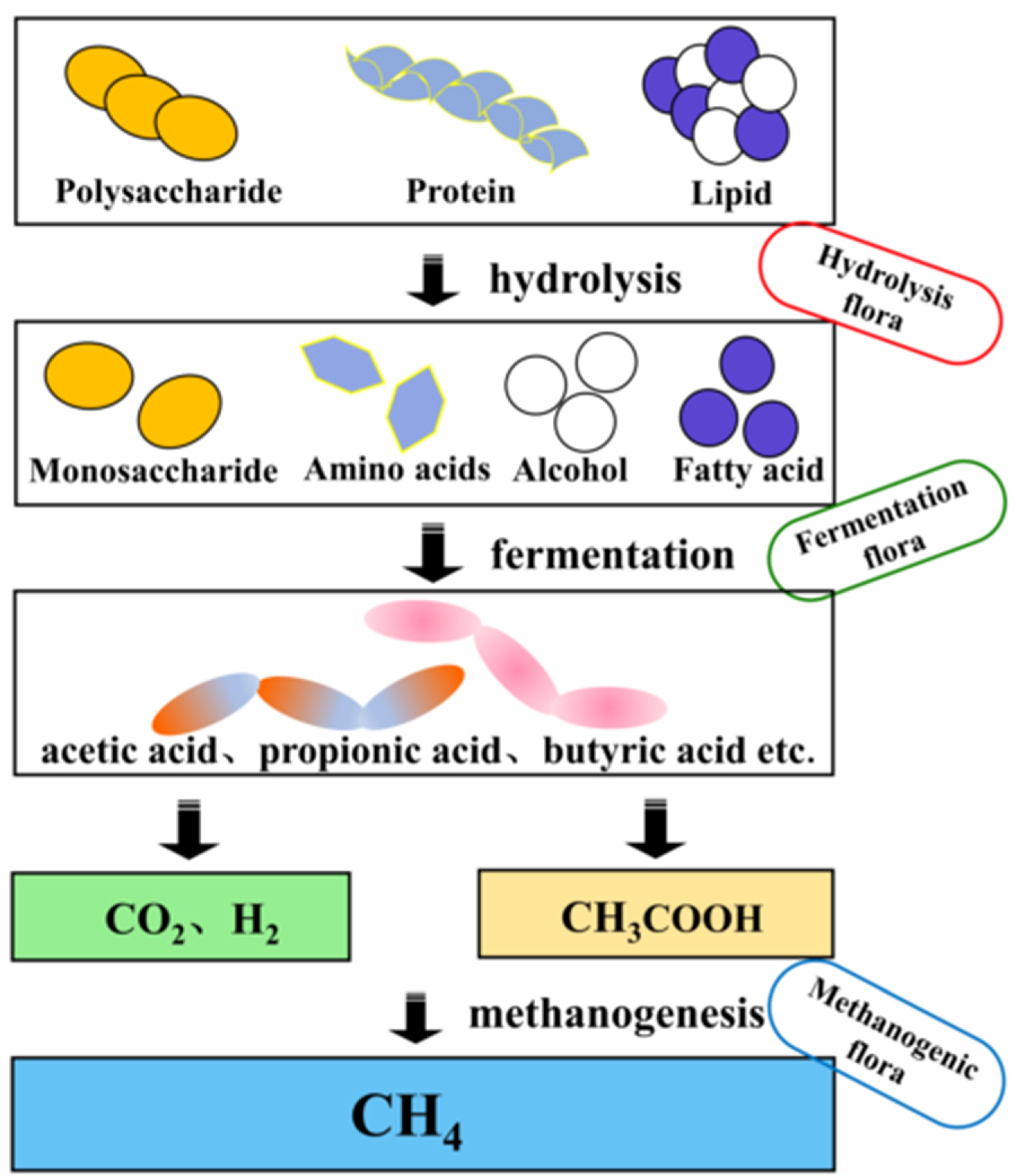
1. Introduction
CO2 + 8H→CH4 + 2H2O (Conducted by methanogenic bacteria)
4H2 + CO2→CH4 + 2H2O
2CH3COOH→2CH4 + 2CO2
2. The Modes and Mechanisms of Electron Transfer
2.1. MIET
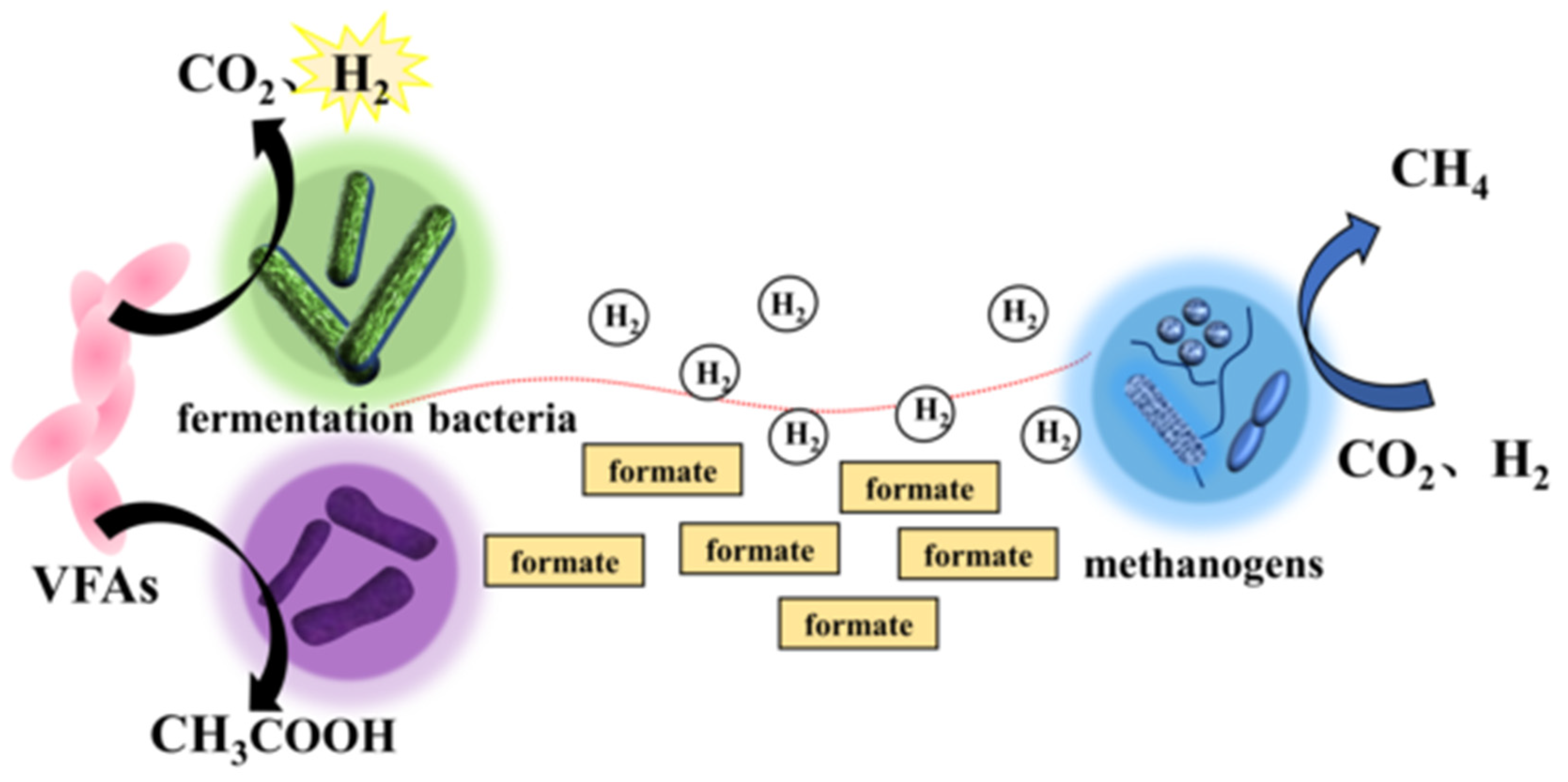
2.1.1. Hydrogen-Mediated MIET
2.1.2. MIET Mediated by Formic Acid
2.1.3. E-Transmitter Mediated MIET
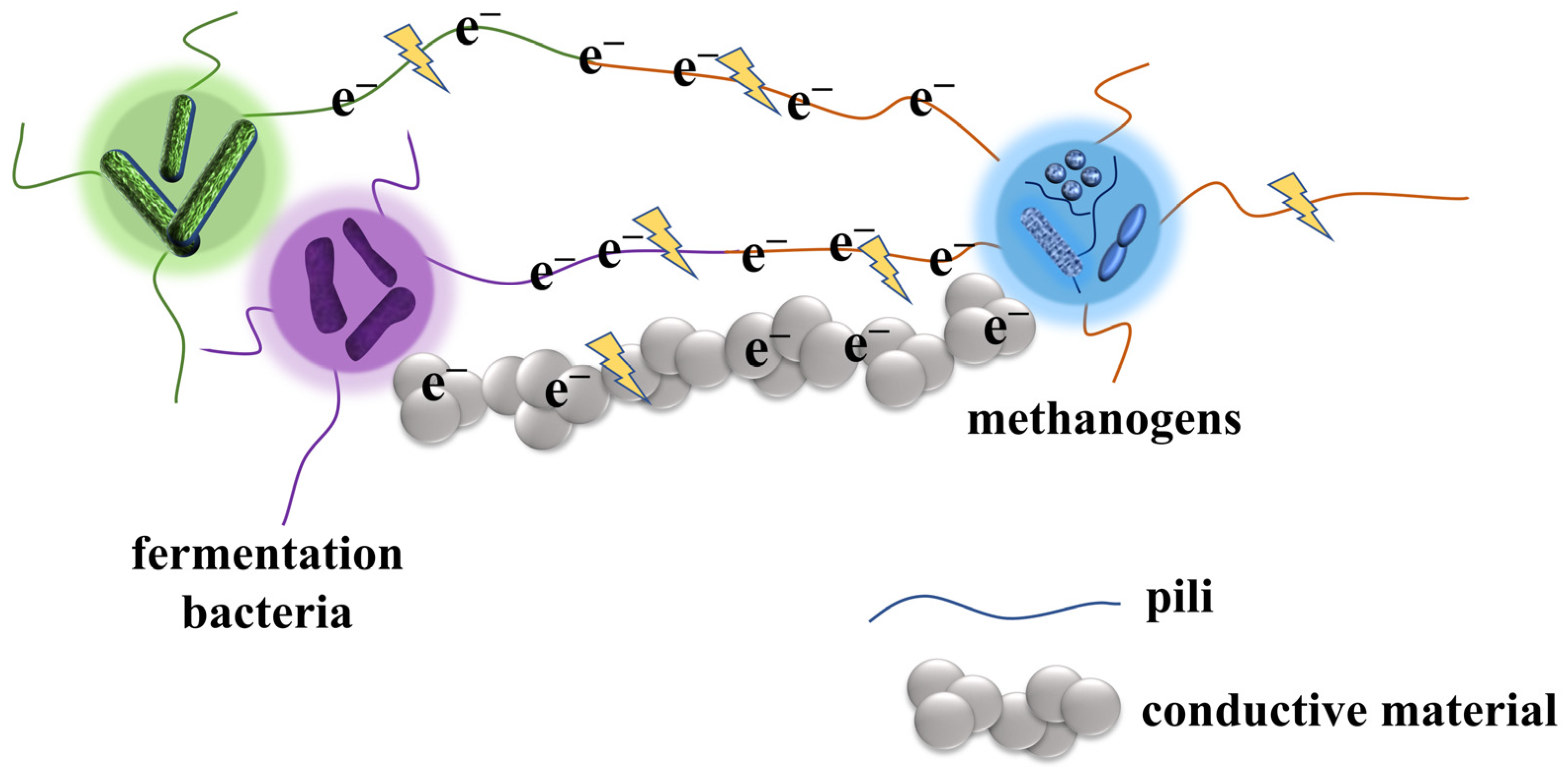
2.2. DIET
2.2.1. DIET via Bioelectric Connection
2.2.2. DIET Connected by Conductive Material
3. Comparison of DIET and MIET
3.1. Effect of DIET and MIET on Methanogenic Performance
3.2. Comparison of Degradation Process from Thermodynamic and Kinetic Perspective
3.3. Differences in the Microbial Communities Involved in MIET and DIET
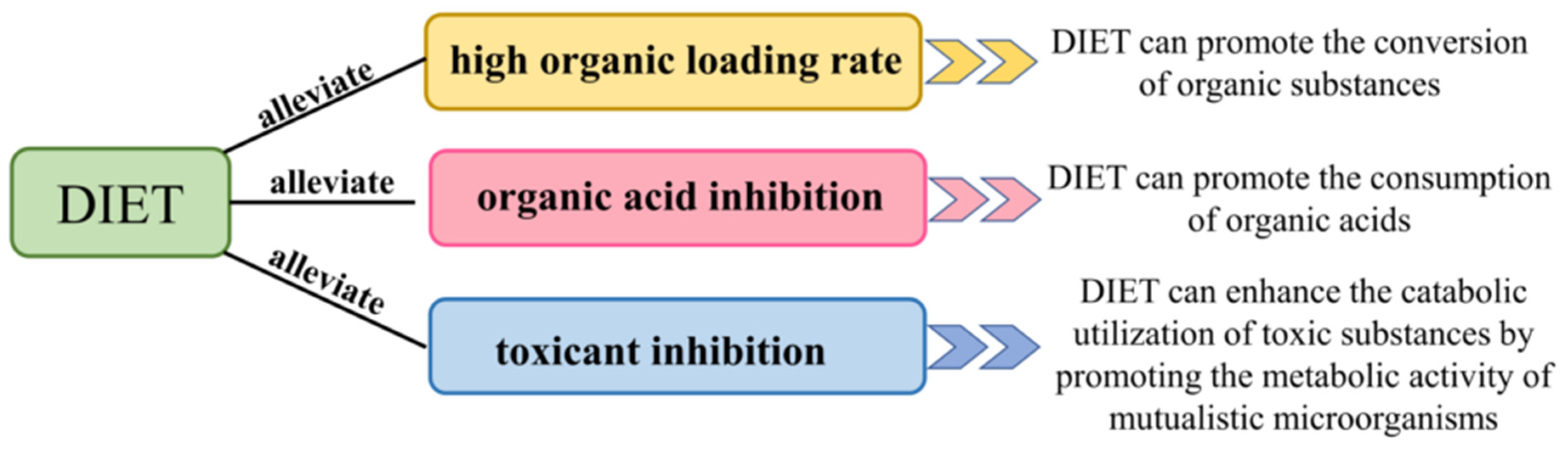
4. DIET Alleviates Inhibition in Anaerobic Methanogenesis
4.1. Mitigation of the Inhibitory Effect of DIET on High Organic Loading Rate
4.2. Mitigation of Organic Acid Inhibition by DIET
4.3. Mitigation of Toxicant Inhibition by DIET
5. Conclusions and Prospects
- Discovery of more anaerobic methanogenic bacteria capable of direct interspecies electron transfer with electron-producing microorganisms. With the development of bioelectrochemistry, electron-producing microorganisms and their pathways of electron transfer have received extensive attention. However, a limited number of microorganisms capable of driving IET have been identified. Among the methanogenic groups, only Methanosarcinales and Methanobacterium have been found to be capable of DIET with electrogenic microorganisms; however, there are a large number of unexplored microorganisms involved in the DIET process. For example, Methanomicrobia, Methanobacter, Methanolinea, and Methanospirillum were found to have potential DIET capabilities in a study by Kang et al., and these methanogenic bacteria could also be the target of future DIET research [56];
- Using molecular biology and cryoelectron microscopy to clarify how the electron transport chain between electron donor microorganisms and electron acceptor microorganisms completing DIET transfers electrons. The research on DIET is still in the early stage. Although a large number of studies in recent years have proven that CH4 production can be enhanced by DIET, the reported evidence is indirect, and direct evidence of DIET needs to be collected. The cryo-electron microscopy technique has higher resolution, and the microorganisms are better maintained in their original state when frozen at low temperature than when dried, resulting in more objective and direct observations. The filamentous protein appendages known as “microbial nanowires” have been found to be composed not of pili but of the cytochromes OmcS and OmcZ;
- How DIETs can adapt to extreme weather at very high or low temperatures without compromising their electron transfer efficiency. In recent years, global environmental degradation has led to global warming, ozone layer depletion, acid rain, freshwater crisis, energy shortage, sharp decrease in forest resources, land desertification, accelerated species extinction, garbage disaster, toxic chemical pollution, and many other aspects of environmental problems. The study of IET is of great importance for biogeochemical cycles, such as carbon cycle, nitrogen cycle, methane production, and greenhouse gas emissions. A future research direction could involve using the DIET process to adapt to extreme weather, such as very high or very low temperature, without affecting its electron transport efficiency. This could also examine microorganisms that produce electricity and are resistant to high or low temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.; Kang, H.; Park, K.; Park, H. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.Q.; Hu, Z.Q. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ. Sci.-Process. Impacts 2013, 15, 39–48. [Google Scholar] [CrossRef]
- Parawira, W.; Nyandoroh, M.G.; Zvauya, R. A study of industrial anaerobic treatment of opaque beer brewery wastewater in a tropical climate using a full-scale UASB reactor seeded with activated sludge. Process. Biochem. 2005, 40, 593–599. [Google Scholar] [CrossRef]
- Li, Y.B.; Park, S.Y.; Zhu, J.Y. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.J.; Zhou, J.T. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2017, 224, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Roopnarain, A.; Rama, H.; Ndaba, B.; Bello-Akinosho, M.; Bamuza-Pemu, E.; Adeleke, R. Unravelling the anaerobic digestion ‘black box’: Biotechnological approaches for process optimization. Renew. Sustain. Energy Rev. 2021, 152, 111717. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Liu, Y.C.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef]
- Ueno, Y.; Yamada, K.; Yoshida, N.; Maruyama, S.; Isozaki, Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 2006, 440, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Syntrophy goes electric: Direct interspecies electron transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, M.; Wang, G.; Chen, R.; Qiao, W.; Wang, X. Biochar assisted thermo-philic co-digestion of food waste and waste activated sludge under high feedstock to seed sludge ratio in batch experiment. Bioresour. Technol. 2018, 249, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.H.; Zeikus, J.G. Control of interspecies electron flow during anaerobic digestion: Significance of formate transfer versus hydrogen transfer during syntrophic methanogenesis in flocs. Appl. Environ. Microb. 1988, 54, 20–29. [Google Scholar] [CrossRef]
- Logan, B.E.; Oh, S.E.; Kim, I.S.; Van Ginkel, S. Biological hydrogen productionmeasured in batch anaerobic respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef]
- Martins, G.; Salvador, A.F.; Pereira, L.; Alves, M.M. Methane production and conductive materials: A critical review. Environ. Sci. Technol. 2018, 52, 10241–10253. [Google Scholar] [CrossRef]
- Peth, S.; Horn, R.; Beckmann, F.; Donath, T.; Fischer, J.; Smucker, A. Three-di-mensional quantification of intra-aggregate pore-space features using synchrotron-radiation-based microtomography. Soil Sci. Soc. Am. J. 2008, 72, 897–907. [Google Scholar] [CrossRef]
- Cruz Viggi, C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic co-culture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Lesnik, K.L.; Hong, L. Stay connected: Electrical conductivity of microbial aggregates. Biotechnol. Adv. 2017, 35, 669. [Google Scholar]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.L.; Reguera, G.; Beyenal, H.; Beyenal, H.; Lu, A.H.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Stams, A.J.M.; de Bok, F.A.M.; Plugge, C.M.; van Eekert, M.H.A.; Schraa, G. Exocellular electron transfer in anaerobic microbial communities. Environ. Microbiol. 2006, 8, 371–382. [Google Scholar] [CrossRef]
- Bryant, M.P.; Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Microbiol. 1967, 59, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Live wires: Direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 2011, 4, 4896–4906. [Google Scholar] [CrossRef]
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotech. 2009, 20, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hickey, R.F.; Jain, M.K.; Zeikus, J.G. Energetics and regulations of formate and hydrogen metabolism by Methanobacterium formicicum. Arch. Microbiol. 1993, 159, 57–65. [Google Scholar] [CrossRef]
- De Bok, F.A.M.; Plugge, C.M.; Stams, A.J.M. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 2004, 38, 1368–1375. [Google Scholar] [CrossRef]
- Nozhevnikova, A.N.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.N.; Zhuravleva, E.A.; Nikitina, A.A. Syntrophy and Interspecies Electron Transfer in Methanogenic Microbial Communities. Microbiology 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Huang, L.Y.; Liu, X.; Ye, Y.; Chen, M.; Zhou, S.G. Evidence for the coexistence of direct and riboflavin-mediated interspecies electron transfer in Geobacter co-culture. Environ. Microbiol. 2020, 22, 243–254. [Google Scholar] [CrossRef]
- Abbas, Y.; Yun, S.N.; Wang, Z.Q.; Zhang, Y.W.; Zhang, X.M.; Wang, K.J. Recent advances in bio-based carbon materials for anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2021, 135, 110378. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.G.; Wu, J. Nhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.L.; Angenent, L.T.; Zhang, T. Extracellular electron uptake: Among autotrophs and mediated by surfaces. Trends Biotechnol. 2017, 35, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Malvankar, N.S.; Franks, A.E.; Summers, Z.M.; Giloteaux, L.; Rotaru, A.E.; Rotaru, C.; Lovley, D.R. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio 2011, 2, e00159-11. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct Interspecies Electron Transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microb. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- Rotaru, A.; Shrestha, P.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy. Environ. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- McGlynn, S.E.; Chadwick, G.L.; Kempes, C.P.; Orphan, V.J. Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 2015, 526, 531–535. [Google Scholar] [CrossRef]
- Ueki, T.; Nevin, K.P.; Rotaru, A.E.; Wang, L.Y.; Ward, J.E.; Woodard, T.L.; Lovley, D.R. Geobacter strains expressing poorly conductive pili reveal constraints on direct interspecies electron transfer mechanisms. mBio 2018, 9, e01273-18. [Google Scholar] [CrossRef]
- Liu, X.; Zhuo, S.Y.; Rensing, C.; Zhou, S.G. Syntrophic growth with direct interspecies electron transfer between pili-free Geobacter species. Isme. J. 2018, 12, 2142–2151. [Google Scholar] [CrossRef]
- Wang, Z.X.; Wang, T.F.; Si, B.C.; Watson, J.; Zhang, Y.H. Accelerating anaerobic digestion for methane production: Potential role of direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 145, 111069. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, S.H.; Lim, T.G.; Park, J.H.; Kim, B.; Buffiere, P.; Park, H.D. Recent advances in methanogenesis through direct interspecies electron transfer via conductive materials: A molecular microbiological perspective. Bioresour. Technol. 2021, 322, 124587. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.H.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting interspecies electron transfer with biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Zhang, Y.B.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef]
- Liu, F.H.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ. Microbiol. 2015, 17, 648–655. [Google Scholar] [CrossRef]
- Wang, G.J.; Li, Q.; Gao, X.; Wang, X.C.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef]
- Yan, W.W.; Shen, N.; Xiao, Y.Y.; Chen, Y.; Sun, F.Q.; Tyagi, V.K.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344. [Google Scholar] [CrossRef]
- Lin, R.C.; Cheng, J.; Zhang, J.B.; Zhou, J.H.; Cen, K.F.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef]
- Liang, Y.G.; Li, X.J.; Zhang, J.; Zhang, L.G.; Cheng, B.J. Effect of microscale ZVI/magnetite on methane production and bioavailability of heavy metals during anaerobic digestion of diluted pig manure. Environ. Sci. Pollut. Res. 2017, 24, 12328–12337. [Google Scholar] [CrossRef]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Yamada, C.; Kato, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J. Biosci. Bioeng. 2015, 119, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.G.; Xu, J.L.; Yang, G.Q.; Zhuang, L. Methanogenesis affected by the co-occurrence of iron(III) oxides and humic substances. FEMS Microbiol. Ecol. 2014, 88, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Ambuchi, J.J.; Zhang, Z.H.; Shan, L.L.; Liang, D.D.; Zhang, P.; Feng, Y.J. Response of anaerobic granular sludge to iron oxide nanoparticles and multi-wall carbon nanotubes during beet sugar industrial wastewater treatment. Water Res. 2017, 117, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, A.D.; Ren, G.P.; Zhou, T.; Zhang, G.M.; Zhou, S.G. Red mud enhances methanogenesis with the simultaneous improvement of hydrolysis-acidification and electrical conductivity. Bioresour. Technol. 2018, 247, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Vu, M.T.; Johir, M.A.; Pernice, M.; Ngo, H.H.; Zdarta, J.; Jesionowski, T.; Nghiem, L.D. Promotion of direct interspecies electron transfer and potential impact of conductive materials in anaerobic digestion and its downstream processing-a critical review. Bioresour. Technol. 2021, 341, 125847. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, W.; Wu, S.L.; Ni, B.J. Zerovalent Iron Effectively Enhances Medium-Chain Fatty Acids Production from Waste Activated Sludge through Improving Sludge Biodegradability and Electron Transfer Efficiency. Environ. Sci. Technol. 2020, 54, 10904–10915. [Google Scholar] [CrossRef]
- Yan, W.W.; Mukherjee, M.; Zhou, Y. Direct interspecies electron transfer (DIET) can be suppressed under ammonia-stressed condition-Reevaluate the role of conductive materials. Water Res. 2020, 183, 116094. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Direct interspecies electron transfer mechanism in enhanced methanogenesis: A mini-review. Bioresour. Technol. 2021, 330, 124980. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.Y.; Ma, C.X.; White, J.C.; Zhao, Z.Q.; Duan, C.; Zhang, Y.L.; Adeel, M.; Rui, Y.K.; Li, G.X.; et al. Carbon nanomaterials induce residue degradation and increase methane production from livestock manure in an anaerobic digestion system. J. Clean. Prod. 2019, 240, 118257. [Google Scholar] [CrossRef]
- Shi, Y.P.; Liu, T.; Chen, S.; Quan, X. Accelerating anaerobic hydrolysis acidification of dairy wastewater in integrated floating-film and activated sludge (IFFAS) by using zero-valent iron (ZVI) composite carriers. Biochem. Eng. J. 2021, 177, 108226. [Google Scholar] [CrossRef]
- Jiao, P.B.; Zhang, X.X.; Qiu, S.W.; Zhou, X.Y.; Tian, Z.X.; Liang, Y.J.; Zhang, Y.F.; Ma, L.P. Pyrite-enhanced Sludge Digestion via Stimulation of Direct Interspecies Electron Transfer between Syntrophic Propionate- and Sulfur-oxidizing Bacteria and Methanogens: Genome-centric Metagenomics Evidence. Chem. Eng. J. 2023, 456, 141089. [Google Scholar] [CrossRef]
- Jing, Y.H.; Wan, J.J.; Angelidaki, I.; Zhang, S.C.; Luo, G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Res. 2017, 108, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, S.; Liang, D.H.; Li, N. Conductive materials in anaerobic digestion: From mechanism to application. Bioresour. Technol. 2020, 298, 122403. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.D.; Yang, S.; Wang, Z.Z.; Xing, L.Z.; Wu, G.X. Clarifying electron transfer and metagenomic analysis of microbial community in the methane production process with the addition of ferroferric oxide. Chem. Eng. J. 2018, 333, 216–225. [Google Scholar] [CrossRef]
- Yin, Q.; He, K.; Liu, A.; Wu, G. Enhanced system performance by dosing ferro-ferric oxide during the anaerobic treatment of tryptone-based high-strength waste-water. Microbiol. Biot. 2017, 101, 3929–3939. [Google Scholar] [CrossRef]
- Yin, Q.; Miao, J.; Li, B.; Wu, G. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon. Biodeter. Biodegrad. 2017, 119, 104–110. [Google Scholar] [CrossRef]
- Kaden, J.; Galushko, A.S.; Schink, B. Cysteine-mediated electron transfer in syntrophic acetate oxidation by cocultures of Geobacter sulfurreducens and Wolinella succinogenes. Arch. Microbiol. 2002, 178, 53–58. [Google Scholar] [CrossRef]
- Biebl, H.; Pfennig, N. Growth yields of green sulfur bacteria in mixed cultures with sulfur and sulfate reducing bacteria. Arch. Microbiol. 1978, 117, 9–16. [Google Scholar] [CrossRef]
- Smith, J.A.; Nevin, K.P.; Lovley, D.R. Syntrophic growth via quinone-mediated interspecies electron transfer. Arch. Microbiol. 2015, 6, 121. [Google Scholar] [CrossRef]
- Beckmann, S.; Welte, C.; Li, X.M.; Oo, Y.M.; Kroeninger, L.; Heo, Y.; Zhang, M.M.; Ribeiro, D.; Lee, M.; Bhadbhade, M.; et al. Novel phenazine crystals enable direct electron transfer to methanogens in anaerobic digestion by redox potential modulation. Energy Environ. Sci. 2016, 9, 644–655. [Google Scholar] [CrossRef]
- Cheng, Q.W.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef]
- Storck, T.; Virdis, B.; Batstone, D.J. Modelling extracellular limitations for mediated versus direct interspecies electron transfer. ISME J. 2016, 10, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.P.; Campbell, L.L.; Reddy, C.A.; Crabill, M.R. Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl. Environ. Microb. 1977, 33, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Johnson, R.L.; Liu, Y. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Appl. Environ. Microb. 1989, 55, 1735–1741. [Google Scholar] [CrossRef]
- Holmes, D.E.; Rotaru, A.E.; Ueki, T.; Shrestha, P.M.; Ferry, J.G.; Lovley, D.R. Electron and proton flux for carbon dioxide reduction in Methanosarcina barkeri during direct interspecies electron transfer. Front. Microbiol. 2018, 9, 3109. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ma, J.L.; Tang, J.; Tang, Z.Y.; Zhou, S.G. Cysteine-accelerated methanogenic propionate degradation in paddy soil enrichment. Microb. Ecol. 2017, 73, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Plugge, C.M.; Balk, M.; Stams, A.J.M. Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum subsp. nov. a thermophilic, syntrophic, propionate-oxidizing, spore-forming bacterium. Int. J. Syst. Evol. Microbiol. 2002, 52, 391–399. [Google Scholar] [CrossRef]
- Malvankar, N.S.; Vargas, M.; Nevin, K.P.; Franks, A.E.; Leang, C.; Kim, B.C.; Inoue, K.; Mester, T.; Covalla, S.F.; Johnson, J.P.; et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 2011, 6, 573–579. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Link between capacity for current production and syntrophic growth in Geobacter species. Front. Microbiol. 2015, 6, 744. [Google Scholar] [CrossRef]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 2015, 526, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Zhang, H.X.; Li, Y.; Zhang, H.; Wang, O.M.; Zhang, J.; Liu, F.H. Co-occurrence of Methanosarcina mazei and Geobacteraceae in an iron (III)-reducing enrichment culture. Front. Microbiol. 2015, 6, 941. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Liu, F.H.; Wang, B.C.; Zhang, Y.C.; Lovley, D.R. Methanobacterium capable of direct interspecies electron transfer. Environ. Sci. Technol. 2020, 54, 15347–15354. [Google Scholar] [CrossRef] [PubMed]
- Ha, P.T.; Lindemann, S.R.; Shi, L.; Dohnalkova, A.C.; Fredrickson, J.K.; Madigan, M.T.; Beyenal, H. Syntrophic anaerobic photosynthesis via direct interspecies electron transfer. Nat. Commun. 2017, 8, 13924. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.H.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. 2013, 5, 8982–8989. [Google Scholar] [CrossRef]
- Sieber, J.R.; Le, H.M.; McInerney, M.J. The importance of hydrogen and formate transfer for syntrophic fatty, aromatic and alicyclic metabolism. Environ. Microbiol. 2014, 16, 177–188. [Google Scholar] [CrossRef]
- Walker, D.J.F.; Nevin, K.P.; Holmes, D.E.; Rotaru, A.E.; Ward, J.E.; Woodard, T.L.; Zhu, J.X.; Ueki, T.; Nonnenmann, S.S.; McInerney, M.J.; et al. Syntrophus conductive pili demonstrate that common hydrogen-donating syntrophs can have a direct electron transfer option. ISME J. 2020, 14, 837–846. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, M.; Liu, Y.; Liu, F.H. Desulfovibrio feeding Methanobacterium with electrons in conductive methanogenic aggregates from coastal zones. Water Res. 2021, 202, 117490. [Google Scholar] [CrossRef]
- Yee, M.O.; Rotaru, A.E. Extracellular electron uptake in Methanosarcinales is independent of multiheme c-type cytochromes. Sci. Rep. 2020, 10, 372. [Google Scholar] [CrossRef]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 224, 698–707. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhang, Y.B. Application of ethanol-type fermentation in establishment of direct interspecies electron transfer: A practical engineering case study. Renew. Energy 2019, 136, 846–855. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhang, Y.B.; Yu, Q.L.; Dang, Y.; Li, Y.; Quan, X. Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate. Water Res. 2016, 102, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Demirer, G.N. Volatile Fatty Acid Production from Organic Fraction of Municipal Solid Waste Through Anaerobic Acidogenic Digestion. Environ. Eng. Sci. 2009, 26, 1443–1450. [Google Scholar] [CrossRef]
- Stams, A.J.M. Metabolic interactions between anaerobic bacteria in methanogenic environments. Anton. Leeuw. Int. G. 1994, 66, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.Q.; Sun, D.Z.; Dang, Y.; Chen, H.M.; Zhao, Z.Q.; Zhang, Y.B.; Holmes, D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016, 222, 270–276. [Google Scholar] [CrossRef]
- Lopes, S.I.C.; Dreissen, C.; Capela, M.I.; Lens, P.N.L. Comparison of CSTR and UASB reactor configuration for the treatment of sulfate rich wastewaters under acidifying conditions. Enzym. Microb. Tech. 2008, 143, 471–479. [Google Scholar] [CrossRef]
- Bertolet, B.L.; West, W.E.; Armitage, D.W.; Jones, S.E. Organic matter supply and bacterial community composition predict methanogenesis rates in temperate lake sediments. Limnol. Oceanogr. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef]
- Zhuang, L.; Ma, J.L.; Yu, Z.; Wang, Y.Q.; Tang, J. Magnetite accelerates syntrophic acetate oxidation in methanogenic systems with high ammonia concentrations. Microb. Biotechnol. 2018, 11, 710–720. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Lee, C. A long-term study on the effect of magnetite supplementation in continuous anaerobic digestion of dairy effluent: Enhancement in process performance and stability. Bioresour. Technol. 2016, 222, 344–354. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.Y.; Zhu, P.R.; Yong, X.Y.; Wang, Y.J.; An, W.; Jia, H.H.; Zhou, J. Enhancing biomethane production and pyrene biodegradation by addition of bio-nano FeS or magnetic carbon during sludge anaerobic digestion. Bioresour. Technol. 2020, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Tang, Z.Y.; Ma, J.L.; Yu, Z.; Wang, Y.Q.; Tang, J. Enhanced anaerobic biodegradation of benzoate under sulfate-reducing conditions with conductive iron-oxides in sediment of pearl river estuary. Front. Microbiol. 2019, 10, 374. [Google Scholar] [CrossRef]
- Zhou, H.B.; Qiu, G.Z. Inhibitory effect of ammonia nitrogen on specific methanogenic activity of anaerobic granular sludge. J. Cent. South Univ. 2006, 13, 63–67. [Google Scholar] [CrossRef]
- Shi, X.C.; Lin, J.; Zuo, J.N.; Li, P.; Li, X.X.; Guo, X.L. Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. J. Environ. Sci. 2017, 55, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Masse, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Siles, J.A.; Brekelmans, J.; Martin, M.A.; Chica, A.F.; Martin, A. Impact of ammonia and sulphate concentration on thermophilic anaerobic digestion. Bioresour. Technol. 2010, 101, 9040–9048. [Google Scholar] [CrossRef]
- Lee, J.; Koo, T.; Yulisa, A.; Hwang, S. Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition. J. Environ. Manag. 2019, 241, 418–426. [Google Scholar] [CrossRef]
- Lu, T.D.; Zhang, J.Y.; Wei, Y.S.; Shen, P.H. Effects of ferric oxide on the microbial community and functioning during anaerobic digestion of swine manure. Bioresour. Technol. 2019, 287, 121393. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhao, Z.Q.; Zhang, Y.B. Potential of direct interspecies electron transfer in synergetic enhancement of methanogenesis and sulfate removal in an up-flow anaerobic sludge blanket reactor with magnetite. Sci. Total Environ. 2019, 677, 299–306. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.B.; Yang, Y.F.; Quan, X.; Zhao, Z.Q. Potentially direct interspecies electron transfer of methanogenesis for syntrophic metabolism under sulfate reducing conditions with stainless steel. Bioresour. Technol. 2017, 234, 303–309. [Google Scholar] [CrossRef]
| Conductive Materials | Dose | Operation Mode | Substrate | Main Effects and Impacts of Promotion | References |
|---|---|---|---|---|---|
| Biochar | 1.25 g/L | Reactor | Ethanol | CH4 production rate increased by 30–45%. | [44] |
| Granular activated carbon | 10 g/L | Bacth | Glucose | CH4 production rate increased by 168%; Accelerated substrate hydrolysis. | [48] |
| Graphene | 1.0 g/L | Bacth | Ethanol | Accelerated hydrolysis and acidification of substrates. | [49] |
| Carbon Cloth | 10 g/L | Bacth | Ethanol | Accelerated hydrolysis and acidification of substrates. | [42] |
| NZVI | 20 mg/L | Reactor | Pig manure | Adding 20 mg/L NZVI increased CH4 by up to 126% for digesting of pig manure. | [50] |
| 0.1% wet wight of sludge | Bacth | Sludge | Accelerated hydrolysis and acidification of substrates. | [51] | |
| Magnetite | 25 mM | Bacth | Acetate | Magnetite supplementation accelerated thermophilic methanogenesis; CH4 production rate increased by 130%. | [52,53] |
| Iron Oxide | 750 mg/L | Reactor | Beet sugar industrial wastewater | Accelerated hydrolysis process of substrates. | [54] |
| Red mud | 20 g/L | Bacth | Waste activated sludge | 136% increase in methane production compared to the control group. | [55] |
| MIET | DIET | References | |
|---|---|---|---|
| Mechanism | Mutual symbiosis using electronic carriers (Electronic carriers: Hydrogen, Formic acid, L-Cysteine, Sulfide, Quinones, Riboflavin, Phenazine) | Mutual symbiosis using direct contact of the bacterium (Self-structure of bacteria: Conductive pili, Cyt-c; External conductive material: Activated Carbon, Magnetite, etc.) | [12,29,68,69,70,71] |
| Advantages | Longer distance electron transfer is possible |
| [28,35,36,72] |
| Limitations |
|
| [20,73] |
| Electron-Donating Microorganism | Electron-Accepting Microorganism | IET Pattern | References |
|---|---|---|---|
| S strain | M. ruminantium | MIET (H2-mediated) | [23] |
| D. acatoxidans | P. aestuarii | MIET (Sulfide-mediated) | [69] |
| G. sulfurreducens | W. succinogenes | MIET (L-cystine/cysteine-mediated) | [68] |
| D. vulgaris | Methanobacterium formicicum | MIET (Formate-mediated) | [74] |
| Syntrophomonas wolfei | M. barkeri | MIET (Formate-mediated) | [75] |
| Pelotomaculum | Methanobacteriaceae | MIET (Cysteine-mediated) | [77] |
| G. metallireducens | G. sulfurreducens | DIET | [18] |
| Geobacteraceae | M. mazei | DIET | [82] |
| G. metallireducens | Methanobacterium sp. YSL | DIET | [83] |
| Desulfovibrio sp. | Methanobacterium electrotrophus | DIET | [88] |
| Rhodoferrax ferrireducens | Mx. harundinacea | DIET | [89] |
| G. hydrogenophilus | M. barkeri | DIET | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, K.; Li, L.; Wang, Q.; Cao, R. A Review on the Interspecies Electron Transfer of Methane Production in Anaerobic Digestion System. Fermentation 2023, 9, 467. https://doi.org/10.3390/fermentation9050467
Su K, Li L, Wang Q, Cao R. A Review on the Interspecies Electron Transfer of Methane Production in Anaerobic Digestion System. Fermentation. 2023; 9(5):467. https://doi.org/10.3390/fermentation9050467
Chicago/Turabian StyleSu, Kai, Linxiao Li, Qin Wang, and Rong Cao. 2023. "A Review on the Interspecies Electron Transfer of Methane Production in Anaerobic Digestion System" Fermentation 9, no. 5: 467. https://doi.org/10.3390/fermentation9050467
APA StyleSu, K., Li, L., Wang, Q., & Cao, R. (2023). A Review on the Interspecies Electron Transfer of Methane Production in Anaerobic Digestion System. Fermentation, 9(5), 467. https://doi.org/10.3390/fermentation9050467





