Abstract
The cosmetic segment is a rapidly growing industry that has been challenged in recent years due to the origin and impact of its ingredients and manufacturing techniques. With a focus on reducing carbon dioxide emissions and improving the degradability of products, many conventional ingredients are being dismissed to meet more exigent regulations and consumer ethical demands. Biotechnology, and fermentation as the core technology, is a solution to support and drive more sustainable growth for the cosmetic industry. This review presents the latest research and development in fermentation applied to cosmetics and showcases multiple examples throughout all classes of ingredients: from functional compounds, such as oil and surfactants, to multi-faceted molecules with a wide spectrum of formulations and skin benefits derived from their emulsifying, antimicrobial or antioxidant properties. The bottlenecks associated with the commercialization of such ingredients, together with successful examples, are also discussed. The shift towards a bio-based beauty industry requires a combination of technical, regulatory and marketing efforts. Fermentation strategies to better utilize low-cost substrates and optimize microorganisms and processes will reduce overall costs, reducing the price gap with traditional methods of production. The testing, standardization and regulation of these new ingredients need to catch up with the fast research happening in the field. Finally, consumer communication is key to achieve a successful introduction of biotech ingredients in the market.
Keywords:
biotechnology; skin care; sustainability; circular economy; bioprocesses; microorganisms; metabolite; biomass 1. Fermentation: An Ancient Technology with a Prosperous Future
Biotechnology can be generally defined as the application of scientific and engineering principles to the processing of materials using biological agents. Bioprocesses are commonly defined as (specific) biochemical reactions carried out by living cells or their components and whose objective is the production of desired components. The metabolic process that converts certain organic substrates to products is called fermentation and, thus, is the heart of bioprocessing [1].
Although the word fermentation is more popular than ever, the use of living organisms (or their parts) to generate desired products has been practiced since the dawn of civilization. It is known that ancient Egyptians were regular consumers of fermented beverages, such as beer, wine or mead. Other fermented products such as tofu, tempeh, natto, bread, kefir and cheese have been essential for diets in Eastern and Western cultures for centuries [2].
Today, fermentation offers sustainable solutions for many industries, ranging from pharmaceuticals and food to the textile and construction sectors, the production of chemicals, consumer goods and energy. The dependance on renewable feedstocks (biomass) and normally mild operation conditions (temperature, pression) make fermentation a process with a much lower environmental footprint than conventional (petro)chemical synthesis. Feedstocks can be in the form of crops (with the consequent uptake of carbon dioxide during growth), by-products or wastes from other industries or sometimes even carbon dioxide itself [3,4]. This fact aligns perfectly with the axiomata of the circular economy and represents a great asset in the reduction of greenhouse gas emissions. The cosmetic industry is a rapidly growing business (valued at USD 262 billion in 2022 and forecast to expand at a compound annual growth rate (CAGR) of 4.2% until 2030 [5]) with, in many cases, an arguably expansion required to obtain virgin raw materials.
As for the food market, the interest in fermentation for the cosmetic sector emerged in Asia (mainly Korea, Japan and China). A more traditional awareness and acceptance of fermented foods’ benefits was probably what extended the application of fermentation to cosmetics in a natural and logical manner. One of the first best-selling cosmetic ingredients was Pitera by Procter & Gamble’s Japanese SKII. According to the International Nomenclature of Cosmetics Ingredients (INCI), Pitera is actually Galactomyces ferment filtrate. The company claims that after realizing that the hands of elderly women working at a sake brewery were soft and youthful-looking, they started examining the potential of yeast fermentation by-products as potent anti-aging ingredients [6].
With the advent of chemistry and technology, the production of cosmetics moved away from natural ingredients (such as goat’s milk or honey) to synthetic chemicals that can be produced on a large scale. However, this trend is reversing, and consumers are demanding natural products that are safe, respectful to the environment and do not compromise future generations. At the same time, fermentation has emerged as a technological field disrupted with new innovations which enable more efficacious ingredients. Skin-related benefits from fermentation are attributed to different factors. During fermentation, substrates (such as soybean or wheat bran) are broken down to smaller molecules, increasing the bioavailability of nutrients in the final fermented product. In other words, good molecules “trapped” in the substrate structure are liberated after fermentation and become available and prone to be absorbed by the skin. Secondly, numerous reactions happening in the cells are responsible for the formation of multiple metabolites, many of which occur and can be recognized in our own body. For example, fermentation can result in organic acids such as malic, fumaric and citric acids. These are intermediates in the Krebs cycle and act as quick energy substrates for skin-cell mitochondria. They act also as essential mineral (Ca, Mg, Zn, Cu) chelator agents, which in turn reduces the toxicity of skin care formulations [7].
Furthermore, it is the value of fermentation delivering on sustainability pledges which will make biotechnology the future of many fields, including the skin care segment. New policies around bioeconomy are already putting pressure on the market to use bio-based and biodegradable materials. It was, for example, the need to eliminate non-degradable microplastics from formulations that pushed the development of bioplastics. As depicted in Figure 1, the number of scientific publications produced on the topic of fermentation related to cosmetics has exponentially grown in recent years, which reflects the interest of using biotechnology in skin applications. The objective of the present review is to showcase how fermentation can provide the cosmetic industry with countless alternatives to replace controversial ingredients. The advantages and limitations in the development of some of these alternatives are critically described.
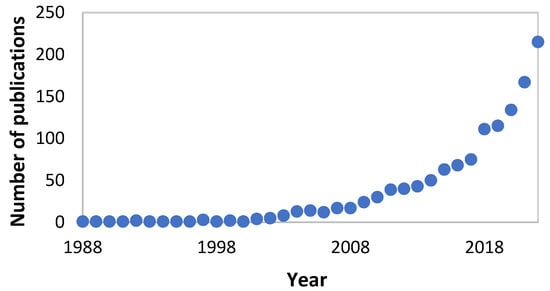
Figure 1.
Scopus search with “fermentation” and “cosmetics” in the title, abstract or keywords.
2. Introduction to Cosmetic Formulations
The skin is the largest organ in the human body and acts as a protective shield against external aggressors, enables tactile sensation and oversees the regulation of body temperature, among other functions (vitamin D synthesis, wound healing, skin flora). The skin consists of three functional layers: epidermis, dermis, and subcutis/hypodermis. The epidermis is the uppermost skin layer and hence the first line of defense against the environment. The most exposed 0.015 mm of skin constitute the stratum corneum, which is composed of a laminated structure formed by the corneocytes (brick) and specialized lipids (ceramides, fatty acids, sterols) that bind the cells together as a kind of mortar [8].
Cosmetics can be defined as any substances or mixtures intended to be placed in contact with the external parts of the human body (epidermis, hair system, nail and external genital organs) or with the teeth and the mucous membranes of the oral cavity with the exclusive or main intention of cleaning them, perfuming them, changing their appearance, protecting them (including sun tanning), keeping them in good condition or correcting body odors [9]. It is important to remark that this definition includes not only items fulling specific actions such as skin whitening or minimizing the appearance of lines in the face and body, but also important products such as toothpaste, sun cream, washing gels and deodorants, which are essential according to our modern hygienic and healthy habits.
Skin care formulations, often referred to as creams, are often water in oil, oil in water emulsions or hydrogels. An emulsion is a dispersion of two immiscible liquids (e.g., oil and water) that do not dissolve in each other. To stabilize the dispersed liquid, they require one or more emulsifiers, which are small molecular surface-active substances. Hydrogels are three-dimensional polymeric networks held together by cross-linked covalent bonds and weak cohesive forces in the form of either hydrogen bonds or ionic bonds [10]. Several raw materials are thus used in cosmetic preparation and are selected to comply with regulations which may vary and depend on a region and/or country. The most common types of molecules besides water, together with their function in skin care formulations, are presented in Figure 2.
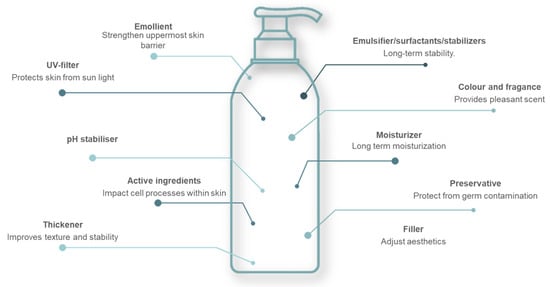
Figure 2.
Role of the main ingredients contained in a skin product in the form of emulsion.
3. Fermentation in the Cosmetic Industry
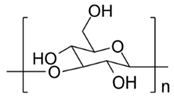
Fermentation is no stranger in Western cosmetics neither [11]. Very well-known brands and cornerstone ingredients are manufactured via fermentation, e.g., ubiquinone (Q10), hyaluronic acid and resveratrol. Ubiquinone is a co-enzyme produced by the human body with an important function in cellular energy production [12]. Microbial production of the coenzyme Q10 has become a research topic with an increasing interest for pharmaceutical and cosmetical applications. Hyaluronic acid is a very popular moisturizer and anti-aging compound that can be extracted from animal sources or produced via fermentation. Similarly, resveratrol is a secondary metabolite produced in plants that can also be synthesized by yeasts such as Saccharomyces cerevisiae or Pichia pastoris. Other functional and very common ingredients such as xanthan gum and lactic acid (LA), used as a thickener and a preservative, respectively, have been included in formulations for decades [13,14].
As we can see, sometimes fermentation is the only process to produce a molecule; other times, chemical and biological routes lead to the same compounds and the latter is often a more sustainable approach. In fact, fermentation enables the switch from extracting raw material to growing ingredients under controlled conditions. A good example to showcase the environmental advantages of biotech production is the case of squalene. Traditionally sourced from shark liver and later replaced by an impure/inconsistent olive-derived squalene, this natural emollient can be instead manufactured via sugarcane fermentation [15]. The main benefits of fermentation-based production in skin care are shown in Figure 3.
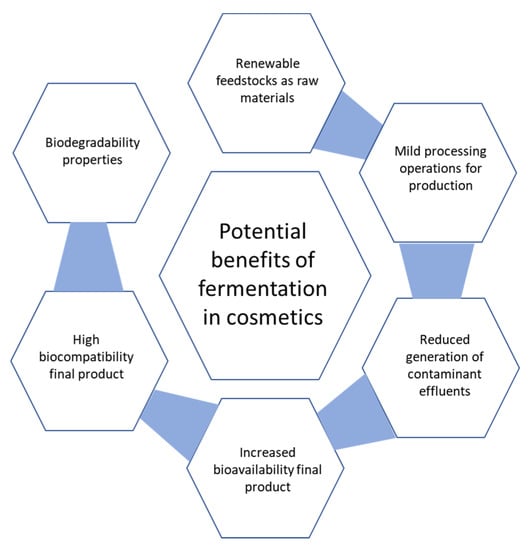
Figure 3.
Possible advantages of fermentation-derived ingredients for cosmetic purposes compared to conventional chemical counterparts.
The beauty of fermentation and the incredible potential in skin care is related to the enormous portfolio of ingredients that can be synthesized by microorganisms and be suitable for a wide range of cosmetic products. Table 1 shows examples of the main ingredient classes. Although there are some recent reviews on this topic in the literature, the majority focus on active ingredients or plant extracts [16,17,18,19]. The present work instead aims to provide a more complete overview on the different molecules and functionalities possible within fermentation, highlighting the additional benefit brought through its incorporation into a skin care product. A chemical substance can play many different roles in skin care formulation, e.g., film former, rheology modifier or emollient. For practicality, we decided to group fermented molecules by the function they have in the final product and not based on their chemical structure. In this way, the reader can find under the same section, for example, the different biomolecules that act as emollients.

Table 1.
Examples of fermentation-derived products with application in the cosmetic industry.
4. Emollients
Emollients constitute the largest ingredient group (volume-wise) in skin care products and are generally responsible for the main carbon emission contribution. They can comprise between 5 to 30% of an oil-in-water formulation [27]. Emollients are used to soothe and maintain hydration of the skin through creating a hydrophobic protective layer that prevents water loss. From a chemical point of view, they can have diverse structures: fats and oils (triglycerides and fatty acids), hydrocarbons, esters, ethers, alcohols, siloxanes and silanes (precursors of silicon) [28]. In recent years, many oil-derived emollients (e.g., mineral oils and silicones) have been replaced by naturally sourced compounds (e.g., vegetable oils and waxes). Although this reduces fossil fuel dependance, indiscriminate usage of such compounds has put pressure on the sustainability aspect of their sourcing. Palm oil, with a global production of 70 million tons per year [29], is a perfect case to illustrate the deforestation and loss of biodiversity caused by one of the most common vegetable oils used in cosmetics [30]. Biotechnology offers solutions for the production and transformation of emollients that can contribute to greenhouse emission savings, not requiring extensive land use and not competing with food purposes [31].
Algae and oleaginous yeasts (lipid content can exceed 20% of the dry biomass weight) have been extensively researched for their natural capacity to accumulate lipids, often as survival mechanisms to cope with stress conditions such as lack of carbon sources or nutritionally unbalanced growing environments [32,33]. Advances in microbial production from microalgae, fungi and bacteria for the synthesis of the major emollient types are described next.
4.1. Hydrocarbons
Hydrocarbons, used as emollients, are generally saturated with more than 16 carbon atoms in their molecules. These are normally mineral oils produced from petroleum with high stability, tolerability and an affordable price. Bioproduction of hydrocarbon in bacteria, although possible, has some inherent challenges and most of the research in the area is directed to the production of jet fuels and diesel [34]. Nonetheless, there are some examples of hydrocarbons synthesized through biological means intended for use in personal care. Global Bioenergies has developed a process to produce isobutene from renewable resources (agricultural feedstock and residues) via a genetically modified microorganism. The fermentation product can be later processed into other derivatives such as polyisobutenes [35]. The threats associated with establishing bio-based hydrocarbon production from cheap feedstocks relate to the cost of developing efficient and robust microbial cell factories and establishing more efficient routes for biomass hydrolysis to sugars [36].
Squalene
Previously mentioned as an already-available biotechnological cosmetic ingredient, squalene is a naturally occurring isoprenoid compound and has been discovered as a basic intermediate in the biosynthesis of sterols, hopanoids and triterpenes as well as a key precursor in cholesterol production [37]. The addition of several organic supplements and genetic modification of microbial strains to, for example, achieve an overexpression of rate-limiting enzymes, has been attempted to enhance the squalene content and to reduce production costs derived from the lower microbial content compared to plants such as Amaranthus seed oil and olive oil [15,38]. Xu et al. engineered Rhodopseudomonas palustris to use as a host organism for squalene production due to its natural ability to grow photosynthetically [39]. The biotechnology company Apprinova has commercialized Brazilian-sugar-cane-derived squalene under the trade name Neossance, which is a stable and lot-to-lot consistent, highly pure hydrocarbon, as opposed to natural-based squalene from plants (olive) or animals (shark) which typically has high levels of impurities [40]. Microbial processes relying on metabolically engineered Yarrowia lipolytica and S. cerevisiae strains can overcome the limitations of native producers (slow growth and low titers) [41]. A shorter alkane (C13-15) produced through fermentation (hemisqualane) and commercialized with the name Neossance Hemisqualane has been made available as a replacement for silicone (dimethicone) by Apprinova, too [42].
4.2. Fatty Acids
Fatty acids (FAs) containing oils are another class of common emollients. Most fatty acids used in cosmetics are unsaturated and polyunsaturated fatty acids (PUFAs) present in triglycerides (TGs) of vegetable origin, but microorganisms are also a great source for this valuable compound [43,44,45]. Essential fatty acids (EFAs) such as linoleic acid (omega-6) and α-linolenic acid (omega-3) are known to have beneficial effects in the prevention and treatment of inflammatory skin diseases [46]. The degree of saturation and the length of the fatty acid chain play an important role in the properties of the emollient and the function of the formula. Normally, a combination of emollients with different physical and chemical properties is required to achieve the desired product performance (stability, spreadability, sensory, etc.).
The production of medium-chain fatty acids, e.g., caprylic/capric acid, through fermentation is limited since the natural metabolism of lipids is more often wired to produce longer-chain fatty acids (C > 14). Nonetheless, strain engineering has allowed the production of C8/C10 free fatty acids (FFAs) using E. coli with yields of 0.35 to 0.37 g/g, but the process has not been scaled up yet [47,48].
Direct microbial production (with no chain elongation required) of long-chain fatty acids (C > 14) is feasible and extracellular production of FFA has been achieved. Genetically modified cyanobacteria can achieve an increased FFA secretion, although the cytotoxic effects of overproduction seem to have a detrimental effect on the cells [49]. On the other hand, intracellular lipid accumulation can be achieved with relative high titers, and the long-chain molecules synthetized within the cells can be processed afterwards to reach high levels of FFAs. This approach exhibits higher maturity levels for commercial purposes. Actually, the first process for microbial oil production developed obtained an oil rich in γ-linoleic acid, an attractive nutraceutical useful in the treatment of eczema [50].
Corbion, through its global collaboration agreement with Lubrizol, has introduced a highly stable algae oil in the market under the trade name AlgaPūr™ (INCI name: triolein). The manufacturing process involves the bioconversion of sugarcane-derived sugars (one of the crops with the highest yields) in triglycerides inside algae cells, which are later pressed, and the contained oil is extracted and refined. Those downstream operations are powered by the combustion of sugarcane waste material, which improves the economic feasibility of the process. When compared to vegetable oils, this process is claimed to have a lower carbon footprint and lower water consumption [51].
C16 Biosciences in the US and Colipi in Hamburg make use of oleaginous yeast to produce palm oil alternatives [52]. NoPalm Ingredients in the Netherlands valorizes agricultural wastes and other by-product streams as a nutrient source for oil-producing yeast. The Dutch company has recently signed a collaboration agreement with Colgate-Palmolive to intensify their pilot studies and scaling-up efforts [53]. A similar approach is taken by the Clean Food Group that makes use of ready-to-waste food stocks to produce palm oil substitutes for food and cosmetics via fermentation with a non-GM yeast and has received the support of the Doehler group to accelerate commercialization [54]. Genomatica and Unilever have also joined forces to scale plant-based alternatives based on biotechnology [55].
4.3. Fatty Alcohols
Fatty alcohols can be microbially synthetized from fatty acyl-CoAs or free fatty acids using E. coli and S. cerevisiae strains [56]. Guo et al. aimed at the utilization of xylose instead of glucose for the production of 1-hexadecanol via an engineered S. cerevisiae [57]. Y. lipolytica has also been extensively researched as a host microorganism for this matter [56].
4.4. Wax Ester
Wax ester emollients are long-chain esters of fatty acids esterified to fatty alcohols with chain lengths of 12 carbons or more. Rhyme Biotechnology harnesses a genetically modified bacterium, naturally present in soil from the genus Rhodococcus, to synthetize a wax-ester-based emollient [58]. Life cycle assessments (LCAs) performed on the chemical and enzymatic production of a typical emollient ester used in cosmetics (myristyl myristate) revealed that the biotechnological approach outperformed, in a positive way, traditional synthesis in all the categories assessed: energy consumption, global warming potential, acidification potential, nutrient enrichment potential and smog formation potential [59].
The US start-up Upwell Cosmetics has recently licensed their technology to produce a marine-microalgae-derived wax. According to the company’s research team, the ingredient, a long-chain alkenone sourced from Isochyrsis sp., has the potential to substitute petroleum-based and other types of waxes in various applications such as lip stick, shampoo, deodorant and sunscreen, among others [60].
In addition to the direct production of emollients, biotechnology tools also allow to adjust the lipid profile of existing emollients or to create tailored-made emollients to satisfy specific requirements relying on synthetic biology or enzyme transformation. E. coli is neither a natural oleaginous bacteria nor a high FA producer but an easily genetically modifiable microbe and has been established as a model organism for the next generation of tailored FAs [61]. As an example of how biotechnology can be used for lipid transformation, enzymes can be applied to produce new branched esters. In the study from Papadaki et al., microbial oils were converted to wax ester via enzymatic catalysis [62]. Enzymatic synthesis of bio-based wax esters from palm and soybean fatty acids has been performed using crude lipases produced on agricultural residues [63]. The Korean company LABIO ferments vegetable oils with Pseudozymas spp. to generate emollients with enhanced properties [64].
5. Humectants
For the skin to look and feel healthy, the water content in the stratum corneum should be between 10 and 30%. As water is lost through evaporation, it must be replenished from water in deeper layers to maintain a value within that range. When the natural skin barrier is compromised, the transepidermal water loss (TEWL) exceeds healthy values and skin problems such as xerosis can occur (disorder of the natural barrier function and or lack of moisturizing factors of the skin which lead to a reduced hydration of the skin). The role of moisturizers is thus to restore skin water content [65].
Some of the compounds previously described, such as hydrocarbons, fatty acids, fatty alcohols or wax esters, also serve as moisturizers through creating an occlusive layer on the skin that prevents water loss and are thereby often referred to as occlusive moisturizers. However, in the current section, the focus is on humectant molecules that attract water from the deeper epidermis and dermis, as depicted in Figure 4. When that happens, the skins feels smoother as the holes in the stratum corneum are filled through swelling [66]. Common humectants used in cosmetics include glycerin, sodium lactate, urea, propylene glycol, sorbitol, hyaluronic acid, sodium pyrrolidone carboxylic acid (PCA), gelatin, etc. [67]. Most of them can nowadays be produced via fermentation.
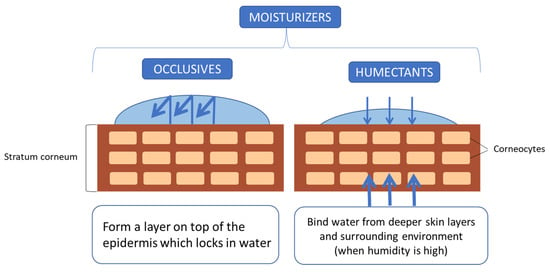
Figure 4.
Mechanisms of action of main types of skin moisturizers.
5.1. Glycerin
Glycerin is the most widely used humectant in skincare. Although it is a metabolic product from many yeasts, the current low price of the glycerol obtained as a by-product of biodiesel production makes research on its biotechnological synthesis not so attractive [68]. Glycerol can be produced with conversion efficiencies around 40% using genetically modified yeasts, in which the synthesis of by-products (ethanol, acetic acid, acetaldehyde) is minimized. Zhuge and co-workers achieved a glycerol yield above 50% using an osmotolerant yeast isolated from environmental conditions of high osmotic pressure growing in an optimized glycerol production medium [69].
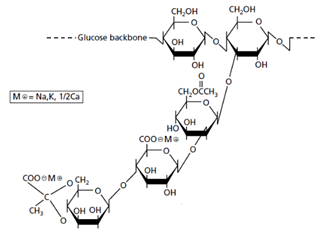
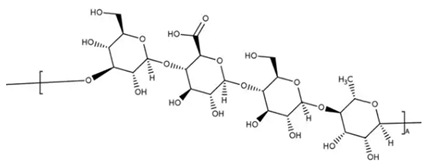
5.2. Hyaluronic Acid (HA)
HA is an abundant glycosaminoglycan in human and other mammalian tissues. Its property of forming viscoelastic hydrogels in aqueous solutions makes it a first-class humectant, in addition to finding many other applications in the esthetic and medical fields [70]. It was precisely the increasing demand of this polysaccharide linked to the low yield of its extraction from animal sources (e.g. rooster combs) which encouraged the commercial introduction of the biotechnologically produced version. Research on microbial synthesis of HA focuses primarily on the search for safe and high-performing producing strains and the control of its molecular weight. The original Gram-positive Streptococcus strain used in the production of high-molecular-weight HA in the 1980s contains potentially harmful compounds such as endotoxins and proteins in the membrane that need to be removed in a series of filtration steps. The use of recombinant B. subtilis, which secretes HA into the medium, eliminates these tedious purification stages and delivers an HA powder obtained after high-temperature spray drying of the fermentation medium [71].
Depending on its molecular weight, HA may serve different purposes in the formulation [72]; a simplified representation can be observed in Figure 5. High-molecular HA (around 1 MDa) reduces TEWL (and therefore has a positive effect on hydrating the upper layers of the epidermis) through increasing the viscosity and stability of the product film formed on the skin. As molecular weight decreases to approximately 250 kDa, HA can penetrate deeper into the skin and interact with skin cells and extracellular components (serving as signal molecule to trigger natural HA production), enabling more profound hydration and wrinkle reduction. It has been claimed that a lower molecular weight results in more potent anti-aging properties [73]. Since the molecular weight has such an impact, research has been carried out to modify the enzymes responsible for HA biosynthesis (hyaluronan synthase) and, in this way, alter the molecular weight of the polymer [74]. An overview of titers and polymer length obtained with different microorganisms and different culture conditions can be found in the study by Liu et al. [75].
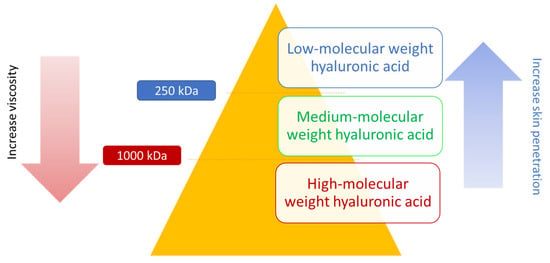
Figure 5.
Classification and properties of hyaluronic acid based on molecular weight.
5.3. Natural Moisturizing Factors (NMFs)
NMFs are molecules present in the skin that promote moisture binding capacity. A lack of NMFs is thus directly associated with dry skin, and such molecules (urea, LA, glycolic acid and amino acids) are often included in moisturizing products. Many of them are also metabolic intermediates in microbial catabolism. The so-called alpha hydroxyl acids (lactic and glycolic acid), very popular as moisturizers and exfoliators, can be obtained with fermentation-based methods. LA is a perfect paradigm of a molecule that has traditionally been produced via fermentation. Unlike chemical synthesis, the biotechnological production of this molecule allows us to obtain a single enantiomer. Most of the applications require L-LA at high purities, and only specific bacteria can metabolize such an enantiomer [76]. Furthermore, several studies have been devoted to the production of LA from renewable sources instead of the traditional simple sugars from sugarcane, cassava or corn [77,78,79].
5.4. Butylene Glycol
Butylene glycol is another humectant commonly used in cosmetic formulations. The California-based company Genomatica has commercialized natural butylene glycol under the trade name of Brontide. Produced from sugar instead of crude oil, the fermentation process is more sustainable and safer than the petrochemical route since the mutagen and group 1 carcinogen acetaldehyde used in the latter is no longer an intermediate. Plant-based dextrose can be converted to 1,3-butanediol, also known as butylene glycol or BD, through an engineered strain of E. coli in batch fermentations. Microfiltration and nanofiltration of the fermentation broth, followed by ion-exchange evaporation and distillation, are carried out to manufacture cosmetic-grade 1,3 BD. Genomatica performed an LCA to compare Brontide and petroleum-based 1,3-BD. LCA data showed that reductions in global warning potential (51% greenhouse gases emissions) and the use of non-renewable resources can be achieved when using the bio-based production route. The petrochemical route, however, outperformed Brontide in indicators/impact categories such as acidification and eutrophication, smog formation potential and blue water consumption. The greater impact of Brontide on those metrics is attributed to the cultivation of corn used as feedstock. Careful selection of cultivation conditions and processes, together with an eventual selection of secondary/third generation feedstock, would improve the score of these less important, but still relevant, categories in the future [80].
5.5. 1,3-Propanediol
1,3-propanediol (PDO) is another diol used to hold moisture and is employed as a replacement for synthetic propylene glycol. Different microbial strains including Klebsiella, Clostridia, Citrobacter, Enterobacter and Lactobacilli metabolize 1,3 PDO using glucose and/or glycerol as a carbon source [81,82]. Royal DSM collaborated with Metex Novista, a subsidiary of Metabolic Explorer, to develop the first European fermentation process for 1,3 PDO. The product was launched as TILAMAR® PDO with NØØVISTA and is made in France [83].
5.6. Erythritol
Erythritol is produced by microorganisms as an osmoprotectant and is nowadays commonly used as a natural sweetener. Yeasts such as Moniliella sp. are used in industrial production of erythritol. Its high retail price has limited its popularity across the food, pharmaceutical and cosmetics sectors. Research on yeast metabolism and genetic engineering to increase yield and productivity and on the utilization of low-cost substrates is directed to reduce production costs. Recent studies show how Y. lipolytica can utilize pure or crude glycerol for erythritol production [84] and Candida magnoliae is able to assimilate the sucrose content of cane molasses and convert it to erythritol [85].
5.7. Xylitol and Sorbitol
Xylitol and sorbitol are among the top 12 high-value-added building block intermediate chemicals listed by the US Department of Energy that can be produced from renewable sources and, similar to erythritol, has found applications in the pharma, food and cosmetics sectors [86]. Xylitol can be produced via fermentation using C. guilliermondii, while sorbitol can be biosynthetized from glucose after an isomerization stage to fructose using Streptomyces sp. Galán et al. modeled an integrated biorefinery for the production of xylitol and sorbitol from lignocellulosic biomass [87]. Xylitol is also used in toothpaste formulations to combat gingivitis and other serious oral problems [88].
5.8. Sacran
Sacran is a freshly discovered sulfated polysaccharide with a megamolecular weight (>107 g/mol). It is secreted by the cyanobacterium Aphanothece sacrum and exhibits unique physicochemical characteristics and assembly properties. For instance, sacran is able to form complexes in a gel-like sheet form in presence of polyols [89]. When used in topical applications, it creates an artificial barrier that protects against external stimuli and inhibits excessive water evaporation. This high moisture retention capacity confers superior hydration, but sacran has also been investigated in dermatology due to its anti-inflammatory properties and potential wound dressing application [90,91]. Studies describing its use against atopic dermatitis have also been published since its recent finding [92,93]. Algae, producer of sacran, has been used in the Japanese diet for a long time.
5.9. Schizophyllan
Schizophyllan is an extracellular polysaccharide secreted by the fungi Schizophyllum commune and is considered a bioactive polymer in the pharmaceutical sector and used as a humectant in cosmetics [94,95]. Traditionally produced from the fermentation of glucose, low-cost agricultural feedstocks have been recently evaluated to reduce production costs. Novel substrates include cellulosic materials such as corn fiber, distiller’s dried grains and date syrup [96,97,98,99]. Co-production of the biopolymer together with cellulolytic enzymes from bagasse can constitute another cost-effective route [100].
6. Biosurfactants
Surfactants are compounds with ambivalent character as they contain a water-insoluble tail with hydrophobic groups and a water-soluble head with hydrophilic groups. Synthetic surfactants of petrochemical origin, traditionally used across a wide range of industries including cosmetics, are being replaced by plant-based and microbial-based biosurfactants due to their lower eco-toxicity, biodegradability and use of renewable sources including waste streams [101]. Furthermore, the critical micelle concentration of biosurfactants, frequently used to measure the efficiency of a surfactant, is often lower than that of the conventional non-bio-based counterparts [102]. Extensive literature on the topic of biosurfactants and their comparison to synthetic surfactants is available [103,104,105,106,107] as the biosurfactant market size is expected to grow at 15.7% CAGR until 2030. The global market distribution of surfactants according to their origin is shown in Figure 6 [108]. In cosmetics, surfactants fulfill different functions, including emulsification, foaming and solubilization, and can serve as wetting agents and cleansers depending on the hydrophilic–lipophilic balance [109].
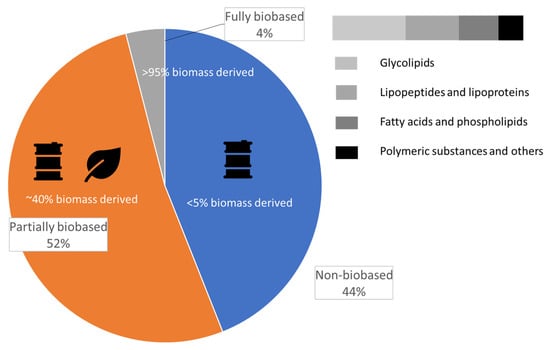
Figure 6.
Market distribution of surfactants according to their origin.
Biosurfactants (methyl ester sulphonates, alky polyglucosides, sucrose esters, etc.) produced through chemical synthesis (using green chemistry principles) from renewable sources are normally classified according to the nature of the polar group as anionic (e.g., methyl ester sulfonates, lignosulfonates), cationic (e.g., amides), non-ionic (e.g., sugar-based) and amphoteric (e.g., cocoamidopropyl) [103]. However, the classification of microbially produced surfactants is based on their molecular structure. Bacteria from soil and marine environments as well as fungi and yeasts produce biosurfactants in different stages of microbial growth and with various purposes (cell motility, biofilm, pathogenesis, substrate solubilization) [110].
6.1. Glycolipids
Glycolipids are the most important group of biosurfactants, from a commercial point of view, and are already produced in high quantities [111]. Yeast and bacteria from the genus Pseudomonas are the main producers. Within glycolipids, sophorolipids, rhamnolipids and mannosylerythritol lipids are the most relevant groups regarding industrial application for skin care. Examples of commercially available glycolipids are listed in Table 2.

Table 2.
Principal biosurfactants in the cosmetic market with corresponding producers.
6.1.1. Sophorolipids
Sophorolipids constitute the most-studied type of low-molecular-weight biosurfactant and very recent reviews on the topic have been published by Pal et al. and Cho et al. [112,113]. They are formed by a carbohydrate linked to a hydroxyaliphatic acid or a long-chain FA. Starmerella bombicola, for some time classified as Candida bombicola, is a yeast with a high capacity of sophorolipid production (exceeding 400 g/L) over a wide range of substrate choices [114,115,116,117]. Additionally, S. bombicola offers the possibility of regulating the structural diversity of sophorolipids either via genetic manipulation or adjustment of the fermentative conditions. Other strains with high potential for industrial application, together with different fermentation configurations and production process metrics, are summarized by Roelants et al. [109].
6.1.2. Rhamnolipids
Rhamnolipids are formed by one or two rhamnose molecules attached to one or two molecules of β-hydroxydecanoic acid. Rhamnolipids have been typically obtained from Pseudomonas sp., an opportunistic pathogen, with very high yields [118]. Similar to sophorolipids, they are known to exhibit antimicrobial activities against various microorganisms [119,120]. To circumvent safety and regulatory limitations caused by the risk of pathogenicity, non-harmful strains, such as those from the genus Burkholderia, have been intensively investigated [121,122,123]. Another production strategy is the use of recombinant strains; such an approach can allow for growth-decoupled production of rhamnolipids, as opposed to what happens with P. aeruginosa, and constant rhamnolipid production can be achieved even when the stationary phase of cell growth is reached [124].
6.1.3. Mannosylerythritol Lipids (MELs)
MELs have created great interest as a cosmetic ingredient, especially in Japan [125]. Their self-assembling properties make them suitable to form lamellar structures similar to those of the natural lipid bilayers of our own skin [126]. Ceramela is the trade name used by Toyobo for such an ingredient positioned as a ceramide replacement. Studies conducted with cultured human skin fibroblasts were aimed at investigating the antioxidant properties of several MEL derivatives; they confirmed the hypothesis and showed a protective effect in human cells, suggesting that MELs could be ideal candidates as anti-aging ingredients [127].
6.2. Lipopeptides and Oligopeptides
Lipo- and oligopeptides are often cyclic structures whose best-known producers are bacteria. The most popular example is surfactin, a strong and commercially available biosurfactant with antibacterial and antiviral properties. Its commercial production process uses Bacillus subtilis and has a fermentation titer that does not exceed 10 g/L. [128].
6.3. Polymeric Substances
Polymeric substances such as emulsan have also been applied in cosmetics as emulsifiers. Acinetobacter calcoaceticus secretes this lipopolysaccharide, which acts as a potent surfactant able to emulsify hydrocarbon in ratios from 1:100 to 1:1000 [129].
All in all, microbial biosurfactants constitute interesting molecules that can bring additional benefits to a cosmetic’s formulation beyond its functional role. Many of them can be considered microbiome-friendly compounds when compared to chemical surfactants due to their prebiotic character [120,130]. Low toxicity, moisturizing capacities and even skin penetration enhancement have been reported in the literature [107,131].
There are still several bottlenecks preventing microbial biosurfactants from reaching a broader market. They currently are more expensive than chemically derived surfactants, and the structural variation is determined by the microorganisms and substrates selected; therefore, the versatility can be reduced when compared to chemical synthesis. Thus, there is a need for structural variation that can expand applicability and properties. Nonetheless, biotechnology methods (special substrate utilization and better pathway understanding, genetic engineering, molecular tools, genomic and enzymatic information) can assist with this task. It is important to mention here that microbial biosurfactants are normally produced as a mixture of congeners and not as single compounds, which can be challenging from a toxicology point of view. That is why rigorous characterization and subsequent evaluation of their interaction with the environment is essential for successful commercialization [132]. A systematic study performed by Briem et al. highlighted the importance of conducting more LCAs to ensure the green credentials of bio-based surfactants [133].
7. Rheology Modifiers
Present in almost all products in cosmetics, including leave-on and rinse-off products, and every personal care sector, the role of rheology modifiers is to thicken and stabilize formulations. They can also act as texturizing agents and are therefore very influential on consumer perception. Besides that, they have a direct impact on the formula’s behavior during use; this means aspects such as the pick-up from a jar, the flowing out of a bottle, spreading performance on the skin or the immediate and after-feel sensation. They are often referred to as thickeners [134].
The portfolio of rheology modifiers is composed of synthetic polymers, such as acrylates, biopolymers, fatty alcohols, polyethylene glycol (PEG), soaps and other minor groups [135]. Due to the recalcitrant properties of traditionally used synthetic rheology modifiers such as polyacrylate/acrylate copolymers (carbomers), there is a need for biodegradable alternatives that replace materials with higher negative environmental impact (toxic/harmful to aquatic life). Such biopolymers can be of natural (e.g., gelatin, collagen, chitin) or plant sources (e.g., pectin, Locust bean gum, guar gum, tara gum, gum arabic, corn/tapioca/potato starch, agarose, alginate, carrageenan) or can be obtained from living microorganisms, i.e., bacteria, fungi or microalgae. There are also semi-synthetic rheology modifiers which include modified cellulose, obtained from vegetal or microbial origin, that has been altered to generate carboxymethyl cellulose, methyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose and dehydroxyxanthan gum [136,137,138]. Microbial species can secrete polysaccharides (exopolysaccharides or EPSs) under certain environmental conditions, such as stress, or can produce them intracellularly as integral components of the cellular wall. In this section, we describe some of the most-researched microbial biopolymers with applications in the cosmetic sector.
7.1. Xanthan Gum
Xanthan gum is probably the most famous microbial polysaccharide in skin care and its applications extend to the food industry and even oil drilling [139]. It is a high-molecular-weight and branched anionic polymer composed of glucose, mannose and glucuronic acid monomers. It is soluble in water and increases formulation viscosity even at low concentrations. Furthermore, it maintains its viscosity in the presence of electrolytes and can withstand a wide range of pHs and temperatures. Beyond thickening, it is used as a stabilizer for pigment suspension and as solubilizer for antidandruff or antiacne agents across many different cosmetic categories [14]. Kelco was the first company to commercialize its production using the Xanthomonas campestris strain grown on corn syrup molasses and has recently partnered with ExoPolymer to develop the next generation of functional biopolymers. Optimization of xanthan gum production from cheese whey conducted by Mesomo and co-workers resulted in concentrations close to 50 g/L [140].
7.2. Succinoglycan Gum
Succinoglycan gum is an acidic heteropolysaccharide with glucose, galactose, pyruvate and succinate units. It exhibits similar properties to xanthan gum and can also be used in a wide range of pHs and electrolyte-containing media for thickening and stabilizing purposes. It is produced by Agrobacterium tumefaciens and other species of Pseudomonas and Rhizobium [141]. It has been commercialized under the trade name of Rheozan SH by Solvay Novacare.
7.3. Gellan Gum
Gellan gum is another water-soluble anionic polysaccharide that is also used in the food sector as a gelling agent. It has a tetrasaccharide repeating unit formed by two glucose units, one glucuronic and one rhamnose unit. This hydrocolloid has a high-acyl and a low-acyl form. The first produces hard, non-elastic and transparent gels while the latter creates soft, elastic and opaque gel products. [14]. It is produced by the bacterium Sphingomonas elodea and was brought to the market by CP Kelco. High-acyl gellan gum is also commercialized for the cosmetic sector by Jungbunzlauer.
7.4. Sclerotium Gum
Sclerotium gum is a homopolysaccharide of glucose used as emulsifier, stabilizer and thickener in the food and beauty industries. It is produced from the fungus Sclerotium rolfsii. In general, the term scleroglucan is used to designate similar EPSs produced from fungi such as Schizophyllum rolfsii, S. glucaniucm, S. commune, Botrytis cinerea and Epicoccum nigrum [142]. Sclerotium gum has been commercialized under the Amigel and Actigum trade names.
7.5. Chitin and Chitosan
Chitin is a very abundant polysaccharide distributed in the animal (e.g., exoskeleton of crustaceans) and vegetal kingdoms. Chemically, it is a co-polymer of glucosamide and N-acetylglucossamide. Chitosan is the N-deacetylated derivative of chitin and only found in some fungi. Chitosan is soluble in acidic media and is normally produced from chitin extracted from shellfish waste. Chitosan and its derivatives have been researched for use in oral, hair, nail and skin care with UV-protection properties [143]. Among the benefits of the fungal approach for chitin and chitosan synthesis is the fact that different properties can be obtained (e.g., lower molecular weight) through varying species and culture conditions [144]. KitoZyme, Mycoved and Chibio are manufacturers of these multifunctional polysaccharides via fugal fermentation. Solid-state fermentation has been regarded as a promising alternative to submerged fermentation for this matter, but further studies on the scalability of this technology are necessary.
7.6. Bacterial Cellulose
Bacterial cellulose (BC) is another example of a biopolymer gaining importance in the cosmetic market. Oliveira et al. carried out a systemized review that compiles the main research works on BC exclusively for cosmetic use [145]. Its wide spectrum of biological properties makes BC not a simple rheology modifier but rather a cosmetic ingredient with great biocompatibility, antimicrobial and water retention capacities. BC can be produced in static conditions or in stirred mode when cellulose precursors are provided. Layers with different thicknesses and 3D forms are produced under static conditions while pellets, granules or cocoons are produced with an agitated set-up [146]. The most active producer is Gluconacetobacter xylinus (Acetobacter xylinum) [147]. BC is also used for the fabrication of beauty masks [148].
7.7. Alginate
Alginate is not only used for thickening, gel-forming and stabilizing purposes but also as an encapsulating agent for hydrophilic compounds in cosmetic and pharmaceutical formulations, including nanoparticles [149]. Its easy chemical modification allows the production of materials with, for example, different mechanical properties. In the biomedical field, alginate is especially valuable for wound healing and tissue engineering thanks to its high biocompatibility and extremely low toxic risks [150]. Alginate is found in the cell walls of red and brown macroalgae but also in some microalgae. There exist alginate producers in the bacteria domain, e.g., Pseudomonas and Azotobacter spp. [151].
7.8. Dextran
Dextran is another exopolysaccharide produced from sucrose substrates. The Leuconostocaceae family contains the most prominent wild dextran producers even though mutants from Streptococcus have also been exploited for EPS synthesis [19]. Besides thickening, dextran and its derivatives can exhibit moisturizing, soothing and anti-inflammatory properties [152].
In conclusion, there is a long list of naturally occurring polysaccharides, some of which are already used in the food industry, that find applications in the beauty sector as well; these include levan, schizophylan, ß-glucan, dextran, welan, rhamsan, pullulan and curdlan. Some of the most common molecules are listed in Table 3. Through extending their application use and therefore increasing the market demand, these microbial counterparts could finally become competitive with plant-derived alternatives.

Table 3.
Microbial-origin polysaccharides used in the cosmetic industry.
8. Active Ingredients
In the pharmaceutical industry, an active ingredient is a biologically active substance present in a drug; the term “active” in cosmetics refers to a molecule/compound or extract that has a proven (perceivable) skin benefit, e.g., wrinkle reduction, anti-acne, sebum reduction, skin whitening, boost collagen production, etc. Actives are normally efficacious substances that, even in low concentrations, are responsible for various product claims (anti-ageing, firm skin, stretch mark reduction, etc.). In the past, actives could have a synthetic, animal or plant origin. With the advent of biotechnology, the possibilities for the discovery, design and reproduction of existing actives in a more sustainable manner or enhancement of current actives are countless. In addition, the non-utilization of animals and the lack of dependance on synthetic raw materials can alleviate the concerns of the most eco-friendly consumers.
8.1. Fermentation as a More Sustainable Process to Produce Existing Actives
8.1.1. Resveratrol
Resveratrol (3,4′,5-trihydroxystilbene) is a secondary metabolite found in several plants, including grapes, berries, nuts and Japanese knotweed. It exhibits many interesting features for derma/skin care and therapeutical applications. It is a strong antioxidant with anti-inflammatory and anti-aging properties and has already been used for skin disease control [153]. Its natural production in plants, harvested seasonally, occurs at low concentrations, and the isolation and purification are tedious multistep processes. Transgenic plants designed to yield larger amounts of resveratrol still require very specific growth conditions—normally, stress conditions and the presence of elicitors—and have not yet been so effective. The chemical synthesis of resveratrol generates other by-products and requires the use of toxic solvents [154]. Metabolic engineering of microorganisms for high production of resveratrol could be a solution to meet the increasing demand of this molecule. S. cerevisiae and Y. lipolytica appear as potential workhorses for the task, although the yields obtained with such host yeasts are still low [155]. Endophytic fungi have also demonstrated good resveratrol production ability and metabolic engineering techniques applied on them could enable large-scale production [156].
8.1.2. Collagen
Collagen is a structural protein that accounts for one-third of the proteins in the human body and is present in skin, bones, sinews, blood vessels and teeth. Composed of glycine, proline and hydroxyproline, collagen molecules are fiber-forming proteins used in sports orthopedics and regenerative medicine (tissue engineering) but also in cosmetics, for a plumping and smoothing effect on the skin and hair strengthening. Its animal origin has raised concerns among many consumers who demand safer and more sustainable alternatives [157]. Therefore, the production of collagen via fermentation coupled to synthetic biology tools appears as a hot area of research [157]. Evonik has developed a microbial fermentation process to produce high-quality human-like collagen of different types through implanting the genetic information that encodes a specific collagen structure into bacteria or yeast cells [158]. Other companies such as Cambrium, Jellatech and Geltor are also in the business of animal-free and biotechnology-derived collagen.
8.1.3. Ceramides
Ceramides is the common name given to a heterogeneous group of sphingolipids, which, along with free fatty acids and cholesterol, form the lamellar structure in our stratum corneum that greatly contributes to the barrier function of the skin [159]. Ceramide-containing formulas are trendy cosmetic products, which claim to be efficacious even with very low concentrations of sphingolipids. Plants as well as animals are a source of ceramides, but the extraction process is difficult and expensive. Therefore, ceramides in the cosmetic market are generally of synthetic origin. Companies such as Evonik have developed fermentation technologies to produce ceramides which can be later converted to human-skin-identical molecules. Croda has recently acquired the Korean supplier Solus Biotech in order to strengthen their position in the ceramide market [160]. Wickerhamomyces ciferrii (formerly known as Pichia ciferrii), S. cerevisiae and Y. lioplytica have been reported as ceramide producers [14,161].
8.1.4. Plant Cell Culture
Plant cell culture, or the growth of plant cell tissues in vitro, can be seen as not just a means of reducing the environmental impact of growing the entire plant used for extract generation (soil, water, fertilizer and pesticide use) but also as a way of controlling the production process with the highest-quality standards and consistency, avoiding the impacts of seasonality or climate change and simplifying supply chain logistics [162]. Moreover, selected growing conditions can trigger the overproduction of metabolites of interest which, in the end, can have an effect on the potency of the extract [163,164]. A representation of plant cell culture procedures is shown in Figure 7. Mibelle Biochemistry is regarded as a pioneer in the field of plant cell culture and introduced the first ingredient using an endangered species of a Swiss apple variety [165]. Although nowadays, there are several suppliers of plant stem cells and their extracts, the development in plant culture is not as advanced as in microbial fermentation due to the intrinsic difficulties of cell culture, e.g., slower growth [166]. The Horizon-2020-funded European research and innovation project InnCoCells is precisely working in the development of naturally sourced cosmetic ingredients obtained through biotechnological means, such as plant cell culture, and new production methods such as aeroponic farming.
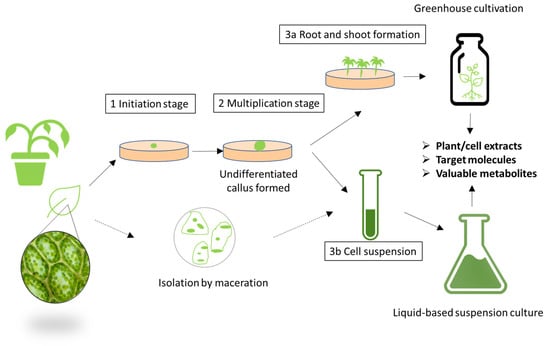
Figure 7.
Possible plant cell culture procedures to produce cosmetic active ingredients.
Besides the described ingredients, vitamins (vitamin A or retinol, vitamin B5 or panthenol), hydroxyacids (ellagic and gallic acids), aminoacids (leucine) and even cannabidiol (CBD) are all examples of beauty actives of high demand that can currently also be produced via fermentation [167,168,169].
8.2. Fermentation for Enhancement of Current Active Ingredients/Active Ingredient Booster
Fermentation can also be used in cosmetics to further improve the biological compounds of existing active ingredients, plant extracts or even agriculture by-products [170]. For some of these fermented ingredients, clinical studies have been carried out to demonstrate their superior efficacy; a few examples are presented next.
8.2.1. Ginseng
Red ginseng fermented with L. brevis was found to be richer in ginsenosides, flavonoids, polyphenols and uronic acid compared to non-fermented red ginseng. Tyrosinase- and elastase-inhibitory activities were increased compared to non-fermented red ginseng, while irritation and sensitizing potential were reduced [171]. Besides that, the fermentation of black ginseng using S. cerevisiae was proved to have anti-wrinkle activity in cultured human fibroblasts first and also a non-cytotoxic effect [7]. A clinical study on 23 subjects confirmed the anti-wrinkle and skin whitening effect of fermented black ginseng at 1% concentration in a cream after eight weeks [172].
8.2.2. Soybean
Soybean and soybean milk ferments, rich in polyamines and isoflavones (genistein and daidzein), have also been tested for topical application [173,174]. Improvement in skin elasticity was achieved after six weeks due to an increase in natural HA production [175]. Another study comparing fermented and non-fermented soybean extracts showed that antioxidant and skin-whitening properties are greater in the former after determination of DPPH, superoxide radical and hydroxyl radical scavenging activities, linoleic acid inhibition activity and tyrosinase inhibition activity [176]. LA bacteria (LAB) and Bifidobacteria and B. subtilis have been employed in the fermentation of soybeans [177,178,179].
8.2.3. Aloe Vera
Fermentation of aloe vera mediated by L. plantarum led to a fourfold increase of the moisturization capacities of the extract [180]. Malic acid present in the plant was converted to LA, which together with fructose increases the skin water content. A rise in serine, alanine and glycine levels was detected after fermentation, and these amino acids constitute natural moisturizing factors in human skin. An in vivo study showed that fermented aloe has a positive impact on the production of eosinophils and fibroblasts, which explains the good anti-inflammatory and wound-healing properties of fermented aloe [10]. The reduction of the molecular weight of the polysaccharides present in the plant (with 20% of the total in the 600–900 range) can be linked to the attributed anti-wrinkle activities [181].
8.2.4. Kombucha
Niziol-Lukaszewska and colleagues studied the effect of different kombucha ferments as potential cosmetic ingredients. Kombucha is the name given to a functional tea brewed using a symbiotic culture of bacteria and yeast (SCOBY). When using this mixed culture for the fermentation of green coffee beans, red berries and berry leaf extracts, ingredients with various beneficial dermatological and cosmetic properties were found [182,183].
Examples of other fermented raw materials with cosmetic properties include coffee, pomegranate, berries, milk, honey and also by-products especially from the food industry such as rice bran [16]. The effect of fermentation also varies with the type of microorganisms and conditions used in fermentation.
8.3. Fermentation for Novel Actives Production
Fermentation can also be regarded as a discovery tool for the generation of new active compounds or tailor-made substances to fulfill concrete problems.
8.3.1. Enzymes
Enzymes have been used in the cosmetic industry already for some years with various purposes and action mechanisms. Enzymes such as lysyl and prolyl hydoxylases promote collagen formation, which in turn contributes to skin firmness [184]. Superoxide dismutase (SOD) converts free radicals into less reactive forms and proteolytic enzymes, such as keratinases and other proteases, act as exfoliating agents removing dead cells from the skin [185]. Photolyases have also gained lot of attention for their capacity to protect against solar radiation through reversing lesions present in the DNA [186].
8.3.2. Extremolytes
Extremolytes are low-molecular-weight organic molecules which protect macromolecules and the cell structures of extremophiles, these are organisms that inhabit hostile environments, such as hot geysers, arctic areas or volcanoes. Such molecules act as multifunctional agents against various stress sources (cytotoxins, radiation or salts). Ectoince, hydroxyectoine, proline and trehalose are extremolytes of interest in the cosmetic, medical and food industries where protection of sensitive compounds is sought. Biotechnological production of these compounds is already possible using non-GMO (natural extremolyte producers) or metabolically engineered E. coli, C. glutamicum and S. cerevisiae [187].
8.3.3. Growth Factors
Growth factors are signal molecules of protein nature that promote various cellular processes, including growth. Exogenous supplementation of growth factor either via injection or topical application is perceived as promising tool to fight the signs of skin aging, although further studies on tolerability, stability and safety are needed. Human epidermal growth factors can be exploited, for example, in the treatment of age spots, wrinkles or freckles. Eukaryotic systems such as S. cerevisiae can be used to produce these in greater amounts than prokaryotic cells, and the formation of inclusion bodies in the cell host is avoided [188].
8.3.4. Stem Cells
Stem cells are unspecialized cells with a long-term self-renewal capacity. Such an internal limitless repair system enables the replenishment of cells lost due to disease or injury in an adult tissue. The second important feature of stem cells is that they can be induced to become any specialized cell, exactly as it happens in the early stages of an embryo [189]. There are cosmetics-manufacturing companies already marketing (plant) stem cell technology for the production of some of their ingredients, even though the formulations do not contain live stem cells [190]. A report contrasting product claims with scientific evidence was published by Trehan and colleagues [162].
8.3.5. Bacteriophages
Bacteriophages, or simply phages, are bacteria-infecting viruses that have drawn the attention of the scientific community as antibiotic resistance among the human population continues to rise. Phages have the advantage of specifically targeting certain prokaryotic cells. Therefore, they could be used to combat pathogenic bacteria linked to skin diseases without harming beneficial species. Such an approach could, in principle, replace antibiotic-based treatments for skin problems such as acne [191,192]. Challenges in the manufacturing, formulation and short longevity of phages on the skin surface still need to be addressed for the successful implementation of this kind of biotech active ingredient in the future [193].
9. Preservatives
Cosmetics, same as many food products, require some form of preservation to guarantee a certain shelf-life (maintenance of product properties) without jeopardizing consumers’ safety [194]. The most common form of preservation is the addition of synthetic or natural compounds that act as antimicrobial agents and have proved their activity in the formulation in suitable microbiological tests, i.e., challenge tests [195]. Some of these chemicals (triclosan, phenoxyethanol) can expose the consumer to other undesirable effects (toxicity), and there has been a shift towards the use of natural compounds, with some preservatives that had been commonly utilized for a long time (i.e., parabens) being banned [194,196]. Although the idea of preservative-free or self-preserving cosmetics is already quite mature [197], new physical tools and multifunctional ingredients, such as fermented ingredients with antimicrobial effect, and suitable packaging designs can contribute to existing methods in so-called “Hurdle Technology” [198].
9.1. Organic Acids
Organic acids, such as lactic, citric, acetic, fumaric, propionic, malic and levulinic acid, prevent the growth of pathogenic and spoilage microorganisms through creating unfavourable conditions for their propagation. These acids can be produced via carbohydrate conversion in the metabolism of different species of bacteria and fungi. LAB are well known for the synthesis of such metabolites and are often used in food preservation [199]. Recently, even anisic acid derivatives have been produced via fermentation using Rhizopycnis vagum isolated from the tissues of Nicotiana tabacum [200].
9.2. Lactobacillus Ferment
Lactobacillus ferment is the INCI name of different trade name ingredients positioned as a multifunctional ingredient with strong preservation capacity. Lactobacillus spp. are also capable of producing bacteriocins, which, in combination with lactic acid and the aforementioned organic acids, confer antimicrobial properties [201,202].
9.3. Bacteriocins
Bacteriocins are a complex group of protein-consisting compounds with a bactericidal action towards organisms that closely resemble the producer. Nisin is an example of bacteriocin belonging to the lantibiotic class produced by Lactococcus lactis [201,203].
9.4. Enzymes
Enzymes such as lisozymes, lactoperoxidase and other lytic enzymes, initially researched for their application in food preservation, could become attractive for cosmetic use provided regulatory clearance is achieved, which often requires high investments. It is foreseen that biotechnological techniques will play an important role in the development of effective antimicrobials in the form of engineered/recombinant peptides [204].
Antimicrobial compounds with specific activity against fungi are desired as current preservatives tend to have weak antifungal activity. A recent review on antifungal compounds produced from the fermentation of food waste has been published by Martí-Quijal et al. [205]. Plants and algae are also known to synthesize an impressive array of antimicrobial compounds, which will enlarge the options for preservative systems in the future [206].
10. Pre/Pro/Postbiotics
The terms pre/pro/postbiotics have rapidly gained popularity among cosmetics users during the last years [207]. Prebiotics in skin care refers to fermentation metabolites that have a positive impact on maintaining a healthy skin microbiome, serving as nutrients for the “good bacteria” in our skin. Formulations with probiotics contain living microorganisms in a dormant stage that are reactivated when the product is applied. These microorganisms help to populate the skin with beneficial microorganisms and combat pathogens or other microorganisms linked to skin disorders. Postbiotics, on the other hand, are just cell lysates or inanimate/non-viable microorganisms but not live forms [208,209,210]. The difference between the three terms is shown in Figure 8.
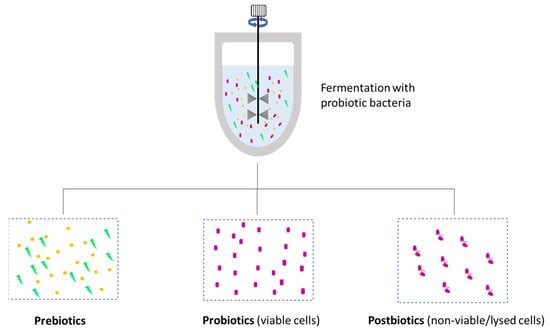
Figure 8.
Schematic representation of the origin and differentiation between pre/pro and postbiotic molecules.
Interest in the topic of skin microbiome started after an exploration of the so-called gut-to-skin axis, since many skin alterations are accompanied by an altered gut microbiome [211]. Products intended to restore and regulate or modulate a healthy skin microbiome were first found in the form of pills and capsules and were to be administered orally. Nowadays, there are also pre/pro/postbiotics formulations for skin care to be applied topically.
Bifidobacterium, Lactobacillus and Streptococcus thermophilus are the most common strains used, alone or as blends, as probiotics. Their production, compared to pre- or postbiotics, presents additional challenges to formulate stable forms of viable microorganisms. Microencapsulation and oil suspension have been investigated as possible formats to deliver skin-friendly microorganisms that can colonize and rebalance the cutaneous microbiota. A list of microorganisms associated with probiotic properties for dermal health can be found in [212]. Postbiotics are often derived from the Lactobacillus genera and S. cerevisiae and comprise different metabolites such as polysaccharides or teichoic acids, which have biological properties ranging from anti-inflammatory to anti-oxidants [213]; their shelf-life and manufacturing process is of course simpler than the case of probiotics.
LAB, generally recognized as safe (GRAS), constitute an easy entry point for the manufacture of ferments to be sold as prebiotics in skin care. Indeed, LA and other metabolites have revealed to cause an improvement of the skin condition and even prevent atopic diseases [214]. Lipoteichoic acid is reported to increase dermal cellular defence against bacterial infection [212]. Other examples of prebiotics include oligo- and polysaccharides, amino acids, vitamins, lipids and proteins. Such compounds can be used as nutrients, induce the growth or activity of a microorganism and be utilized as an energy source for the production of biomolecules. The number of compounds with potential prebiotic activity is hence very high, and rapid methods for detecting prebiotic activity need to be developed [215,216]. At the same time, there is a lack of a regulatory framework for cosmetic ingredients specifically intended to work with the skin microbiome.
Breakthroughs in the field of sequencing and bioinformatics have catapulted the understanding of microbiome composition. Even though insights on the biochemical routes of specific strains and metabolic routes of given compounds within the skin microbiome are needed, these new technologies open the door to innovative therapy platforms for the treatment of skin conditions through, for example, genetically engineered probiotics that can perform certain tasks [217,218].
11. Other Compounds
11.1. Aromas and Delivery Systems
Aromas and perfumes are frequently added to skin care products to mask the natural smell of certain raw materials and produce a pleasant sensation for the consumer in the using phase. The production of fragrances demands a high consumption of raw materials [219] and biotechnological means could be used to replicate essential oils and produce volatile compounds (e.g., terpens) with pleasant notes [220]. Common fragrance compounds such as sandalwood, patchouli and vanilla have already being produced by fermentation processes [221,222,223,224]. A more exhaustive list of natural-identical fragrances obtained via biotransformation can be found in [225].
Cyclodextrins (CDs) are oligosaccharides of cyclic structure produced from the enzymatic conversion of starch. They have attracted considerable attention as active delivery systems for pharmaceutical and cosmetic applications. CDs encapsulate active molecules and increase their stability and solubility through creating a reservoir effect on the stratum corneum, which ensures a sustained release [226]. They can also be used to reduce ester volatility in perfumes. B. subtilis, B. agaradhaerensis, Brevibacterium terrae and Microbacterium terrae are examples of bacteria investigated for cyclodextrinase enzymes [19].
The LA polymer (polylactic acid or PLA) is used in the preparation of biodegradable microbeads as a substitute for plastic non-degradable particles [227]. PLA has been approved by the US Food and Drug Administration for food, pharmaceutical and cosmetic applications due to its non-toxicity and high biocompatibility properties [228].
11.2. Pigments and UV Filters
Synthetic colorants were frequently added to cosmetic formulations to modify the product appearance and make it more appealing to consumers. The side effects, including health and environmental problems related to such toxic compounds, have motivated the search for natural and harmless substances. Bacteria (cyanobacteria or prokaryotic microalgae), fungi and microalgae (eukaryotic microalgae) are all sources of microbial pigments, which have been the subject of many studies in recent years [229,230]. Due to their photosynthetic activity, microalgae are rich in chlorophyll, carotenoids (lycopene, astaxanthin, β-carotene), phycobiliproteins, astaxanthin, xanthophyl and phycocyanin, to name some of the most well-known. These compounds have multifaceted roles and can serve not only as pigments for the food and cosmetic industries but also as antioxidant-active compounds and microbial enzymes. Additionally, photoprotective formulations can be achieved using these or similar cyanobacteria secondary metabolites, investigated for their UV radiation properties [231]. An example of a promising UV screen is scytonemin [232]. Extremophiles living in areas of high solar incidence can accumulate carotenoids in response to environmental stress and are thus good candidates for photoprotectant biofactories [233]. Although UV filters are the segment of cosmetics with the strictest regulations, current chemical filters have raised the alarm of consumers due to potential human and environmental toxicity (e.g., coral and marine life). Nature-inspired and biotechnologically produced compounds, such as the secondary metabolites of plants and cyanobacteria, could constitute safer alternatives to avobenzone and octrocrylene [234].
Chlorella is a genus of single-cell green algae with photoautotroph, heterotroph and mixotroph metabolism. Its industrial application started in 1960 as a food additive. Nowadays, there are more than 70 industrial producers of the microalgae with a total supply of 2000 tons/year [235]. In cosmetics, the species C. vulgaris, C. fusca, C. minutissima, C. sorokiniana and C. pyrenoidosa have been investigated for the production of sporopollenin and mycoscoporine-like amino acids, which can protect the skin from UV radiation [236]. Dermachlorella (Chlorella vulgaris extract) is the name of the cosmetic ingredient positioned as a firming and anti-agent active that also prevents the formation of black circles [237]. Based on SuperPro Designer simulations using data from their bibliography, Caicedo and co-workers calculated that a production volume of 48 to 70 m3 is required for a commercially viable production of biomass and extracellular metabolites of C. vulgaris intended for cosmetic application [238].
12. Conclusions
Many cosmetic ingredients have been scrutinized for their environmental impact and/or consumer risk. The search for new ingredients and ingredient sources is pivotal for the sustainable growth of this sector. Biotechnology, and fermentation as the core technology, will have a predominant role in the composition of the future skin care raw materials portfolio. Bio-sourced compounds often constitute highly biocompatible multifunctional ingredients with a lower ecological footprint than those derived from fossil-based or plant-based materials. Furthermore, biotechnology is a great source for innovation, since the endless combinations of microorganisms and substrates favor the discovery of new molecules with interesting cosmetic attributes. Nowadays, it is already possible to formulate a cosmetic product containing only microbial-derived ingredients (including packaging material). To speed up the transition into biotech-based beauty, challenges in regulations and new method development for testing still need to be addressed (see Figure 9). The final decision about an ingredient should be made based on comprehensive evaluation, e.g., cradle-to-grave LCA, where the supposed fermentation benefits are not standalone indicators of sustainability but rather inputs for the assessment.
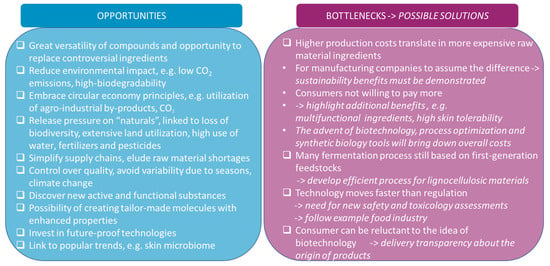
Figure 9.
Summary of opportunities and bottlenecks in the adoption of fermentation-derived ingredients for skin care.
Author Contributions
Both authors have equally contributed to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Beiersdorf AG for the support in the preparation of this manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| BD | Butanediol |
| BC | Bacterial cellulose |
| CAGR | Compound annual growth rate |
| CBD | Cannabidiol |
| CD | Cyclodextrins |
| EFA | Essential fatty acid |
| EPS | Exopolysaccharide |
| FA | Fatty acids |
| FFA | Free fatty acids |
| GRAS | Generally recognized as safe |
| HA | Hyaluronic acid |
| INCI | International nomenclature of cosmetic ingredients |
| LA | Lactic acid |
| LAB | Lactic acid bacteria |
| LCA | Life cycle assessment |
| LTA | Lipoteichoic acid |
| MEL | Mannosylerythritol lipid |
| NMF | Natural moisturizing factor |
| PCA | Pyrrolidone carboxylic acid |
| PDO | Propanediol |
| PEG | Polyethylene glycol |
| PLA | Polylactic acid |
| PUFA | Polyunsaturated fatty acid |
| TEWL | Transepidermal water loss |
| TG | Triglycerides |
References
- Doran, P.M. Bioprocess Engineering Principles; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mora-Villalobos, J.-A.; Aguilar, F.; Carballo-Arce, A.-F.; Vega-Baudrit, J.-R.; Trimino-Vazquez, H.; Villegas-Peñaranda, L.R.; Stöbener, A.; Eixenberger, D.; Bubenheim, P.; Sandoval-Barrantes, M.; et al. Tropical agroindustrial biowaste revalorization through integrative biorefineries—Review part I: Coffee and palm oil by-products. Biomass Convers. Biorefinery 2023, 13, 1469–1487. [Google Scholar] [CrossRef]
- Cosmetics Market Size, Share & Trends Analysis Report by Product (Skin Care, Hair Care, Makeup, Fragrance), by End-User (Men, Women), by Distribution Channel, by Region, and Segment Forecasts, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/cosmetics-market#:~:text=Report (accessed on 15 December 2022).
- Chan, C.-F.; Huang, C.-C.; Lee, M.-Y.; Lin, Y.-S. Fermented Broth in Tyrosinase- and Melanogenesis Inhibition. Molecules 2014, 19, 13122–13135. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.L.; Jang, H.-J.; Kim, K.-B. Anti-wrinkle effect of fermented black ginseng on human fibroblasts. Int. J. Mol. Med. 2017, 39, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Chuong, C.M.; Nickoloff, B.J.; Elias, P.M.; Goldsmith, L.A.; Macher, E.; Maderson, P.A.; Sundberg, J.P.; Tagami, H.; Plonka, P.M.; Thestrup-Pederson, K.; et al. What is the “true” function of skin? Exp. Dermatol. 2002, 11, 159–187. [Google Scholar] [CrossRef]
- Gagliardi, L.; Dorato, S. General Concepts. Current Legislation on Cosmetics in Different Countries. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–28. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Soetaert, W. Personal Care Products via Fermentation and Biocatalysis Processes. In Biotechnology in Personal Care, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 27–56. [Google Scholar]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q 10-containing formulas improves skin’s Q10 level and provides antioxidative effects. BioFactors 2015, 41, 383–390. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Techno-Economic Analysis of a Hyaluronic Acid Production Process Utilizing Streptococcal Fermentation. Processes 2021, 9, 241. [Google Scholar] [CrossRef]
- Sajna, K.V.; Gottumukkala, L.D.; Sukumaran, R.K.; Pandey, A. White Biotechnology in Cosmetics. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 607–652. [Google Scholar] [CrossRef]
- Waltz, E. Cosmetics: When biotech is better than nature. Nat. Biotechnol. 2022, 40, 626–628. [Google Scholar] [CrossRef]
- Majchrzak, W.; Motyl, I.; Śmigielski, K. Biological and Cosmetical Importance of Fermented Raw Materials: An Overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Sousa Lobo, J.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Mellou, F.; Varvaresou, A.; Papageorgiou, S. Renewable sources: Applications in personal care formulations. Int. J. Cosmet. Sci. 2019, 41, 517–525. [Google Scholar] [CrossRef]
- Gupta, P.L.; Rajput, M.; Oza, T.; Trivedi, U.; Sanghvi, G. Eminence of Microbial Products in Cosmetic Industry. Nat. Prod. Bioprospecting 2019, 9, 267–278. [Google Scholar] [CrossRef]
- Moon, N.J.; Hammond, E.G.; Glatz, B.A. Conversion of Cheese Whey and Whey Permeate to Oil and Single-Cell Protein. J. Dairy Sci. 1978, 61, 1537–1547. [Google Scholar] [CrossRef]
- Sakai, K.; Taniguchi, M.; Miura, S.; Ohara, H.; Matsumato, T.; Shirai, Y. Making Plastics from Garbage A Novel Process for Poly- L -Lactate. J. Ind. Ecol. 2004, 7, 63–74. [Google Scholar] [CrossRef]
- Qin, Z.; Guo, W.; Liu, J.; Zhao, G.; Liu, M.; Song, X. Reduced-Cost Production of Sophorolipids by Starmerella bombicola CGMCC1576 Grown on Cottonseed Molasses and Cottonseed Oil-Based Medium. Int. J. Mol. Sci. 2023, 24, 5759. [Google Scholar] [CrossRef]
- Kamer, D.D.A.; Gumus, T.; Palabiyik, I.; Demirci, A.S.; Oksuz, O. The fermentation-based production of gellan from rice bran and the evaluation of various qualitative properties of gum. Int. J. Biol. Macromol. 2022, 207, 841–849. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, D.; Shang, L.; Ma, X.; Luo, Y.; Xue, W.; Gao, P. Optimization of Fermentation Process for Human-like Collagen Production of Recombinant Escherichia coli Using Response Surface Methodology. Chin. J. Chem. Eng. 2010, 18, 137–142. [Google Scholar] [CrossRef]
- Wolf, L.; Cummings, T.; Müller, K.; Reppke, M.; Volkmar, M.; Weuster-Botz, D. Production of β-carotene with Dunaliella salina CCAP19/18 at physically simulated outdoor conditions. Eng. Life Sci. 2021, 21, 115–125. [Google Scholar] [CrossRef]
- Yamada, M.; Okada, Y.; Yoshida, T.; Nagasawa, T. Vanillin production using Escherichia coli cells over-expressing isoeugenol monooxygenase of Pseudomonas putida. Biotechnol. Lett. 2008, 30, 665–670. [Google Scholar] [CrossRef]
- Alander, J.T. Chemical and Physical Properties of Emollients. In Treatment of Dry Skin Syndrome; Springer: Berlin/Heidelberg, Germany, 2012; pp. 399–417. [Google Scholar] [CrossRef]
- Chao, C.; Génot, C.; Rodriguez, C.; Magniez, H.; Lacourt, S.; Fievez, A.; Len, C.; Pezron, I.; Luart, D.; van Hecke, E. Emollients for cosmetic formulations: Towards relationships between physico-chemical properties and sensory perceptions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 156–164. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Forests and Deforestation. OurWorldInData.org. 2021. Available online: https://ourworldindata.org/forests-and-deforestation (accessed on 4 January 2023).
- Vijay, V.; Pimm, S.L.; Jenkins, C.N.; Smith, S.J. The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss. PLoS ONE 2016, 11, e0159668. [Google Scholar] [CrossRef] [PubMed]
- Schörken, U.; Kempers, P. Lipid biotechnology: Industrially relevant production processes. Eur. J. Lipid Sci. Technol. 2009, 111, 627–645. [Google Scholar] [CrossRef]
- Wynn, J.P.; Ratledge, C. Microbial oils: Production, processing and markets for specialty long-chain omega-3 polyunsaturated fatty acids. In Long-Chain Omega-3 Specialty Oils; Elsevier: Amsterdam, The Netherlands, 2012; pp. 43–76. [Google Scholar] [CrossRef]
- Rajak, R.C.; Rajlakshmi; Saravanabhupathy, S.; Banerjee, R. Microbial lipids production by oleaginous yeasts. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–189. [Google Scholar] [CrossRef]
- Castro, A.R.; Cavaleiro, A.J.; Pereira, M.A. Oil and Hydrocarbon-Producing Bacteria. In Biogenesis of Hydrocarbons; Springer International Publishing: Cham, Switzerland, 2019; pp. 471–487. [Google Scholar] [CrossRef]
- Delcourt, M.; Rocle, R. Hydrocarbons produced by industrial biology: Global Bioenergies’ background and prospects. Real. Ind. 2017, 48, 44–47. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:48079411 (accessed on 5 April 2023).
- Zhou, Y.J.; Kerkhoven, E.J.; Nielsen, J. Barriers and opportunities in bio-based production of hydrocarbons. Nat. Energy 2018, 3, 925–935. [Google Scholar] [CrossRef]
- Patel, A.; Bettiga, M.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microbial genetic engineering approach to replace shark livering for squalene. Trends Biotechnol. 2022, 40, 1261–1273. [Google Scholar] [CrossRef]
- Xu, W.; Yao, J.; Liu, L.; Ma, X.; Li, W.; Sun, X.; Wang, Y. Improving squalene production by enhancing the NADPH/NADP+ ratio, modifying the isoprenoid-feeding module and blocking the menaquinone pathway in Escherichia coli. Biotechnol. Biofuels 2019, 12, 68. [Google Scholar] [CrossRef]
- Xu, W.; Chai, C.; Shao, L.; Yao, J.; Wang, Y. Metabolic engineering of Rhodopseudomonas palustris for squalene production. J. Ind. Microbiol. Biotechnol. 2016, 43, 719–725. [Google Scholar] [CrossRef]
- McPhee, D.; Pin, A.; Kizer, L.; Perelman, L. Deriving Renewable Squalane from Sugarcane. 2014. Available online: https://www.cosmeticsandtoiletries.com/research/methods-tools/article/21835220/deriving-renewable-squalane-from-sugarcane (accessed on 5 April 2023).
- Paramasivan, K.; Mutturi, S. Recent advances in the microbial production of squalene. World J. Microbiol. Biotechnol. 2022, 38, 91. [Google Scholar] [CrossRef]
- Apprinova. The Best-Performing Natural Silicone Replacement. 2023. Available online: https://aprinnova.com/neossance-hemisqualane/ (accessed on 5 April 2023).
- Shah, A.M.; Yang, W.; Mohamed, H.; Zhang, Y.; Song, Y. Microbes: A Hidden Treasure of Polyunsaturated Fatty Acids. Front. Nutr. 2022, 9, 827837. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Cho, I.J.; Choi, K.R.; Lee, S.Y. Microbial production of fatty acids and derivative chemicals. Curr. Opin. Biotechnol. 2020, 65, 129–141. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Wang, L.; Chauliac, D.; Moritz, B.E.; Zhang, G.; Ingram, L.O.; Shanmugam, K.T. Metabolic engineering of Escherichia coli for the production of butyric acid at high titer and productivity. Biotechnol. Biofuels 2019, 12, 62. [Google Scholar] [CrossRef]
- Stamatopoulou, P.; Malkowski, J.; Conrado, L.; Brown, K.; Scarborough, M. Fermentation of Organic Residues to Beneficial Chemicals: A Review of Medium-Chain Fatty Acid Production. Processes 2020, 8, 1571. [Google Scholar] [CrossRef]
- Eungrasamee, K.; Lindblad, P.; Jantaro, S. Enhanced productivity of extracellular free fatty acids by gene disruptions of acyl-ACP synthetase and S-layer protein in Synechocystis sp. PCC 6803. Biotechnol. Biofuels Bioprod. 2022, 15, 99. [Google Scholar] [CrossRef]
- Akoh, C.C. Handbook of Functional Lipids; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Trimbur, D.E.; Im, C.-S.; Dillon, H.F.; Day, A.G.; Franklin, S.; Coragliotti, A. Production of Oil in Microorganisms; WO2008151149A2; World Intellectual Property Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Ong, S. This Forgotten Technology Could Solve the World’s Palm Oil Problem. National Geographic. 2023. Available online: https://www.nationalgeographic.com/environment/article/forgotten-technology-could-solve-world-palm-oil-problem-microbe (accessed on 5 April 2023).
- NOPalm Ingredients. NoPalm Ingredients Collaboration with Colgate-Palmolive. 2023. Available online: https://www.nopalm-ingredients.com/news/nopalm-ingredients-collaboration-with-colgate-palmolive (accessed on 6 March 2023).
- Long, M. Food Matters Live, Doehler Invests in Clean Food Goup to Accelerate the Market Approval of Its Fermented Palm Oil Altern. 2022. Available online: https://foodmatterslive.com/article/doehler-clean-food-group-fermented-palm-oil-alternative/ (accessed on 26 February 2023).
- Genomatica Inc. Geno Unilever Launch $120m Venture to Scale Alternative to Palm Oil Fossil Fuels. Geno. 2022. Available online: https://www.genomatica.com/news-content/genomatica-and-unilever-launch-venture/ (accessed on 27 February 2023).
- Zhang, J.-L.; Cao, Y.-X.; Peng, Y.-Z.; Jin, C.-C.; Bai, Q.-Y.; Zhang, R.-S.; Liu, D.; Yuan, Y.-J. High production of fatty alcohols in Yarrowia lipolytica by coordination with glycolysis. Sci. China Chem. 2019, 62, 1007–1016. [Google Scholar] [CrossRef]
- Guo, W.; Sheng, J.; Zhao, H.; Feng, X. Metabolic engineering of Saccharomyces cerevisiae to produce 1-hexadecanol from xylose. Microb. Cell Factories 2016, 15, 24. [Google Scholar] [CrossRef]
- Rhyme Biotechnology. Available online: https://www.rhymebiotechnology.com/technology/ (accessed on 13 April 2023).
- Thum, O.; Oxenboll, K.M. Biocatalysis—A Sustainable Method for the Production of Emollient Esters. SÖFW J. 2008, 134, 44–47. [Google Scholar]
- McIntosh, K.; Smith, A.; Young, L.; Leitch, M.; Tiwari, A.; Reddy, C.; O’Neil, G.; Liberatore, M.; Chandler, M.; Baki, G. Alkenones as a Promising Green Alternative for Waxes in Cosmetics and Personal Care Products. Cosmetics 2018, 5, 34. [Google Scholar] [CrossRef]
- Kassab, E.; Fuchs, M.; Haack, M.; Mehlmer, N.; Brueck, T.B. Engineering Escherichia coli FAB system using synthetic plant genes for the production of long chain fatty acids. Microb. Cell Factories 2019, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Mallouchos, A.; Efthymiou, M.-N.; Gardeli, C.; Kopsahelis, N.; Aguieiras, E.C.G.; Freire, D.M.G.; Papanikolaou, S.; Koutinas, A.A. Production of wax esters via microbial oil synthesis from food industry waste and by-product streams. Bioresour. Technol. 2017, 245, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Aguieiras, E.C.G.; Papadaki, A.; Mallouchos, A.; Mandala, I.; Sousa, H.; Freire, D.M.G.; Koutinas, A.A. Enzymatic synthesis of bio-based wax esters from palm and soybean fatty acids using crude lipases produced on agricultural residues. Ind. Crop. Prod. 2019, 139, 111499. [Google Scholar] [CrossRef]
- Ciardiello, T.; Pinto, D.; Marotta, L.; Giuliani, G.; Rinaldi, F. Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota. Cosmetics 2020, 7, 34. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- Flynn, T.C.; Petros, J.; Clark, R.E.; Viehman, G.E. Dry skin and moisturizers. Clin. Dermatol. 2001, 19, 387–392. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ann, J.; Othman, N.; Khan, J.; Alolayan, S.O.; Al thagfan, S.S.; Kaleemullah, M. A Review of Moisturizers; History, Preparation, Characterization and Applications. Cosmetics 2022, 9, 61. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Zhuge, J.; Fang, H.-Y.; Wang, Z.-X.; Chen, D.-Z.; Jin, H.-R.; Gu, H.-L. Glycerol production by a novel osmotolerant yeast Candida glycerinogenes. Appl. Microbiol. Biotechnol. 2001, 55, 686–692. [Google Scholar] [CrossRef]
- ŠMejkalová, D.; Huerta Angeles, G.; Ehlova, T. Hyaluronan (Hyaluronic Acid): A natural moisturizer for skincare. In Harry’s, 9th ed.; Chen, C.W., Ed.; Chemical Publishing Company: Palm Springs, CA, USA, 2015; pp. 605–622. [Google Scholar]
- Ciriminna, R.; Scurria, A.; Pagliaro, M. Microbial production of hyaluronic acid: The case of an emergent technology in the bioeconomy. Biofuels Bioprod. Biorefining 2021, 15, 1604–1610. [Google Scholar] [CrossRef]
- Mohan, N.; Balakrishnan, R.; Sivaprakasam, S. Optimization and effect of dairy industrial waste as media components in the production of hyaluronic acid by Streptococcus thermophilus. Prep. Biochem. Biotechnol. 2016, 46, 628–638. [Google Scholar] [CrossRef]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J. Drugs Dermatol. 2011, 10, 990–1000. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22052267 (accessed on 17 January 2023).
- Valcarcel, J.; García, M.R.; Varela, U.R.; Vázquez, J.A. Hyaluronic acid of tailored molecular weight by enzymatic and acid depolymerization. Int. J. Biol. Macromol. 2020, 145, 788–794. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Factories 2011, 10, 99. [Google Scholar] [CrossRef]
- Alexandri, M.; Neu, A.-K.; Schneider, R.; López-Gómez, J.P.; Venus, J. Evaluation of various Bacillus coagulans isolates for the production of high purity L-lactic acid using defatted rice bran hydrolysates. Int. J. Food Sci. Technol. 2018, 54, 1321–1329. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Latorre-Sánchez, M.; Unger, P.; Schneider, R.; Coll Lozano, C.; Venus, J. Assessing the organic fraction of municipal solid wastes for the production of lactic acid. Biochem. Eng. J. 2019, 150, 107251. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Unger, P.; Schneider, R.; Pierrard, M.; Venus, J. Upgrading pasta wastes through lactic acid fermentations. Food Bioprod. Process. 2022, 135, 135–142. [Google Scholar] [CrossRef]
- Marzo-Gago, C.; Venus, J.; López-Gómez, J.P. Production of lactic acid from pasta wastes using a biorefinery approach. Biotechnol. Biofuels Bioprod. 2022, 15, 128. [Google Scholar] [CrossRef]
- Pacheco, R.; Huston, K. Life cycle assesment (LCA) of naturally-sourced and petroleum-based glycols commonly used in personal care products. SOFW J. 2018, 144, 12–15. [Google Scholar]
- Ju, J.-H.; Wang, D.; Heo, S.-Y.; Kim, M.-S.; Seo, J.-W.; Kim, Y.-M.; Kim, D.-H.; Kang, S.-A.; Kim, C.-H.; Oh, B.-R. Enhancement of 1,3-propanediol production from industrial by-product by Lactobacillus reuteri CH53. Microb. Cell Factories 2020, 19, 6. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Gao, H.; Wang, H.; Wan, Z.; Jiang, Y.; Xin, F.; Zhang, W.; Jiang, M. Current advances in microbial production of 1,3-propanediol. Biofuels Bioprod. Biorefining 2021, 15, 1566–1583. [Google Scholar] [CrossRef]
- TILAMAR® PDO with NØØVISTATM. Available online: https://www.dsm.com/personal-care/en_US/products/multifunctional-ingredients/tilamar-pdo-with-noovista.html (accessed on 14 April 2023).
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef]
- Seshadrinathan, S.; Chakraborty, S. Fermentative Production of Erythritol from Cane Molasses Using Candida magnoliae: Media Optimization, Purification, and Characterization. Sustainability 2022, 14, 10342. [Google Scholar] [CrossRef]
- Holladay, J.E.; Bozell, J.J.; White, J.F. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas, II; U.S. Department of Energy: Washington, DC, USA, 2004.
- Galán, G.; Martín, M.; Grossmann, I.E. Integrated Renewable Production of Sorbitol and Xylitol from Switchgrass. Ind. Eng. Chem. Res. 2021, 60, 5558–5573. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Agurto, A.; Bravo, M.; Magán-Fernandez, A.; López-Toruño, A.; Muñoz, R.; Ferrer, J.; Mesa, F. Randomized clinical trial on the clinical effects of a toothpaste containing extra virgin olive oil, xylitol, and betaine in gingivitis. Sci. Rep. 2023, 13, 6294. [Google Scholar] [CrossRef]
- Okajima, M.K.; Miyazato, S.; Kaneko, T. Cyanobacterial Megamolecule Sacran Efficiently Forms LC Gels with Very Heavy Metal Ions. Langmuir 2009, 25, 8526–8531. [Google Scholar] [CrossRef]
- Puluhulawa, L.E.; Joni, I.M.; Mohammed, A.F.A.; Arima, H.; Wathoni, N. The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications. Molecules 2021, 26, 3362. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int. J. Biol. Macromol. 2016, 89, 465–470. [Google Scholar] [CrossRef]
- Ngatu, N.R.; Okajima, M.K.; Yokogawa, M.; Hirota, R.; Eitoku, M.; Muzembo, B.A.; Dumavibhat, N.; Takaishi, M.; Sano, S.; Kaneko, T.; et al. Anti-inflammatory effects of sacran, a novel polysaccharide from Aphanothece sacrum, on 2,4,6-trinitrochlorobenzene–induced allergic dermatitis in vivo. Ann. Allergy Asthma Immunol. 2012, 108, 117–122.e2. [Google Scholar] [CrossRef]
- Fukushima, S.; Motoyama, K.; Tanida, Y.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Tanaka, T.; Ihn, H.; Arima, H. Clinical Evaluation of Novel Natural Polysaccharides Sacran as a Skincare Material for Atopic Dermatitis Patients. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 9–18. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Leathers, T.D.; Biresaw, G.; Nunnally, M.S.; Bischoff, K.M. Simplified process for preparation of schizophyllan solutions for biomaterial applications. Prep. Biochem. Biotechnol. 2016, 46, 313–319. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom Cosmetics: The Present and Future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Leathers, T.D.; Nunnally, M.S.; Price, N.P.J.; Biresaw, G. Utilization of agricultural biomass in the production of the biopolymer schizophyllan. J. Ind. Microbiol. Biotechnol. 2013, 40, 105–112. [Google Scholar] [CrossRef]
- Leathers, T.D.; Nunnally, M.S.; Stanley, A.M.; Rich, J.O. Utilization of corn fiber for production of schizophyllan. Biomass Bioenergy 2016, 95, 132–136. [Google Scholar] [CrossRef]
- Jamshidian, H.; Shojaosadati, S.A.; Vilaplana, F.; Mousavi, S.M.; Soudi, M.R. Characterization and optimization of schizophyllan production from date syrup. Int. J. Biol. Macromol. 2016, 92, 484–493. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Leathers, T.D.; Price, N.P. Production of schizophyllan from distiller’s dried grains with solubles by diverse strains of Schizophyllum commune. Springerplus 2013, 2, 476. [Google Scholar] [CrossRef]
- Arunrattanamook, N.; Sornlake, W.; Champreda, V. Co-production of schizophyllan and cellulolytic enzymes from bagasse by Schizophyllum commune. Biosci. Biotechnol. Biochem. 2022, 86, 1144–1150. [Google Scholar] [CrossRef]
- Liyana Ismail, N.; Shahruddin, S.; Othman, J. Perspective Chapter: Overview of Bio-Based Surfactant—Recent Development, Industrial Challenge, and Future Outlook. In Surfactants and Detergents—Updates and New Insights; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Moldes, A.B.; Rodríguez-López, L.; Rincón-Fontán, M.; López-Prieto, A.; Vecino, X.; Cruz, J.M. Synthetic and Bio-Derived Surfactants Versus Microbial Biosurfactants in the Cosmetic Industry: An Overview. Int. J. Mol. Sci. 2021, 22, 2371. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Ashtiani, H.-R.; Baldisserotto, A.; Cesa, E.; Manfredini, S.; Sedghi Zadeh, H.; Ghafori Gorab, M.; Khanahmadi, M.; Zakizadeh, S.; Buso, P.; Vertuani, S. Microbial Biosurfactants as Key Multifunctional Ingredients for Sustainable Cosmetics. Cosmetics 2020, 7, 46. [Google Scholar] [CrossRef]
- Biosurfactants Market, By Product Type (Glycolipids, Lipopeptides & Lipoproteins, Surfactin, Others), By Application (Personal care, Food processing, Others), By Region Forecast to 2030. Biosurfactants Market. 2022. Available online: https://www.emergenresearch.com/industry-report/biosurfactants-market (accessed on 14 April 2023).
- Roelants, S.; Solaiman, D.K.Y.; Ashby, R.D.; Lodens, S.; Van Renterghem, L.; Soetaert, W. Production and Applications of Sophorolipids. In Biobased Surfactants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–119. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants: Minireview. Environ. Microbiol. 2001, 3, 229–236. [Google Scholar] [CrossRef]
- Zibek, S.; Soberón-Chávez, G. Overview on Glycosylated Lipids Produced by Bacteria and Fungi: Rhamno-, Sophoro-, Mannosylerythritol and Cellobiose Lipids. In Biosurfactants for the Biobased Economy; Springer: Berlin/Heidelberg, Germany, 2022; pp. 73–122. [Google Scholar] [CrossRef]
- Pal, S.; Chatterjee, N.; Das, A.K.; McClements, D.J.; Dhar, P. Sophorolipids: A comprehensive review on properties and applications. Adv. Colloid Interface Sci. 2023, 313, 102856. [Google Scholar] [CrossRef]
- Cho, W.Y.; Ng, J.F.; Yap, W.H.; Goh, B.H. Sophorolipids—Bio-Based Antimicrobial Formulating Agents for Applications in Food and Health. Molecules 2022, 27, 5556. [Google Scholar] [CrossRef]
- Daniel, H.J.; Reuss, M.; Syldatk, C. Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in a two stage fed batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotechnol. Lett. 1998, 20, 1153–1156. [Google Scholar] [CrossRef]
- De Graeve, M.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W. Starmerella bombicola, an industrially relevant, yet fundamentally underexplored yeast. FEMS Yeast Res. 2018, 18, foy072. [Google Scholar] [CrossRef]
- Celligoi, M.A.P.C.; Silveira, V.A.I.; Hipólito, A.; Caretta, T.O.; Baldo, C. Sophorolipids: A review on production and perspectives of application in agriculture. Span. J. Agric. Res. 2020, 18, e03R01. [Google Scholar] [CrossRef]
- Qazi, M.A.; Wang, Q.; Dai, Z. Sophorolipids bioproduction in the yeast Starmerella bombicola: Current trends and perspectives. Bioresour. Technol. 2022, 346, 126593. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Piljac, T.; Piljac, G. Use of Rhamnolipids in Wound Healing, Treating Burn Shock, Atherosclerosis, Organ Transplants, Depression, Schizophrenia and Cosmetics. U.S. Patent 7262171B1, 24 August 2020. [Google Scholar]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.; Kamalanathan, I.D.; Martin, P.J. Comparative study of the production of rhamnolipid biosurfactants by B. thailandensis E264 and P. aeruginosa ATCC 9027 using foam fractionation. Process Biochem. 2016, 51, 820–827. [Google Scholar] [CrossRef]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef]
- Hošková, M.; Schreiberová, O.; Ježdík, R.; Chudoba, J.; Masák, J.; Sigler, K.; Řezanka, T. Characterization of rhamnolipids produced by non-pathogenic Acinetobacter and Enterobacter bacteria. Bioresour. Technol. 2013, 130, 510–516. [Google Scholar] [CrossRef]
- Tiso, T.; Thies, S.; Müller, M.; Tsvetanova, L.; Carraresi, L.; Bröring, S.; Jaeger, K.-E.; Blank, L.M. Rhamnolipids: Production, performance, and application. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2017; pp. 587–622. [Google Scholar]
- Saika, A.; Koike, H.; Yamamoto, S.; Sugahara, T.; Kawahara, A.; Sogabe, A.; Morita, T. Improvement of Oil Degradation and MEL Production in a Yeast Strain, Pseudozyma tsukubaensis, by Translation Elongation Factor 1 Promoter-driven Expression of a Lipase. J. Oleo Sci. 2022, 71, ess22089. [Google Scholar] [CrossRef]
- Kim, M.K.; Jeong, E.S.; Kim, K.N.; Park, S.H.; Kim, J.W. Nanoemulsification of pseudo-ceramide by molecular association with mannosylerythritol lipid. Colloids Surf. B Biointerfaces 2014, 116, 597–602. [Google Scholar] [CrossRef]
- Takahashi, M.; Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Glycolipid Biosurfactants, Mannosylerythritol Lipids, Show Antioxidant and Protective Effects against H2O2-Induced Oxidative Stress in Cultured Human Skin Fibroblasts. J. Oleo Sci. 2012, 61, 457–464. [Google Scholar] [CrossRef]
- Janek, T.; Gudiña, E.J.; Połomska, X.; Biniarz, P.; Jama, D.; Rodrigues, L.R.; Rymowicz, W.; Lazar, Z. Sustainable Surfactin Production by Bacillus subtilis Using Crude Glycerol from Different Wastes. Molecules 2021, 26, 3488. [Google Scholar] [CrossRef]
- Zosim, Z.; Gutnick, D.; Rosenberg, E. Properties of hydrocarbon-in-water emulsions stabilized by Acinetobacter RAG-1 emulsan. Biotechnol. Bioeng. 1982, 24, 281–292. [Google Scholar] [CrossRef]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Imura, T.; Morita, T.; Fukuoka, T.; Ryu, M.; Igarashi, K.; Hirata, Y.; Kitamoto, D. Spontaneous Vesicle Formation from Sodium Salt of Acidic Sophorolipid and Its Application as a Skin Penetration Enhancer. J. Oleo Sci. 2014, 63, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Baccile, N.; Seyrig, C.; Poirier, A.; Alonso-de Castro, S.; Roelants, S.L.K.W.; Abel, S. Self-assembly, interfacial properties, interactions with macromolecules and molecular modelling and simulation of microbial bio-based amphiphiles (biosurfactants). A tutorial review. Green Chem. 2021, 23, 3842–3944. [Google Scholar] [CrossRef]
- Briem, A.-K.; Bippus, L.; Oraby, A.; Noll, P.; Zibek, S.; Albrecht, S. Environmental Impacts of Biosurfactants from a Life Cycle Perspective: A Systematic Literature Review. In Biosurfactants for the Biobased Economy; Springer: Berlin/Heidelberg, Germany, 2022; pp. 235–269. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Loh, X.J. Natural rheological modifiers for personal care. Polym. Adv. Technol. 2016, 27, 1664–1679. [Google Scholar] [CrossRef]
- Sakamoto, K.; Lochhead, R.Y.; Maibach, H.I.; Yamashita, Y. (Eds.) Cosmetic Science and Technology: Theoretical Principles and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Kambourova, M.; Oner, E.T.; Poli, A. Exopolysaccharides from Prokaryotic Microorganisms—Promising Sources for White Biotechnology Processes. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 523–554. [Google Scholar] [CrossRef]
- Jindal, N.; Singh Khattar, J. Microbial Polysaccharides in Food Industry. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–123. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Katzbauer, B. Properties and applications of xanthan gum. Polym. Degrad. Stab. 1998, 59, 81–84. [Google Scholar] [CrossRef]
- Mesomo, M.; Silva, M.F.; Boni, G.; Padilha, F.F.; Mazutti, M.; Mossi, A.; de Oliveira, D.; Cansian, R.L.; Di Luccio, M.; Treichel, H. Xanthan gum produced by Xanthomonas campestris from cheese whey: Production optimisation and rheological characterisation. J. Sci. Food Agric. 2009, 89, 2440–2445. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, Y.; Hu, Y.; Jung, S. Bacterial Succinoglycans: Structure, Physical Properties, and Applications. Polymers 2022, 14, 276. [Google Scholar] [CrossRef]
- Kırtel, O.; Avşar, G.; Erkorkmaz, B.A.; Öner, E.T. Microbial Polysaccharides as Food Ingredients. In Microbial Production of Food Ingredients and Additives; Elsevier: Amsterdam, The Netherlands, 2017; pp. 347–383. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- Oliveira, T.J.; Segato, T.C.M.; Machado, G.P.; Grotto, D.; Jozala, A.F. Evolution of Bacterial Cellulose in Cosmetic Applications: An Updated Systematic Review. Molecules 2022, 27, 8341. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized culture conditions for bacterial cellulose production by Acetobacter senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef]
- Bianchet, R.T.; Vieira Cubas, A.L.; Machado, M.M.; Siegel Moecke, E.H. Applicability of bacterial cellulose in cosmetics—Bibliometric review. Biotechnol. Rep. 2020, 27, e00502. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, X.; Li, Y.; Wang, L. Bacterial alginate metabolism: An important pathway for bioconversion of brown algae. Biotechnol. Biofuels 2021, 14, 158. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Ibrahim, G.G.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. Resveratrol Production in Yeast Hosts: Current Status and Perspectives. Biomolecules 2021, 11, 830. [Google Scholar] [CrossRef]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef]
- Thapa, S.B.; Pandey, R.P.; Park, Y.I.; Sohng, J.K. Biotechnological Advances in Resveratrol Production and its Chemical Diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [PubMed]
- Abo-Kadoum, M.A.; Abouelela, M.E.; Al Mousa, A.A.; Abo-Dahab, N.F.; Mosa, M.A.; Helmy, Y.A.; Hassane, A.M.A. Resveratrol biosynthesis, optimization, induction, bio-transformation and bio-degradation in mycoendophytes. Front. Microbiol. 2022, 13, 1010332. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.-X.; Gong, J.-S.; Li, H.; Shi, W.-T.; Jiang, M.; Xu, Z.-H.; Shi, J.-S. Heterologous expression, fermentation strategies and molecular modification of collagen for versatile applications. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef]
- Evonik Achieves Major Biotech Breakthrough with a New Animal-Free and Fermentation-Based Collagen Platform. 2020. Available online: https://healthcare.evonik.com/en/evonik-achieves-major-biotech-breakthrough-with-a-new-animal-free-and-fermentation-based-collagen-pl-125478.html (accessed on 16 April 2023).
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Acquisition of Solus Biotech Expands Fast Growth Beauty Actives Business in Asia. 2023. Available online: https://www.croda.com/en-gb/news/acquisition-of-solus-biotech-expands-fast-growth-beauty-actives-business-in-asia (accessed on 16 April 2023).
- Nafisi, S.; Maibach, H.I. Nanotechnology in Cosmetics. In Cosmetic Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 337–369. [Google Scholar] [CrossRef]
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant stem cells in cosmetics: Current trends and future directions. Futur. Sci. OA 2017, 3, FSO226. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef]
- Barbulova, A.; Apone, F.; Colucci, G. Plant Cell Cultures as Source of Cosmetic Active Ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Jin, Z.; Zhang, D. Advances in metabolic engineering for vitamins production. Sheng Wu Gong Cheng Xue Bao 2021, 37, 1748–1770. [Google Scholar] [CrossRef]
- Qiu, J.; Hou, K.; Li, Q.; Chen, J.; Li, X.; Hou, H.; Wang, L.; Liu, J.; Xue, Q.; Wang, C. Boosting the Cannabidiol Production in Engineered Saccharomyces cerevisiae by Harnessing the Vacuolar Transporter BPT1. J. Agric. Food Chem. 2022, 70, 12055–12064. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Xu, J.-Z.; Zhang, W.-G. Metabolic engineering of l-leucine production in Escherichia coli and Corynebacterium glutamicum: A review. Crit. Rev. Biotechnol. 2019, 39, 633–647. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Abd Rashid, N.Y.; Jamaluddin, A.; Sharifudin, S.A.; Long, K. Enhancement of phenolic acid content and antioxidant activity of rice bran fermented with Rhizopus oligosporus and Monascus purpureus. Biocatal. Agric. Biotechnol. 2015, 4, 33–38. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, M.-R.; Park, Y.; Park, H.J.; Chang, U.J.; Kim, S.Y.; Suh, H.J. Fermenting Red Ginseng Enhances Its Safety and Efficacy as a Novel Skin Care Anti-Aging Ingredient: In Vitro and Animal Study. J. Med. Food 2012, 15, 1015–1023. [Google Scholar] [CrossRef]
- Park, J.J.; An, J.; Lee, J.D.; Kim, H.Y.; Im, J.E.; Lee, E.; Ha, J.; Cho, C.H.; Seo, D.-W.; Kim, K.-B. Effects of anti-wrinkle and skin-whitening fermented black ginseng on human subjects and underlying mechanism of action. J. Toxicol. Environ. Health Part A 2020, 83, 470–484. [Google Scholar] [CrossRef]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented Soy Products and Their Potential Health Benefits: A Review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef]
- Kobayashi, K.; Horii, Y.; Watanabe, S.; Kubo, Y.; Koguchi, K.; Hoshi, Y.; Matsumoto, K.; Soda, K. Comparison of soybean cultivars for enhancement of the polyamine contents in the fermented soybean natto using Bacillus subtilis (natto). Biosci. Biotechnol. Biochem. 2017, 81, 587–594. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hanamizu, T.; Sone, T.; Chiba, K.; Kinoshita, T.; Yoshikawa, S. Topical application of Bifidobacterium-fermented soy milk extract containing genistein and daidzein improves rheological and physiological properties of skin. J. Cosmet. Sci. 2004, 55, 473–479. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15608997 (accessed on 23 February 2023). [CrossRef]
- Chae, G.-Y.; Ha, B.-J. The Comparative Evaluation of Fermented and Non-fermented Soybean Extract on Antioxidation and Whitening. Toxicol. Res. 2011, 27, 205–209. [Google Scholar] [CrossRef]
- Izawa, N.; Sone, T. Cosmetic Ingredients Fermented by Lactic Acid Bacteria. In Microbial Production; Springer: Tokyo, Japan, 2014; pp. 233–242. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K.; Muhammed, A.; Jeyaram, K.; Talukdar, N.C. Enhancement of antioxidant properties of two soybean varieties of Sikkim Himalayan region by proteolytic Bacillus subtilis fermentation. J. Funct. Foods 2015, 14, 650–658. [Google Scholar] [CrossRef]
- Lai, J.; Xin, C.; Zhao, Y.; Feng, B.; He, C.; Dong, Y.; Fang, Y.; Wei, S. Study of Active Ingredients in Black Soybean Sprouts and Their Safety in Cosmetic Use. Molecules 2012, 17, 11669–11679. [Google Scholar] [CrossRef] [PubMed]
- Izawa, N.; Kudo, M.; Nakamura, Y.; Mizukoshi, H.; Kitada, T.; Sone, T. Production of aroma compounds from whey using Wickerhamomyces pijperi. AMB Express 2015, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Ro, H.S.; Jang, H.J.; Kim, G.R.; Park, S.J.; Lee, H.Y. Enhancement of the Anti-Skin Wrinkling Effects of Aloe arborescens Miller Extracts Associated with Lactic Acid Fermentation. Evid.-Based Complement. Altern. Med. 2020, 2020, 2743594. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Wójciak, M.; Szczepanek, D.; Sowa, I. Assessment of Cosmetic and Dermatological Properties and Safety of Use of Model Skin Tonics with Kombucha-Fermented Red Berry Extracts. Int. J. Mol. Sci. 2022, 23, 14675. [Google Scholar] [CrossRef] [PubMed]
- Zofia, N.-Ł.; Aleksandra, Z.; Tomasz, B.; Martyna, Z.-D.; Magdalena, Z.; Zofia, H.-B.; Tomasz, W. Effect of Fermentation Time on Antioxidant and Anti-Ageing Properties of Green Coffee Kombucha Ferments. Molecules 2020, 25, 5394. [Google Scholar] [CrossRef]
- Salo, A.M.; Myllyharju, J. Prolyl and lysyl hydroxylases in collagen synthesis. Exp. Dermatol. 2021, 30, 38–49. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Marizcurrena, J.J.; Martínez-López, W.; Ma, H.; Lamparter, T.; Castro-Sowinski, S. A highly efficient and cost-effective recombinant production of a bacterial photolyase from the Antarctic isolate Hymenobacter sp. UV11. Extremophiles 2019, 23, 49–57. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. Microbial production of extremolytes—High-value active ingredients for nutrition, health care, and well-being. Curr. Opin. Biotechnol. 2020, 65, 118–128. [Google Scholar] [CrossRef]
- Eskens, O.; Amin, S. Challenges and effective routes for formulating and delivery of epidermal growth factors in skin care. Int. J. Cosmet. Sci. 2021, 43, 123–130. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Pérez-Rivero, C. Cellular Systems. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; Volume 2, pp. 9–21. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sardana, C.; Ozturk, M.; Sarwat, M. Plant stem cells and their applications: Special emphasis on their marketed products. 3 Biotech 2020, 10, 291. [Google Scholar] [CrossRef]
- Abo-elmaaty, S.; El Dougdoug, N.K.; Hazaa, M.M. Improved antibacterial efficacy of bacteriophage-cosmetic formulation for treatment of Staphylococcus aureus in vitro. Ann. Agric. Sci. 2016, 61, 201–206. [Google Scholar] [CrossRef]
- Golembo, M.; Puttagunta, S.; Rappo, U.; Weinstock, E.; Engelstein, R.; Gahali-Sass, I.; Moses, A.; Kario, E.; Ben-Dor Cohen, E.; Nicenboim, J.; et al. Development of a topical bacteriophage gel targeting Cutibacterium acnes for acne prone skin and results of a phase 1 cosmetic randomized clinical trial. Ski. Health Dis. 2022, 2, e93. [Google Scholar] [CrossRef]
- Mutti, M.; Corsini, L. Robust Approaches for the Production of Active Ingredient and Drug Product for Human Phage Therapy. Front. Microbiol. 2019, 10, 2289. [Google Scholar] [CrossRef]
- Halla, N.; Fernandes, I.; Heleno, S.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.; Ferreira, I.; Barreiro, M. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef]
- Herman, A. Antimicrobial Ingredients as Preservative Booster and Components of Self-Preserving Cosmetic Products. Curr. Microbiol. 2019, 76, 744–754. [Google Scholar] [CrossRef]
- Kočevar Glavač, N.; Lunder, M. Preservative efficacy of selected antimicrobials of natural origin in a cosmetic emulsion. Int. J. Cosmet. Sci. 2018, 40, 276–284. [Google Scholar] [CrossRef]
- Kabara, J.J. (Ed.) Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practices; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Wang, A.; Li, P.; Zhang, X.; Han, P.; Lai, D.; Zhou, L. Two New Anisic Acid Derivatives from Endophytic Fungus Rhizopycnis vagum Nitaf22 and Their Antibacterial Activity. Molecules 2018, 23, 591. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Li, L.; Jiang, X.; Chen, Z.; Zhao, F.; Yi, Y. Biosynthesis and Production of Class II Bacteriocins of Food-Associated Lactic Acid Bacteria. Fermentation 2022, 8, 217. [Google Scholar] [CrossRef]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; López-Gómez, J.P. Multi-Product Lactic Acid Bacteria Fermentations: A Review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef]
- Ibrahim, O.O. Classification of Antimicrobial Peptides Bacteriocins, and the Nature of Some Bacteriocins with Potential Applications in Food Safety and Bio-Pharmaceuticals. EC Microbiol. 2019, 15, 591–608. [Google Scholar]
- Zhang, S.; Luo, L.; Sun, X.; Ma, A. Bioactive Peptides: A Promising Alternative to Chemical Preservatives for Food Preservation. J. Agric. Food Chem. 2021, 69, 12369–12384. [Google Scholar] [CrossRef] [PubMed]
- Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review. Fermentation 2021, 7, 106. [Google Scholar] [CrossRef]
- Roller, S. The biotechnological development of new food preservatives. Biotechnol. Genet. Eng. Rev. 1991, 9, 183–206. [Google Scholar]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Oliveira, A.C.; de Carvalho Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, C.R. Production, Formulation, and Application of Postbiotics in the Treatment of Skin Conditions. Fermentation 2023, 9, 264. [Google Scholar] [CrossRef]
- Otsuka, A.; Moriguchi, C.; Shigematsu, Y.; Tanabe, K.; Haraguchi, N.; Iwashita, S.; Tokudome, Y.; Kitagaki, H. Fermented Cosmetics and Metabolites of Skin Microbiota—A New Approach to Skin Health. Fermentation 2022, 8, 703. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Impact of prebiotics and probiotics on skin health. Benef. Microbes 2014, 5, 99–107. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Lew, L.-C.; Liong, M.-T. Bioactives from probiotics for dermal health: Functions and benefits. J. Appl. Microbiol. 2013, 114, 1241–1253. [Google Scholar] [CrossRef]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market—An update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar] [CrossRef]
- Huang, H.-C.; Lee, I.J.; Huang, C.; Chang, T.-M. Lactic Acid Bacteria and Lactic Acid for Skin Health and Melanogenesis Inhibition. Curr. Pharm. Biotechnol. 2020, 21, 566–577. [Google Scholar] [CrossRef]
- Anthony, I.; Lanzalaco, C.; Charbonneau, D.L.; Wilson, B. Method of Making Cosmetic Compositions Containing a Prebotc. U.S. Patent US 8,815,538 B2, 26 August 2014. [Google Scholar]
- Dixit, Y.; Wagle, A.; Vakil, B. Patents in the Field of Probiotics, Prebiotics, Synbiotics: A Review. J. Food Microbiol. Saf. Hyg. 2016, 1, 1000111. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Agarwal, S.M.; Thodum, P.; Sharma, V.K. SkinBug: An artificial intelligence approach to predict human skin microbiome-mediated metabolism of biotics and xenobiotics. iScience 2021, 24, 101925. [Google Scholar] [CrossRef]
- Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güell, M.; Paetzold, B. Skin microbiome transplantation and manipulation: Current state of the art. Comput. Struct. Biotechnol. J. 2021, 19, 624–631. [Google Scholar] [CrossRef]
- Fereidani, B.M.; Üçtuğ, F.G. Life cycle assessment of rose oil and rose water production: A case study in Iran. Int. J. Environ. Sci. Technol. 2023, 20, 3831–3848. [Google Scholar] [CrossRef]
- Ghasemy-Piranloo, F.; Kavousi, F.; Kazemi-Abharian, M. Comparison for the production of essential oil by conventional, novel and biotechnology methods. J. Essent. Oil Res. 2022, 34, 455–478. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Soetaert, W. Bioflavours and fragrances via fermentation and biocatalysis. J. Chem. Technol. Biotechnol. 2002, 77, 1323–1332. [Google Scholar] [CrossRef]
- Leffingwell, J.C. Biotechnology—Conquests and Challenges in Flavors & Fragrances. Leffingwell Rep. 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Leffingwell, D.L.J.C. Flavours & fragrances: Recent advances in biotechnology. Spec. Chem. Mag. 2015, 32–34. [Google Scholar]
- Converti, A.; Aliakbarian, B.; Domínguez, J.M.; Vázquez, G.B.; Perego, P. Microbial production of biovanillin. Braz. J. Microbiol. 2010, 41, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.; Mahmoud, K.F.; Amin, A.A.; El Banna, H.A. Application of biotechnology to the production of natural flavor and fragrance chemicals. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2670–2717. [Google Scholar] [CrossRef]
- Ferreira, L.; Mascarenhas-Melo, F.; Rabaça, S.; Mathur, A.; Sharma, A.; Giram, P.S.; Pawar, K.D.; Rahdar, A.; Raza, F.; Veiga, F.; et al. Cyclodextrin-based dermatological formulations: Dermopharmaceutical and cosmetic applications. Colloids Surf. B Biointerfaces 2023, 221, 113012. [Google Scholar] [CrossRef]
- Nam, H.C.; Park, W.H. Eco-friendly poly(lactic acid) microbeads for cosmetics via melt electrospraying. Int. J. Biol. Macromol. 2020, 157, 734–742. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; AL-Samydai, A.; Al-Dujaili, E.A.S. Nano, micro particulate and cosmetic delivery systems of polylactic acid: A mini review. J. Cosmet. Dermatol. 2020, 19, 2805–2811. [Google Scholar] [CrossRef]
- Kiki, M.J. Biopigments of Microbial Origin and Their Application in the Cosmetic Industry. Cosmetics 2023, 10, 47. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; de Carvalho, J.C.M.; Santos de Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio)Technological aspects of microalgae pigments for cosmetics. Appl. Microbiol. Biotechnol. 2020, 104, 9513–9522. [Google Scholar] [CrossRef]
- Morone, J.; Alfeus, A.; Vasconcelos, V.; Martins, R. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals—A new bioactive approach. Algal Res. 2019, 41, 101541. [Google Scholar] [CrossRef]
- Gao, X.; Jing, X.; Liu, X.; Lindblad, P. Biotechnological Production of the Sunscreen Pigment Scytonemin in Cyanobacteria: Progress and Strategy. Mar. Drugs 2021, 19, 129. [Google Scholar] [CrossRef]
- Mendes-Silva, T.d.C.D.; Andrade, R.F.d.S.; Ootani, M.A.; Mendes, P.V.D.; de Sá, R.A.d.Q.C.; da Silva, M.R.F.; Souza, K.S.; Correia, M.T.d.S.; da Silva, M.V.; de Oliveira, M.B.M. Biotechnological Potential of Carotenoids Produced by Extremophilic Microorganisms and Application Prospects for the Cosmetics Industry. Adv. Microbiol. 2020, 10, 397–410. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.; Cidade, H.; Lobo, J.; Almeida, I. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef]
- Rani, K.; Sandal, N.; Sahoo, P.K. A comprehensive review on chlorella-its composition, health benefits, market and regulatory scenario. Pharma Innov. J. 2018, 7, 584–589. Available online: www.thepharmajournal.com (accessed on 5 April 2023).
- José de Andrade, C.; Maria de Andrade, L. An overview on the application of genus Chlorella in biotechnological processes. J. Adv. Res. Biotechnol. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- CODIF Technologie Naturelle. Dermochlorella D. Available online: https://www.codif-tn.com/en/principesactifs/dermochlorella-d/ (accessed on 19 April 2023).
- Caicedo, Y.; Suarez, C.; Gelves, G. Evaluation of preliminary plant design for Chlorella vulgaris microalgae production focused on cosmetics purposes. J. Phys. Conf. Ser. 2020, 1655, 012086. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).