Abstract
This study aimed to explore the dynamic variations in fermentation characteristics, bacterial diversity and community composition at two preservation temperatures as preservation time extended. Six rumen fluid samples collected from high-grain feeding sheep were stored at −20 °C or −80 °C for 0 day, 7 days, 14 days, 30 days, 60 days, 120 days, and 240 days. The results showed that the current preservation temperature did not alter the fermentation characteristics, bacterial diversity and community composition (p > 0.05). The concentrations of ammonia, microbial crude protein, acetate, propionate, butyrate, valerate, and total volatile fatty acids were higher when stored at 60 days (p < 0.05). Preservation time had no influence on bacterial richness and evenness (p > 0.05), whilst the relative abundances of Bacteroidota and Prevotella were numerically higher when stored at 30 days, and the opposite results were observed regarding Firmicutes. Both principal co-ordinates analysis (PCoA) and non-metric multidimensional scaling (NMDS) showed clusters among treatments in terms of preservation time and preservation temperature. Analysis of similarities (ANOSIM) also revealed similarities between treatments (p > 0.05). This study indicates that most fermentation characteristics in rumen fluid were altered after a 60-day preservation, whilst the preservation time for rumen bacterial community profile alteration was 30 days. It is recommended to finish the sample determination of rumen fluid within 30 days. This study may assist decision-making regarding the practicable time for rumen fluid determination, as well as viable preservation conditions for inoculum used for in vitro fermentation testing.
1. Introduction
Rumen fluid is of unique importance to ruminant nutritional research, due to its vital role as indicator of dietary impact on rumen fermentation characteristics and animal health, as well as the recommended inoculum for in vitro fermentation testing to evaluate the nutritional value of certain feeds. Current rumen fluid collection generally requires invasiveness to animals with rumen fistula or esophageal tubes [1]. The application of the former is restricted by its high maintenance cost and the latter is not suitable for reduplicative sampling due to laborious immobilization [2]. These disadvantages have urged researchers to turn to obtaining rumen fluid from abattoirs where animals are sacrificed. However, the slaughterhouse may be located far from the laboratory and long-distance transportation of rumen fluid becomes an unavoidable routine. Therefore, a viable preservation method for rumen fluid appears to be particularly important.
Several methods for the preservation of rumen fluid have been exploited to improve its viability as inoculum for in vitro fermentation testing. The core pursuit of rumen fluid preservation is to retain adequate quality (e.g., microbial activity, physicochemical property) for routine in vitro incubation and subsequently provide reliable assessment of various feedstuffs [3]. For this purpose, an optimized strategy for rumen fluid preservation has focused on anaerobic environment, proper temperature, cryoprotectants, and freeze-drying. Proper temperature storage, such as refrigerating at 4 °C or on crushed ice, freezing at −20 °C or −80 °C or liquid nitrogen, has shown to be a feasible preservation technique in adopting rumen fluid as inoculum for subsequent in vitro fermentation testing, whilst the appropriate storage time varied with temperature [3,4,5,6]. Moreover, inconsistent results were also reported in terms of freeze and freeze-drying of rumen fluid, mainly due to decreased in vitro degradation of feeds [7,8]. Glycerol and dimethyl sulfoxide are two widely used cryoprotectants in the preservation of rumen fluid, and their addition had positive effect on gas production and volatile fatty acid (VFA) production [3,5]. In addition to the above mentioned methods, Fortina et al. [9] found that rumen fluid could retain its fermentative activity for feed digestibility evaluation for as long as 300 min when the rumen fluid was kept at 40 °C, on the premise of anaerobic conditions. Jones et al. [10] also revealed that preserving rumen fluid at 18 °C for up to 48 h was viable for in vitro digestibility evaluation. These studies indicate that the feasibility of preserved rumen fluid as inoculum for in vitro incubation may vary with both storage temperature and storage time.
Dynamic variations in physicochemical properties and microbial activity of rumen fluid under short-term preservation were also investigated. Fabro et al. [2] found similar pH value and VFA concentration when rumen fluid was stored at 4 °C for up to 96 h, whilst prominent differences were observed in the concentration of NH3-N when the storage time exceeded 48 h. Such higher ammonia concentration was also reported in rumen fluid refrozen and thawed twice at 65 d [11]. Dehority et al. [12] reported that the total viable bacterial number and colony counts were comparable when rumen fluid was preserved at 0 °C for 8 h. Martin et al. [13] examined the physicochemical properties and microbiological viabilities of rumen fluid during a 24-h storage at temperatures varying from −18 °C to 38 °C, and found that storage at 38 °C for up to 9 h or 2 h at ambient temperature showed similar properties and viabilities with fresh rumen fluid. Rumen fluid stored at 4 °C for 7 days still retained high fibrolytic activity and provided adequate organic carbon as substrate for methane fermentation of wastepaper [14]. However, decreased microbial activity was observed when fresh rumen fluid was defrosted or lyophilized [15]. Moreover, Fliegerova et al. [16] revealed that rumen fluid preserved at room temperature and −80 °C did not show significant differences on the sample clustering and quantification of Firmicutes and Bacteroidetes. These results suggest that the fermentation characteristics and microbial properties of rumen fluid may be influenced by both preservation temperature and preservation time.
In practice, there may be prolonged within-year time delays between rumen fluid collection and initiation of laboratory determination. To the best of our knowledge, no information was available on the fermentation characteristics and bacterial community of rumen fluid preserved as long as 240 d. In this study, the dynamic variations of fermentation characteristics, bacterial diversity and community composition of rumen fluid, preserved at −20 °C and −80 °C during a 240-d process, were investigated to provide recommendations for the feasible determination time for rumen fluid. It was hypothesized that both storage time and storage temperature would influence the aforementioned indicators.
2. Materials and Methods
2.1. Rumen Fluid Preparation
Animal care and welfare guidelines were provided by the Committee for the Care and Use of Experimental Animals at Jiangxi Agricultural University under protocol number JXAULL−2021036. Rumen fluid was obtained from six sheep fed on a high-grain diet with concentrate to forage ratio of 70:30 for three months. The diet consists of 30.00% peanut straw, 49.38% corn, 1.82% wheat bran, 14.40% soybean meal, 0.15% CaHPO4, 0.25% NaHCO3, 0.50% NaCl, and 3.50% premix, providing 14.66% of crude protein and 10.84 MJ/kg of metabolizable energy. Six Hu sheep (body weight 36.13 ± 1.66 kg) were transported to the slaughterhouse after a 12-h fast. Ruminal content was collected from dorsal, central, and ventral sites immediately after the rumen was separated to improve representativeness, as suggested by Mulder et al. [17], and contents from three sites were evenly pooled into one sample for each sheep. Rumen fluid was obtained by filtering through four layers of gauze, and was divided into dozens of frozen storage tubes.
2.2. Preservation Treatment
Fresh rumen fluid obtained at slaughter was designated as day 0 (D0), and fermentation characteristics determination and DNA extraction were concluded on that day without delay. Other fresh rumen fluid in individual tubes was frozen at −80 °C and −20 °C for 7 days (D7), 14 days (D14), 30 days (D30), 60 days (D60), 120 days (D120), and 240 days (D240), respectively. The selected preservation temperatures are the two typical freezing temperatures in routine laboratory conditions, and were reported to be the two best-performing temperatures regarding in vitro fermentation characteristics [18]. The frozen rumen fluid at each time point was thawed at 0 °C and the temperature was increased gradually (0.33 °C/min) to 39 °C in a water bath after thawing to minimize the temperature shock [3]. All fermentation characteristics determination and DNA extraction were concluded on that day by the same laboratory technicians, with a specific technician for a certain parameter operation throughout the experiment.
2.3. Parameter Determination
The pH value at D0 was measured immediately the rumen was taken out, and pH value of the following time points were determined after thawing; all measurements were made by means of a portable pH meter (Testo 206, Testo AG, Schwarzwald, Germany). The concentrations of ammoniacal nitrogen (NH3-N) and microbial crude protein (MCP) were determined using the methods of phenol-hypochlorite reaction [19] and improved Lowry’s assay [20], respectively. The evaluated VFA in this study included acetate, propionate, isobutyrate, butyrate, isovalerate, and valerate, with the sum of isobutyrate, isovalerate, and valerate defined as branched-chain volatile fatty acids (BCVFA). The individual component of VFA was identified according to the relative retention time and its concentration was quantified referring to the standard curve, which was conducted under the same operational program and parameter setting. The determination was performed on a gas chromatograph (GC-2014 Shimadzu Corporation, Kyoto, Japan) using scheduled procedures and parameter settings as described in Qiu et al. [21].
The DNA of rumen fluid was extracted using a bacterial DNA Kit (OMEGA, Omega Bio-Tek, Norcross, GA, USA) with the two-step method of bead-beating for pretreatment [1]. The integrity and concentration of extracted DNA was evaluated on 1% agarose gels and a spectrophotometer (NanoDrop 2000 Technologies Inc., Wilmington, DE, USA), respectively. A total of 54 examples of high-purity and high-quality DNA were delivered to Allwegene Gene Technology Co., LTD (Nanjing, China) for subsequent amplification and sequencing. The V3-V4 region was selected as the target region for amplification to reveal the bacterial diversity and bacterial community, with the barcoded primers according to Wei et al. [22] as follows: 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). The amplification procedure and its reaction system were the same as described in Qiu et al. [21]. PCR products were used to construct microbial diversity sequencing libraries after checking on a 1% agarose gel and purifying by Agencourt AMPure XP Kits (Beckman, Brea, CA, USA). High throughput sequencing was obtained by means of Illumina Miseq PE300 platform with the form of paired-end reads. The raw data were analyzed using QIIME 2 (https://qiime2.org/, accessed on 30 December 2022; [23]). The criteria of data filtration were according to Wei et al. [22], which allowed sequence lengths of between 250 bp and 500 bp and a quality score greater than 20, as well as removing sequences with ambiguous bases or those mismatched to primers and barcodes. The filtered sequences were merged into tags by Paired-End reAd mergeR (PEAR, version 0.9.6, [24]), where the minimum overlap was set to 10 bp, and allowed a maximum mismatch rate of 0.10. High-quality tags were denoised into amplicon sequence variants (ASVs) using the Deblur algorithm of QIIME 2. The Ribosomal Database Project (RDP) Classifier tool was used for taxonomic classifications with the confidence threshold of 70%, where the bacterial SILVA 138 was adopted as the reference database. Alpha diversity metrics, including Chao 1, observed species, phylogenetic diversity (PD) whole tree, Shannon index, and Simpson index, were introduced to demonstrate the richness and evenness of rumen fluid at various preservation times and preservation temperatures on the basis of ASV information. Alpha diversity metrics were calculated using the Mothur software package (version 1.46.0, Patrick Schloss, Ann Arbor, MI, USA) [25]. Principal coordinates analysis (PCoA) and non-metric multidimensional scaling (NMDS) were carried out to reveal the differences among different preservation times and preservation temperatures based on Bray–Curtis distances. Moreover, analysis of similarity (ANOSIM) was performed to uncover the similarities among different preservation times and preservation temperatures, adopting the vegan package in the R software (version 4.0.2, Lucent Technologies, Murray Hill, NJ, USA). It is worth noting that rumen fluids at day 7, 14, 30, and 60 were equally and evenly mixed from sample 1 and sample 6, sample 2 and sample 5, sample 3 and sample 4. Therefore, only six samples at these time points were used for the subsequent DNA extraction and sequencing.
2.4. Statistical Analyses
Data of fermentation characteristics were analyzed as a repeated measures design in the Mixed Models procedure of SPSS (version 21, IBM Corporation, Armonk, NY, USA) with first-order autoregressive covariance structure. The statistical model is shown as follows:
where Yijt is the continuous dependent parameter determined at preservation time t on the jth sheep at the ith temperature, μ is the overall mean, Ti is the fixed effect of preservation temperature (i = −80 °C, −20 °C), Dt is the fixed effect of preservation time (t = 0, 7, 14, 30, 60, 120, 240 days), Sj is the random effect of the jth sheep, (TD)it is the interaction effect between preservation temperature and preservation time, and eijt is the random error in the jth sheep stored at the ith temperature on preservation time t.
Yijt = μ + Ti + Dt + Sj + (TD)it + eijt
The Mixed Models procedure of SPSS was taken to analyze the rumen bacterial community data, due to asymmetric number of samples at each time point. The statistical model is expressed as described above.
For all statistical analyses, significance was declared at 0.05 (p < 0.05). Simple (first) contrasts of SPSS were performed to show the differences between refrigerated rumen fluid at certain time points and fresh rumen fluid, as well as to search for the allowable preservation time point for each detected parameter.
3. Results
3.1. NH3-N, pH Value, and MCP
As shown in Table 1, preservation temperature (−20 °C or −80 °C) did not influence the pH value, concentrations of NH3-N and MCP, whilst storage time affected the concentrations of the latter two. The concentration of NH3-N was higher in D60 and D240, and was lower in D14 when compared to D0 (p < 0.05). MCP concentration decreased as preservation time extended within 30 days (p < 0.05), whilst increasing abruptly to the highest in D60 and then reaching levels similar to D14 and D30. Higher pH values were observed in D7 and D60 when compared pairwise to D0 (p = 0.036 and 0.016, respectively).
3.2. VFA Concentration
The concentrations of VFA in rumen fluid preserved at −80 °C and −20 °C for various days are presented in Table 2. Preservation time, rather than preservation temperature, affected the concentrations of VFA of rumen fluid. The concentrations of acetate, propionate, butyrate, valerate, and total VFA in D60 were higher than that in D0 (p < 0.05). Isobutyrate, isovalerate and BCVFA concentrations decreased in D30, D120, and D240 when compared pairwise to D0 (p < 0.05).
3.3. VFA Proportion
The proportions of VFA in rumen fluid preserved at −80 °C and −20 °C for various days are listed in Table 3. Similar to the VFA concentration, VFA proportion was also influenced only by preservation time. However, most individual VFA proportions were found to have differences when preservation time exceeded 30 days. The proportion of acetate and acetate to propionate ratio were observed to be higher in D30 when compared to other time points (p < 0.05), whereas the proportions of isobutyrate, isovalerate and BCVFA decreased when the preservation time was greater than 30 days (p < 0.05). Butyrate and valerate proportions increased after preserving rumen fluid for over 60 days (p < 0.05).
3.4. Alpha-Diversity Metrics
Preservation time and preservation temperature, as well as their interactions, showed no significant impacts on richness and evenness of rumen fluid (p > 0.05, Table 4). Moreover, no differences were observed in Chao 1, observed species, PD whole tree, Shannon index, and Simpson index, when those comparisons were made between refrigerated rumen fluid and fresh rumen fluid (p > 0.05).
3.5. Bacterial Relative Abundance at Levels of Phylum and Genus
Table 5 and Table 6 show the dynamic variations in relative abundances of the rumen bacteria community at the levels of phylum and genus, respectively. The relative abundances of Bacteroidota and Firmicutes varied as preservation time advanced, with the former reaching the maximum at D30 and dropping to the minimum at D240, and with the opposite trend for the latter. Six genera were observed with relative abundances greater than 3%, with the relative abundances of Prevotella and Ruminococcus affected by preservation time. The relative abundance of Prevotella increased to the highest at D30 and then decreased as preservation time exceeded 30 days. Ruminococcus abundance was first numerically increased at D7 and then decreased during D14 to D120, and finally reached the highest value at D240.

Table 1.
Ammonia nitrogen, pH value, and microbial crude protein of rumen fluid preserved at −80 °C and −20 °C for various preservation days.
Table 1.
Ammonia nitrogen, pH value, and microbial crude protein of rumen fluid preserved at −80 °C and −20 °C for various preservation days.
| Item | Preservation Time 1 | SEM 3 | p-Value 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D7 | D14 | D30 | D60 | D120 | D240 | Time | Temperature | Interaction | |||
| Ammonia nitrogen, mg/dL | −80 °C | 13.64 | 12.56 | 12.47 | 13.94 | 15.00 | 14.18 | 13.91 | 0.61 | <0.001 | 0.940 | 0.024 |
| −20 °C | 13.64 | 13.38 | 11.88 | 12.67 | 14.41 | 13.60 | 14.95 | |||||
| Average | 13.64 b | 12.97 bc | 12.17 c | 13.30 b | 14.70 a | 13.89 b | 14.48 a | |||||
| Significance 2 | 0.052 | <0.001 | 0.258 | 0.001 | 0.626 | 0.041 | ||||||
| pH value | −80 °C | 6.85 | 6.88 | 6.94 | 6.89 | 6.83 | 6.83 | 6.76 | 0.12 | 0.098 | 0.712 | 0.003 |
| −20 °C | 6.85 | 6.90 | 6.90 | 6.81 | 6.97 | 7.14 | 7.03 | |||||
| Average | 6.85 | 6.89 | 6.92 | 6.85 | 6.90 | 6.98 | 6.89 | |||||
| Significance | 0.036 | 0.085 | 0.933 | 0.016 | 0.063 | 0.211 | ||||||
| Microbial crude protein, mg/L | −80 °C | 541.28 | 500.58 | 452.57 | 439.41 | 703.97 | 367.57 | 383.88 | 42.91 | <0.001 | 0.753 | 0.072 |
| −20 °C | 541.28 | 508.79 | 469.16 | 445.19 | 603.70 | 280.96 | 345.52 | |||||
| Average | 541.28 b | 504.69 bc | 460.86 c | 442.30 c | 653.83 a | 324.26 c | 364.70 c | |||||
| Significance | 0.005 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | ||||||
1 Preservation time D0 indicates rumen fluid was preserved for 0 day, the similar indications for D7, D14, D30, D60, D120, and D240; 2 Significance was obtained by pair-comparing the differences between a certain preservation time and fresh rumen fluid (D0); 3 SEM, standard error of the mean; 4 Interaction indicates interaction effect between preservation time and preservation temperature. Different lowercase letters (“a”, “b”, “c”) within the same row indicate differences, same lowercase letters indicate similarities. The same for the following tables.

Table 2.
Volatile fatty acids concentrations (mmol/L) of rumen fluid preserved at −80 °C and −20 °C for various preservation days.
Table 2.
Volatile fatty acids concentrations (mmol/L) of rumen fluid preserved at −80 °C and −20 °C for various preservation days.
| Item | Preservation Time | SEM | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D7 | D14 | D30 | D60 | D120 | D240 | Time | Temperature | Interaction | |||
| Acetate | −80 °C | 29.97 | 28.29 | 28.11 | 31.67 | 33.61 | 33.60 | 28.33 | ||||
| −20 °C | 29.97 | 29.62 | 29.78 | 28.79 | 37.81 | 29.47 | 30.38 | 2.91 | 0.002 | 0.957 | 0.079 | |
| Average | 29.97 b | 28.95 b | 28.95 b | 30.23 b | 35.71 a | 31.53 b | 29.36 b | |||||
| Significance | 0.384 | 0.263 | 0.743 | 0.002 | 0.063 | 0.696 | ||||||
| Propionate | −80 °C | 8.52 | 8.30 | 8.08 | 8.86 | 10.07 | 10.23 | 8.30 | 2.06 | 0.027 | 0.987 | 0.275 |
| −20 °C | 8.52 | 8.40 | 8.45 | 7.29 | 11.12 | 9.05 | 9.07 | |||||
| Average | 8.52 b | 8.35 b | 8.27 b | 8.08 b | 10.59 a | 9.64 b | 8.69 b | |||||
| Significance | 0.564 | 0.210 | 0.267 | 0.047 | 0.106 | 0.652 | ||||||
| Iso-butyrate | −80 °C | 0.41 | 0.40 | 0.40 | 0.39 | 0.45 | 0.30 | 0.27 | ||||
| −20 °C | 0.41 | 0.41 | 0.42 | 0.33 | 0.43 | 0.26 | 0.31 | 0.02 | 0.001 | 0.846 | 0.450 | |
| Average | 0.41 a | 0.40 a | 0.41 a | 0.36 ab | 0.44 a | 0.28 b | 0.29 b | |||||
| Significance | 0.780 | 0.995 | 0.014 | 0.408 | <0.001 | <0.001 | ||||||
| Butyrate | −80 °C | 7.07 | 6.34 | 6.51 | 6.81 | 9.33 | 8.91 | 7.36 | ||||
| −20 °C | 7.07 | 6.67 | 6.74 | 5.65 | 9.91 | 7.79 | 7.86 | 0.88 | <0.001 | 0.959 | 0.127 | |
| Average | 7.07 b | 6.50 b | 6.62 b | 6.23 b | 9.62 a | 8.35 a | 7.61 ab | |||||
| Significance | 0.177 | 0.198 | 0.022 | <0.001 | 0.001 | 0.108 | ||||||
| Isovalerate | −80 °C | 0.81 | 0.76 | 0.77 | 0.76 | 0.58 | 0.64 | 0.50 | ||||
| −20 °C | 0.81 | 0.80 | 0.80 | 0.61 | 0.60 | 0.54 | 0.55 | 0.07 | <0.001 | 0.902 | 0.043 | |
| Average | 0.81 a | 0.78 a | 0.79 a | 0.69 ab | 0.59 bc | 0.59 bc | 0.52 c | |||||
| Significance | 0.446 | 0.510 | 0.009 | <0.001 | <0.001 | <0.001 | ||||||
| Valerate | −80 °C | 0.56 | 0.53 | 0.53 | 0.55 | 0.75 | 0.83 | 0.65 | ||||
| −20 °C | 0.56 | 0.54 | 0.55 | 0.46 | 0.81 | 0.68 | 0.68 | 0.08 | <0.001 | 0.916 | 0.101 | |
| Average | 0.56 c | 0.54 c | 0.54 c | 0.51 c | 0.78 a | 0.75 a | 0.66 ab | |||||
| Significance | 0.394 | 0.422 | 0.056 | <0.001 | 0.002 | <0.001 | ||||||
| Total volatile fatty acids | −80 °C | 47.33 | 44.62 | 44.41 | 49.04 | 54.79 | 54.50 | 45.41 | ||||
| −20 °C | 47.33 | 46.44 | 46.73 | 43.13 | 60.68 | 47.80 | 48.84 | 5.11 | <0.001 | 0.991 | 0.060 | |
| Average | 47.33 b | 45.53 b | 45.57 b | 46.09 b | 57.73 a | 51.15 ab | 47.13 b | |||||
| Significance | 0.348 | 0.243 | 0.399 | 0.002 | 0.034 | 0.924 | ||||||
| Branched-chain volatile fatty acids | −80 °C | 1.77 | 1.69 | 1.71 | 1.70 | 1.77 | 1.77 | 1.42 | ||||
| −20 °C | 1.77 | 1.75 | 1.76 | 1.40 | 1.84 | 1.48 | 1.54 | 0.15 | <0.001 | 0.896 | 0.018 | |
| Average | 1.77 a | 1.72 a | 1.74 a | 1.55 ab | 1.81 a | 1.63 ab | 1.48 b | |||||
| Significance | 0.471 | 0.542 | 0.014 | 0.725 | 0.038 | <0.001 | ||||||
Different lowercase letters (“a”, “b”, “c”) within the same row indicate differences, same lowercase letters indicate similarities.

Table 3.
Volatile fatty acids proportions (%) of rumen fluid preserved at −80 °C and −20 °C for various preservation days.
Table 3.
Volatile fatty acids proportions (%) of rumen fluid preserved at −80 °C and −20 °C for various preservation days.
| Item | Preservation Time | SEM | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D7 | D14 | D30 | D60 | D120 | D240 | Time | Temperature | Interaction | |||
| Acetate | −80 °C | 64.10 | 64.66 | 64.21 | 65.58 | 62.97 | 62.91 | 63.83 | ||||
| −20 °C | 64.10 | 64.70 | 64.63 | 67.31 | 63.47 | 63.10 | 63.60 | 1.99 | <0.001 | 0.926 | 0.405 | |
| Average | 64.10 b | 64.68 b | 64.42 b | 66.45 a | 63.22 bc | 63.01 c | 63.71 bc | |||||
| Significance | 0.038 | 0.150 | <0.001 | 0.029 | 0.002 | 0.551 | ||||||
| Propionate | −80 °C | 17.38 | 17.35 | 17.23 | 17.05 | 16.77 | 17.52 | 17.24 | ||||
| −20 °C | 17.38 | 17.08 | 17.11 | 16.46 | 17.27 | 17.49 | 17.33 | 2.32 | 0.240 | 0.989 | 0.462 | |
| Average | 17.38 | 17.22 | 17.17 | 16.75 | 17.02 | 17.51 | 17.28 | |||||
| Significance | 0.095 | 0.008 | 0.008 | 0.174 | 0.617 | 0.776 | ||||||
| Acetate to propionate ratio | −80 °C | 4.23 | 4.25 | 4.25 | 4.40 | 4.41 | 4.19 | 4.34 | ||||
| −20 °C | 4.23 | 4.34 | 4.32 | 4.62 | 4.32 | 4.22 | 4.30 | 0.43 | 0.025 | 0.965 | 0.690 | |
| Average | 4.23 b | 4.30 b | 4.29 b | 4.51 a | 4.36 b | 4.21 b | 4.32 b | |||||
| Significance | 0.080 | 0.021 | <0.001 | 0.014 | 0.651 | 0.506 | ||||||
| Iso-butyrate | −80 °C | 0.95 | 0.98 | 0.99 | 0.86 | 0.86 | 0.62 | 0.62 | ||||
| −20 °C | 0.95 | 0.96 | 0.97 | 0.83 | 0.79 | 0.60 | 0.66 | 0.08 | <0.001 | 0.919 | 0.785 | |
| Average | 0.95 a | 0.97 a | 0.98 a | 0.84 b | 0.82 b | 0.61 c | 0.64 bc | |||||
| Significance | 0.164 | 0.012 | <0.001 | 0.016 | <0.001 | 0.001 | ||||||
| Butyrate | −80 °C | 14.60 | 14.07 | 14.55 | 13.77 | 16.93 | 16.21 | 15.75 | ||||
| −20 °C | 14.60 | 14.29 | 14.32 | 12.85 | 16.06 | 16.19 | 15.84 | 0.77 | <0.001 | 0.876 | 0.260 | |
| Average | 14.60 b | 14.18 b | 14.44 b | 13.31 c | 16.50 a | 16.20 a | 15.79 a | |||||
| Significance | 0.101 | 0.373 | <0.001 | <0.001 | <0.001 | 0.001 | ||||||
| Isovalerate | −80 °C | 1.79 | 1.76 | 1.82 | 1.62 | 1.11 | 1.23 | 1.13 | ||||
| −20 °C | 1.79 | 1.79 | 1.79 | 1.48 | 1.05 | 1.18 | 1.16 | 0.12 | <0.001 | 0.913 | 0.471 | |
| Average | 1.79 a | 1.78 a | 1.80 a | 1.55 b | 1.08 d | 1.21 c | 1.15 cd | |||||
| Significance | 0.409 | 0.51 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Valerate | −80 °C | 1.18 | 1.18 | 1.21 | 1.12 | 1.36 | 1.50 | 1.44 | ||||
| −20 °C | 1.18 | 1.17 | 1.18 | 1.07 | 1.36 | 1.44 | 1.41 | 0.07 | <0.001 | 0.842 | 0.742 | |
| Average | 1.18 b | 1.17 b | 1.19 b | 1.09 c | 1.36 a | 1.47 a | 1.43 a | |||||
| Significance | 0.843 | 0.438 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Branched-chain volatile fatty acids | −80 °C | 3.92 | 3.92 | 4.01 | 3.60 | 3.32 | 3.35 | 3.19 | ||||
| −20 °C | 3.92 | 3.92 | 3.94 | 3.38 | 3.20 | 3.22 | 3.23 | 0.24 | <0.001 | 0.884 | 0.500 | |
| Average | 3.92 a | 3.92 a | 3.97 a | 3.49 b | 3.26 b | 3.29 b | 3.21 b | |||||
| Significance | 0.973 | 0.125 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
Different lowercase letters (“a”, “b”, “c”, “d”) within the same row indicate differences, same lowercase letters indicate similarities.

Table 4.
Alpha-diversity metrics of rumen bacteria in rumen fluid preserved at −80 °C and −20 °C for various preservation days.
Table 4.
Alpha-diversity metrics of rumen bacteria in rumen fluid preserved at −80 °C and −20 °C for various preservation days.
| Item | Preservation Time | SEM | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D7 | D14 | D30 | D60 | D120 | D240 | Time | Temperature | Interaction | |||
| Chao1 | −80 °C | 356.33 | 290.36 | 492.88 | 267.29 | 333.21 | 499.73 | 385.86 | ||||
| −20 °C | 356.33 | 234.75 | 412.76 | 558.25 | 453.96 | 513.78 | 480.02 | 74.72 | 0.055 | 0.182 | 0.338 | |
| Average | 356.33 | 262.5 | 452.82 | 412.77 | 393.58 | 506.76 | 432.94 | |||||
| Significance | 0.863 | 0.847 | 0.987 | 0.999 | 0.187 | 0.863 | ||||||
| Observed species | −80 °C | 353.67 | 288.67 | 479.33 | 266.67 | 332.33 | 483.82 | 380.28 | ||||
| −20 °C | 353.67 | 234.33 | 408.00 | 520.00 | 446.67 | 495.32 | 455.48 | 69.82 | 0.059 | 0.221 | 0.412 | |
| Average | 353.67 | 261.50 | 443.67 | 393.33 | 389.50 | 489.57 | 417.88 | |||||
| Significance | 0.833 | 0.848 | 0.997 | 0.998 | 0.219 | 0.913 | ||||||
| PD whole tree | −80 °C | 32.75 | 31.02 | 38.01 | 27.00 | 31.06 | 39.31 | 33.27 | ||||
| −20 °C | 32.75 | 26.72 | 34.26 | 37.37 | 37.67 | 39.48 | 36.87 | 3.59 | 0.107 | 0.356 | 0.501 | |
| Average | 32.75 | 28.87 | 36.13 | 32.19 | 34.37 | 39.40 | 35.07 | |||||
| Significance | 0.928 | 0.961 | 1.000 | 0.999 | 0.270 | 0.984 | ||||||
| Shannon index | −80 °C | 6.22 | 6.44 | 6.62 | 5.85 | 6.63 | 6.39 | 6.34 | ||||
| −20 °C | 6.22 | 6.29 | 6.35 | 5.98 | 6.74 | 6.51 | 6.37 | 0.294 | 0.376 | 0.987 | 0.994 | |
| Average | 6.22 | 6.36 | 6.49 | 5.92 | 6.69 | 6.45 | 6.35 | |||||
| Significance | 0.999 | 0.97 | 0.938 | 0.689 | 0.958 | 0.998 | ||||||
| Simpson index | −80 °C | 0.96 | 0.97 | 0.97 | 0.95 | 0.97 | 0.96 | 0.97 | ||||
| −20 °C | 0.96 | 0.98 | 0.97 | 0.95 | 0.98 | 0.97 | 0.97 | 0.009 | 0.265 | 0.960 | 0.998 | |
| Average | 0.96 | 0.98 | 0.97 | 0.95 | 0.98 | 0.96 | 0.97 | |||||
| Significance | 0.832 | 0.987 | 0.832 | 0.832 | 1.000 | 0.999 | ||||||

Table 5.
Phylum abundance (>1%) of rumen bacteria in rumen fluid preserved at −80 °C and −20 °C for various preservation days.
Table 5.
Phylum abundance (>1%) of rumen bacteria in rumen fluid preserved at −80 °C and −20 °C for various preservation days.
| Phylum Name | Preservation Time | SEM | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D7 | D14 | D30 | D60 | D120 | D240 | Time | Temperature | Interaction | |||
| Bacteroidota | −80 °C | 61.80 | 54.98 | 62.47 | 77.52 | 62.39 | 61.96 | 53.50 | ||||
| −20 °C | 61.80 | 56.08 | 64.24 | 75.30 | 54.10 | 54.75 | 44.32 | 5.91 | 0.002 | 0.290 | 0.912 | |
| Average | 61.80 ab | 55.53 b | 63.36 ab | 76.41 a | 58.25 ab | 58.35 ab | 48.91 b | |||||
| Significance | 0.933 | 1.000 | 0.185 | 0.996 | 0.991 | 0.122 | ||||||
| Firmicutes | −80 °C | 33.13 | 41.23 | 34.32 | 19.95 | 33.70 | 35.06 | 42.21 | ||||
| −20 °C | 33.13 | 40.63 | 34.08 | 22.47 | 42.30 | 42.37 | 51.89 | 5.39 | 0.001 | 0.189 | 0.880 | |
| Average | 33.13 ab | 40.93 a | 34.20 ab | 21.21 b | 38.00 ab | 38.72 a | 47.05 a | |||||
| Significance | 0.766 | 1.000 | 0.299 | 0.969 | 0.857 | 0.038 | ||||||
| Proteobacteria | −80 °C | 3.77 | 2.23 | 2.19 | 1.94 | 2.69 | 1.58 | 1.86 | ||||
| −20 °C | 3.77 | 1.88 | 0.74 | 1.30 | 1.32 | 1.21 | 1.33 | 1.11 | 0.175 | 0.271 | 0.994 | |
| Average | 3.77 | 2.06 | 1.47 | 1.62 | 2.01 | 1.39 | 1.59 | |||||
| Significance | 0.714 | 0.379 | 0.463 | 0.687 | 0.14 | 0.218 | ||||||
Different lowercase letters (“a”, “b”) within the same row indicate differences, same lowercase letters indicate similarities.

Table 6.
Genus abundance (>3%) of rumen bacteria in rumen fluid preserved at −80 °C and −20 °C for various preservation days.
Table 6.
Genus abundance (>3%) of rumen bacteria in rumen fluid preserved at −80 °C and −20 °C for various preservation days.
| Genus Name | Preservation Time | SEM | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D7 | D14 | D30 | D60 | D120 | D240 | Time | Temperature | Interaction | |||
| Prevotella | −80 °C | 47.05 | 37.34 | 49.82 | 63.33 | 47.84 | 46.28 | 38.74 | ||||
| −20 °C | 47.05 | 35.14 | 51.75 | 61.25 | 38.22 | 38.04 | 29.31 | 6.29 | 0.002 | 0.221 | 0.926 | |
| Average | 47.05 ab | 36.24 b | 50.70 ab | 62.29 a | 43.03 ab | 42.16 b | 34.03 b | |||||
| Significance | 0.597 | 0.997 | 0.204 | 0.995 | 0.96 | 0.163 | ||||||
| Rikenellaceae RC9 gut group | −80 °C | 4.91 | 6.23 | 5.52 | 5.17 | 5.29 | 4.82 | 6.69 | ||||
| −20 °C | 4.91 | 8.28 | 5.90 | 5.66 | 6.90 | 6.34 | 7.56 | 2.75 | 0.959 | 0.512 | 1.000 | |
| Average | 4.91 | 7.26 | 5.71 | 5.41 | 6.09 | 5.58 | 7.13 | |||||
| Significance | 0.977 | 1.000 | 1.000 | 0.999 | 1.000 | 0.953 | ||||||
| Christensenellaceae R-7 group | −80 °C | 7.04 | 11.73 | 2.18 | 0.72 | 2.55 | 4.41 | 5.00 | ||||
| −20 °C | 7.04 | 8.53 | 2.10 | 1.26 | 5.89 | 6.05 | 7.43 | 2.67 | 0.064 | 0.647 | 0.944 | |
| Average | 7.04 ab | 10.13 a | 2.14 ab | 0.99 b | 4.22 ab | 5.23 ab | 6.21 ab | |||||
| Significance | 0.903 | 0.522 | 0.273 | 0.935 | 0.980 | 1.000 | ||||||
| Selenomonas | −80 °C | 4.08 | 1.99 | 5.94 | 3.72 | 6.46 | 6.30 | 5.45 | ||||
| −20 °C | 4.08 | 2.39 | 5.51 | 3.99 | 5.75 | 5.30 | 4.28 | 1.58 | 0.254 | 0.660 | 0.997 | |
| Average | 4.08 | 2.19 | 5.73 | 3.86 | 6.11 | 5.80 | 4.87 | |||||
| Significance | 0.887 | 0.938 | 1.000 | 0.852 | 0.828 | 0.996 | ||||||
| Succiniclasticum | −80 °C | 2.67 | 3.50 | 4.34 | 3.66 | 4.05 | 4.05 | 4.60 | ||||
| −20 °C | 2.67 | 2.92 | 4.63 | 2.24 | 2.91 | 3.81 | 1.80 | 1.16 | 0.733 | 0.185 | 0.767 | |
| Average | 2.67 | 3.21 | 4.49 | 2.95 | 3.48 | 3.93 | 3.20 | |||||
| Significance | 0.999 | 0.692 | 1.000 | 0.991 | 0.827 | 0.998 | ||||||
| Ruminococcus | −80 °C | 2.44 | 3.54 | 1.90 | 1.08 | 1.29 | 1.20 | 5.72 | ||||
| −20 °C | 2.44 | 3.18 | 1.83 | 1.31 | 2.22 | 2.04 | 8.27 | 1.96 | 0.019 | 0.582 | 0.985 | |
| Average | 2.44 b | 3.36 b | 1.87 b | 1.19 b | 1.76 b | 1.62 b | 7.00 a | |||||
| Significance | 0.999 | 1.000 | 0.995 | 1.000 | 0.998 | 0.080 | ||||||
Different lowercase letters (“a”, “b”) within the same row indicate differences, same lowercase letters indicate similarities.
3.6. Beta-Diversity Analysis
Both PCoA (Figure 1a) and NMDS (Figure 1b) showed clusters among treatments in terms of preservation time and preservation temperature. ANOSIM also showed similarities between treatments (p > 0.05).
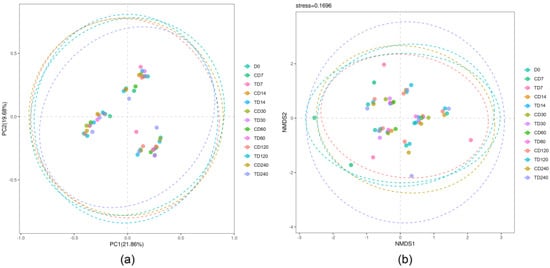
Figure 1.
Beta-diversity of the rumen bacteria in rumen fluid under various preservation temperatures and preservation times. (a) Principal coordinates analysis (PCoA); (b) non-metric multidimensional scaling (NMDS). The first capital letter “C” and “T” indicate preservation temperatures at −80 °C and −20 °C, respectively. The second capital letter “D” indicates preservation days.
4. Discussion
Rumen NH3-N is generally considered as an indicator of nitrogen metabolism for both degradation of dietary protein and ruminal utilization for microbial growth and reproduction [11,26]. Therefore, the NH3-N concentration is influenced by dietary protein provision and factors affecting microbial utilization, i.e., microbial activity, rumen environment, and the storage condition. The current study found that NH3-N concentration was the highest when rumen fluid was stored for 60 days, which is similar to the finding of Nocek et al. [11], who reported that ammonia content was accentuated after refreezing to 65 days. The possible explanation for the elevation may refer to the microbial proteolysis of protein constituents in rumen fluid. However, decreased NH3-N concentration was also observed when rumen fluid was preserved for 14 days, which is inconsistent with the result from Baetz Albert et al. [27], who found stable ammonia concentration after storage at −70 °C for 22 days. The reduction may be due to the volatilization of ammonia because the rumen fluid was not acidified during the whole preservation process. Changes of pH value are expected after preserving for 7 days due to variations in ammonia content, which explained the routine operation of rumen pH value determination, as it is well known that rumen pH value should be concluded immediately after the rumen content was collected. Ruminal MCP is frequently quantitated as an important source of amino acids for ruminants, and its concentration was influenced by dietary nitrogen and carbohydrate, microbial composition and activity [28]. It is interesting to see continuous decline of MCP before 60 days, reaching the highest at 60 days, thereafter maintaining stability. Freezing may reduce the microbial activity through membrane damage and DNA denaturation [5], which partly explained the decline of MCP during storage during the first 60 days. As the freeze extended, the protein constituents in rumen fluid were hydrolyzed into ammonia [11], as well as more energy from carbohydrate degradation, which together contributed to the synthesis of amino acid and further improved the production of MCP. The turning point at 60 days could be indirectly verified by the high concentrations of NH3-N and total VFA, as well as numerically higher rumen bacterial diversity.
The VFA is the main end product of feed and the primary form of energy utilization for ruminants, playing decisive roles in production efficiency and product quality. The concentration and proportion of VFA were not changed by preservation temperature (−20 °C or −80 °C), whilst they were altered by preservation time over 30 days, apart from propionate proportion. Insoluble substrates (e.g., polysaccharides and protein) in feed residue of rumen fluid were degraded into sugars, VFA, and amino acids during the preservation process [14]. Moreover, several metabolic processes in cold shock response during refrigeration, for instance, alteration in membrane lipids and synthesis of proteins, would accelerate the sugar metabolism [2]. Takizawa et al. [14] found that the concentration of VFA in rumen fluid increased during the first two days of preservation. The aforementioned findings and theories supported the varied concentration and proportion of VFA during the refrigeration process, which corresponded well with the increase in ammonia concentration. Another possible explanation for the higher VFA concentration would be the fact that damaged microbes due to freezing could serve as additional substrate for more VFA production [3]. Preservation temperature of rumen fluid is also a decisive factor for VFA variation. A previous study has reported that VFA concentration in rumen fluid was stable when it was stored at 4 °C, whilst increased VFA concentration was observed when the rumen fluid was stored at 20 °C or 35 °C [14]. In this study, no differences in concentrations of VFA were observed between preservation temperature at −20 °C and −80 °C, probably because the rumen fluid was in a frozen state at these two temperatures and showed similar cold shock response to temperature [2].
Rumen microbes play critical roles in dietary nutrient digestion, production efficiency, and body health of ruminants. Most studies reported that exhaustion of insoluble and soluble substrates in rumen fluid alters the microbial community and reduces microbial activity during the process of freezing [3,14]. Changes in microbial community included decreased protozoa count, protozoa viability, and Gram-negative bacteria amount [3,13,14,18], whereas the effects of freezing condition on microbial diversity and specific bacterial species are limited. Here, we tracked the dynamic changes in bacterial diversity and community composition as the storage time extended at two freezing temperatures. Bacterial alpha-diversity results showed that differences in bacterial richness and evenness caused by the preservation temperature and preservation time were small, and beta-diversity data also revealed similarities among treatments. These results might be attributable to the microbial self defense mechanism when encountering environmental stress, such as altering bacterial density and community structure via quorum sensing to maintain stability [29,30]. The explanation could be indirectly seen from the dynamic community composition at the levels of phylum and genus due to storage time, as the predominate two phyla, Bacteroidota and Firmicutes, showed numerically higher and lower relative abundances when rumen fluid was stored at 30 days, respectively. Most Bacteroidota are Gram-negative bacteria and this type of bacteria showed particular sensitivity to freezing [18]. Qiu et al. [21] reported negative correlation between ambient temperature and Bacteroidetes abundance, and the opposite correlation for the phylum Firmicutes. This study observed similar results, probably due to the adaptive capacity of Bacteroidota and Firmicutes to the ambient temperature system [31]. The genus of Prevotella is considered to be particularly active in fermenting starch and protein metabolism [32]. The higher abundance of this genus at 30 days normally indicates higher VFA production and ammonia concentration at that time point. However, these increments were only observed at 60 days, which might be due to the fact that rumen fermentation characteristics were not consequentially in accordance with the rumen bacterial community [33]. Previous studies have revealed that rumen fermentation characteristics required less time to achieve stability than the rumen bacterial community [34]. However, delayed rumen fermentation characteristics due to bacterial abundance was observed in the current study, suggesting that dietary type may affect the crosstalk between fermentation characteristics and bacterial community. Ruminococcus flavefaciens and Ruminococcus albus, two core species belonging to the genus of Ruminococcus, are critical members for degrading the plant cell wall in the rumen community [35]. Higher abundance of Ruminococcus was observed in D240, partly due to the fact that structural carbohydrates are slowly fermentable organic compounds as compared to nonstructural carbohydrates [36]. It is worth mentioning that microbial activity and microorganisms other than bacteria were not determined in this experiment. A long-term and more comprehensive tracking of the rumen microbe, i.e., bacteria, protozoa, and fungi, is required to decide the flexible time for rumen microbe determination, as well as the viable inoculum for the in vitro fermentation test.
5. Conclusions
Taken together, the current preservation temperature (−80 °C and −20 °C) did not alter the fermentation characteristics, bacterial diversity and community composition. Most fermentation characteristics were altered when stored at 60 days, and the preservation time to allow microbial community changes was 30 days. This study indicates that most fermentation characteristics altered after 60-d preservation, whilst the preservation time for rumen bacterial community profile alteration was 30 days. It is recommended to finish the sample determination of rumen fluid within 30 days.
Author Contributions
Conceptualization, Q.Q. and K.O. (Kehui Ouyang); methodology, Q.Q.; validation, Q.Q. and T.L.; formal analysis, T.L., K.O. (Kehan Ouyang); investigation, Q.Q., T.L., K.O. (Kehan Ouyang), X.L., J.Q. and J.Z.; data curation, Y.L. and X.Z.; writing—original draft preparation, Q.Q.; writing—review and editing, K.O. (Kehui Ouyang); visualization, M.Q.; supervision, Q.Q. and K.O. (Kehui Ouyang); project administration, K.O. (Kehui Ouyang); funding acquisition, Q.Q., X.L. and K.O (Kehui Ouyang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, 32160807 and 32260861; Major Discipline Academic and Technical Leaders Training Program of Jiangxi Province, 20213BCJL22043; Innovative training program for college students of Jiangxi Agricultural University, S202210410064; The central government guides local science and technology development fund project of China, 20221ZDF03017; the Earmarked Fund for the Innovation Team of Jiangxi Agricultural University, JXAUCXTD008; China Agriculture Research System of MOF and MARA, CARS-37; and Jiangxi Agriculture Research System, JXARS-13.
Institutional Review Board Statement
The study was approved by the Committee for the Care and Use of Experimental Animals at Jiangxi Agricultural University under protocol number JXAULL−2021036.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data from high-throughput sequencing were deposited in the SRA with the accession number of PRJNA917025.
Acknowledgments
We would like to express our thanks to Kairong Li and Xinfeng Chen from Ganzhou Lvlinwan Agriculture and Animal Husbandry Co. Ltd for their selfless support in the accommodation and animal provisions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paz, H.A.; Anderson, C.L.; Muller, M.J.; Kononoff, P.J.; Fernando, S.C. Rumen bacterial community composition in Holstein and Jersey cows is different under same dietary condition and is not affected by sampling method. Front. Microbiol. 2016, 7, 1206. [Google Scholar] [CrossRef] [PubMed]
- Fabro, C.; Sarnataro, C.; Spanghero, M. Impacts of rumen fluid, refrigerated or reconstituted from a refrigerated pellet, on gas production measured at 24 h of fermentation. Anim. Feed Sci. Technol. 2020, 268, 114585. [Google Scholar] [CrossRef]
- Tunkala, B.Z.; DiGiacomo, K.; Alvarez Hess, P.S.; Dunshea, F.R.; Leury, B.J. Rumen fluid preservation for in vitro gas production systems. Anim. Feed Sci. Technol. 2022, 292, 115405. [Google Scholar] [CrossRef]
- Spanghero, M.; Chiaravalli, M.; Colombini, S.; Fabro, C.; Froldi, F.; Mason, F.; Moschini, M.; Sarnataro, C.; Schiavon, S.; Tagliapietra, F. Rumen inoculum collected from cows at slaughter or from a continuous fermenter and preserved in warm, refrigerated, chilled or freeze-dried environments for in vitro tests. Animals 2019, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Prates, A.; de Oliveira, J.A.; Abecia, L.; Fondevila, M. Effects of preservation procedures of rumen inoculum on in vitro microbial diversity and fermentation. Anim. Feed Sci. Technol. 2010, 155, 186–193. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Giráldez, F.J.; Mora, M.J.; Fernández, B.; Mantecón, Á.R. Effect of preservation on fermentative activity of rumen fluid inoculum for in vitro gas production techniques. Anim. Feed Sci. Technol. 2005, 123–124, 107–118. [Google Scholar] [CrossRef]
- Garcia, T.J.; Brady, J.A.; Guay, K.A.; Muir, J.P.; Smith, W.B. 190 Reduce reuse reRumen: Preservation method of rumen fluid collected from slaughtered cattle affects in vitro digestibility. J. Anim. Sci. 2019, 97, 66–67. [Google Scholar] [CrossRef]
- Chaudhry, A.S.; Mohamed, R. Fresh or frozen rumen contents from slaughtered cattle to estimate in vitro degradation of two contrasting feeds. Czech J. Anim. Sci. 2012, 57, 265–273. [Google Scholar] [CrossRef]
- Fortina, R.; Glorio Patrucco, S.; Barbera, S.; Tassone, S. Rumen fluid from slaughtered animals: A standardized procedure for sampling, storage and use in digestibility trials. Methods Protoc. 2022, 5, 59. [Google Scholar] [CrossRef]
- Jones, R.J.; Stoltz, M.A.; Meyer, J. The effect of rumen fluid storage time on digestive capacity with five forage/browse samples. Trop. Grasslands 1998, 32, 270–272. [Google Scholar]
- Nocek, J.E.; Hart, S.P.; Polan, C.E. Rumen ammonia concentration as influenced by storage time, freezing and thawing, acid preservative, and method of ammonia determination. J. Dairy Sci. 1987, 70, 601–607. [Google Scholar] [CrossRef]
- Dehority, B.A.; Grubb, J.A. Effect of short-term chilling of rumen contents on viable bacterial numbers. Appl. Environ. Microbiol. 1980, 39, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.C.; Hilgert, A.R.; Guirro, E.C.B.d.P. Influence of food, storage temperature, and time on the extracorporeal viability of ruminal fluid of cattle. Semin. Cienc. Agrar. 2018, 39, 1181–1188. [Google Scholar] [CrossRef]
- Takizawa, S.; Baba, Y.; Tada, C.; Fukuda, Y.; Nakai, Y. Preservation of rumen fluid for the pretreatment of waste paper to improve methane production. Waste Manag. 2019, 87, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Belanche, A.; Palma-Hidalgo, J.M.; Nejjam, I.; Serrano, R.; Jiménez, E.; Martín-García, I.; Yáñez-Ruiz, D.R. In vitro assessment of the factors that determine the activity of the rumen microbiota for further applications as inoculum. J. Sci. Food Agric. 2019, 99, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Fliegerova, K.; Tapio, I.; Bonin, A.; Mrazek, J.; Callegari, M.L.; Bani, P.; Bayat, A.; Vilkki, J.; Kopečný, J.; Shingfield, K.J.; et al. Effect of DNA extraction and sample preservation method on rumen bacterial population. Anaerobe 2014, 29, 80–84. [Google Scholar] [CrossRef]
- Mulder, T.; Vandaele, L.; Peiren, N.; Haegeman, A.; Ruttink, T.; De Campeneere, S.; Van De Wiele, T.; Goossens, K. Cow responses and evolution of the rumen bacterial and methanogen community following a complete rumen content transfer. J. Agr. Sci. 2018, 156, 1047–1058. [Google Scholar] [CrossRef]
- Tunkala, B.Z.; DiGiacomo, K.; Alvarez Hess, P.S.; Dunshea, F.R.; Leury, B.J. Impact of rumen fluid storage on in vitro feed fermentation characteristics. Fermentation 2023, 9, 392. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Makkar, H.P.; Sharma, O.P.; Dawra, R.K.; Negi, S.S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Qiu, Q.; Gao, C.; Aziz ur Rahman, M.; Cao, B.; Su, H. Digestive ability, physiological characteristics, and rumen bacterial community of Holstein finishing steers in response to three nutrient density diets as fattening phases advanced. Microorganisms 2020, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Long, T.; Li, Y.; Ouyang, K.; Qiu, Q. Diet shift may trigger LuxS/AI-2 quorum sensing in rumen bacteria. Bioengineering 2022, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Wei, X.; Zhang, L.; Li, Y.; Qu, M.; Ouyang, K. Effect of dietary inclusion of tea residue and tea leaves on ruminal fermentation characteristics and methane production. Anim. Biotechnol. 2021, in press. [Google Scholar] [CrossRef]
- Baetz Albert, L.; Faidley Terry, D.; Allison Milton, J. Automated enzymatic method for the determination of ammonia: Application to rumen fluid, gut fluid, and plasma. Appl. Environ. Microbiol. 1979, 38, 212–215. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Zhao, Y.Y.; Xue, S.L.; Wang, W.; Wang, Y.J.; Cao, Z.J.; Yang, H.J.; Li, S.L. Feeding value assessment of substituting cassava (Manihot esculenta) residue for concentrate of dairy cows using an in vitro gas test. Animals 2021, 11, 307. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q.; Sun, S.; Sun, H.; Wang, Y.; Shen, X.; Zhang, L. Exploring AI-2-mediated interspecies communications within rumen microbial communities. Microbiome 2022, 10, 167. [Google Scholar] [CrossRef]
- Won, M.Y.; Oyama, L.B.; Courtney, S.J.; Creevey, C.J.; Huws, S.A. Can rumen bacteria communicate to each other? Microbiome 2020, 8, 23. [Google Scholar] [CrossRef]
- Resende, J.A.; Godon, J.-J.; Bonnafous, A.; Arcuri, P.B.; Silva, V.L.; Otenio, M.H.; Diniz, C.G. Seasonal variation on microbial community and methane production during anaerobic digestion of cattle manure in Brazil. Microb. Ecol. 2016, 71, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Purushe, J.; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E. Comparative genome analysis of prevotella ruminicola and prevotella bryantii: Insights into their environmental niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Gao, C.; Su, H.; Cao, B. Rumen fermentation characteristics require more time to stabilize when diet shifts. Animals 2021, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J.; Cox, M.S.; Vieira de Paula, T.; Lin, M.; Hall, M.B.; Suen, G. Transient changes in milk production efficiency and bacterial community composition resulting from near-total exchange of ruminal contents between high- and low-efficiency Holstein cows. J. Dairy Sci. 2017, 100, 7165–7182. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, I.R.; Yin, G.; Borovok, I.; Miller, M.E.B.; Yeoman, C.J.; Dassa, B.; Yu, Z.; Mizrahi, I.; Flint, H.J.; Bayer, E.A.; et al. Functional phylotyping approach for assessing intraspecific diversity of Ruminococcus albus within the rumen microbiome. FFEMS Microbiol. Lett. 2015, 362, 1–10. [Google Scholar] [CrossRef]
- Dijkstra, J. Production and absorption of volatile fatty acids in the rumen. Livest. Prod. Sci. 1994, 39, 61–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).