Intermediate Accumulation and Process Stability for Facultative and Obligate Anaerobic Treatment of Leachate from Waste Transfer Stations
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Design and Operation
2.2. Seed Sludge and Feeding
2.3. Analytical Methods
2.4. DNA Extraction, PCR Amplification, and High-Throughput 16S rRNA Gene Sequencing
2.5. Date Analysis
3. Results and Discussion
3.1. The Performance of the ABRs
3.2. The Accumulative Concentration and Type of VFA
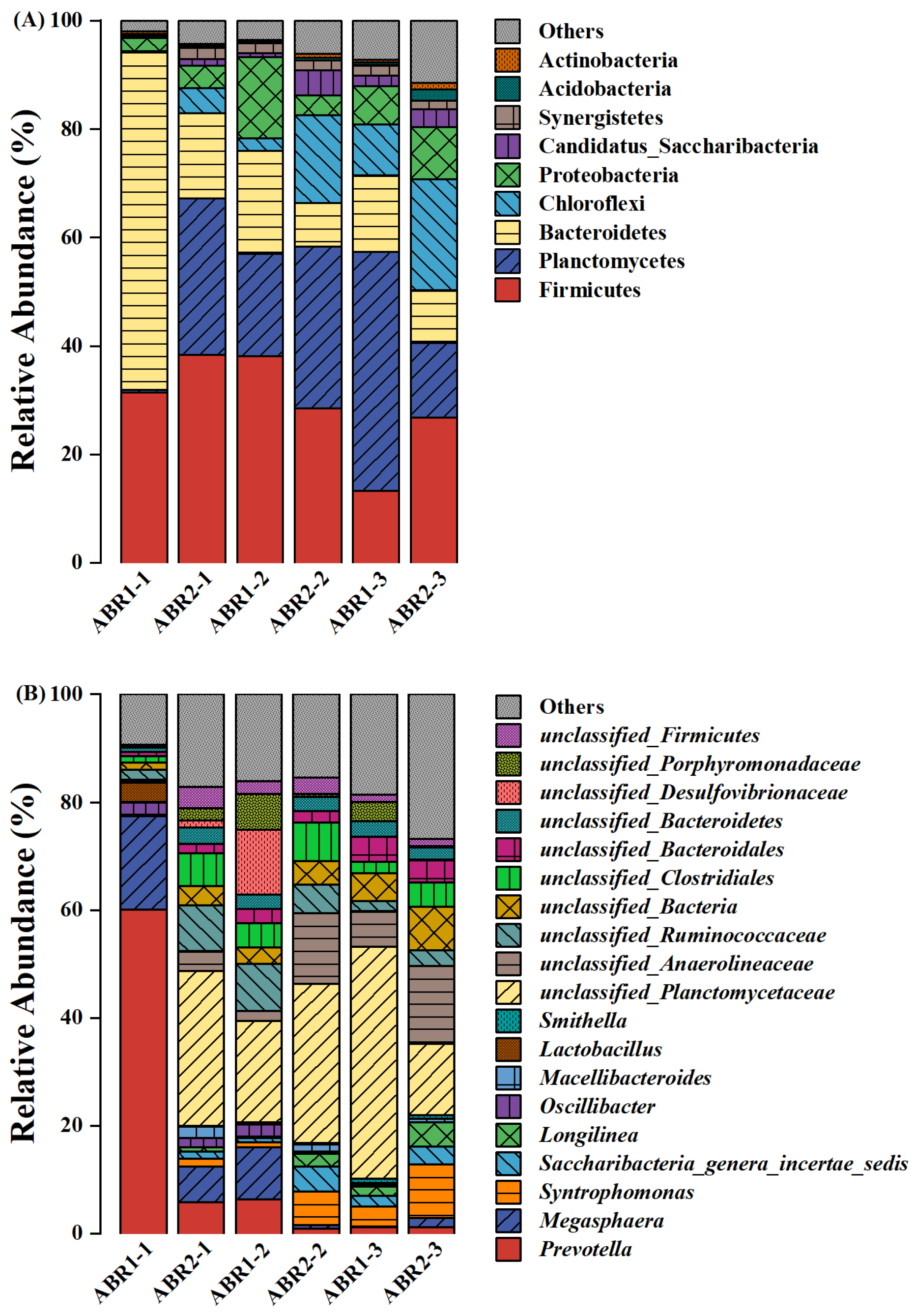
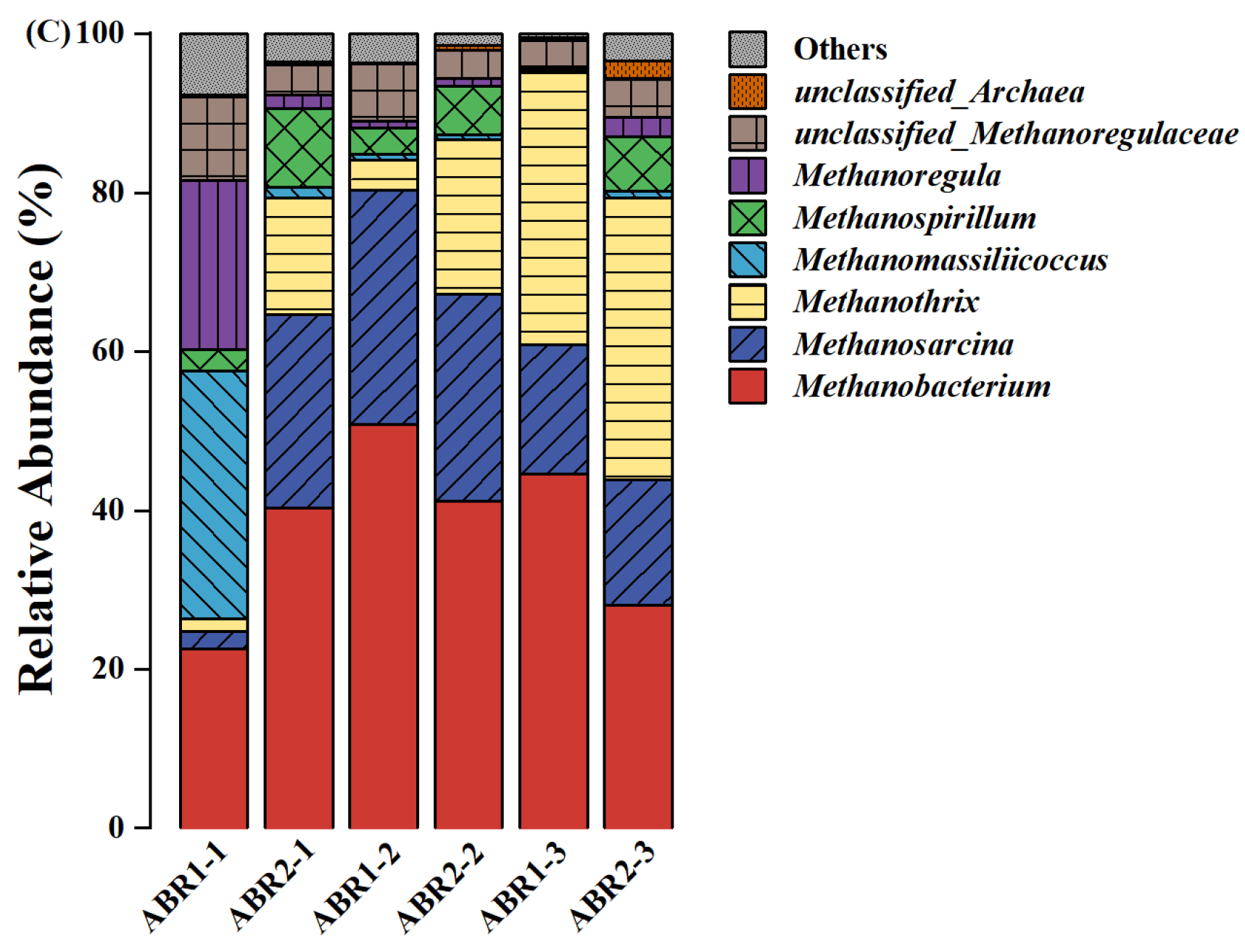
3.3. Microbial Diversity and Community Analysis
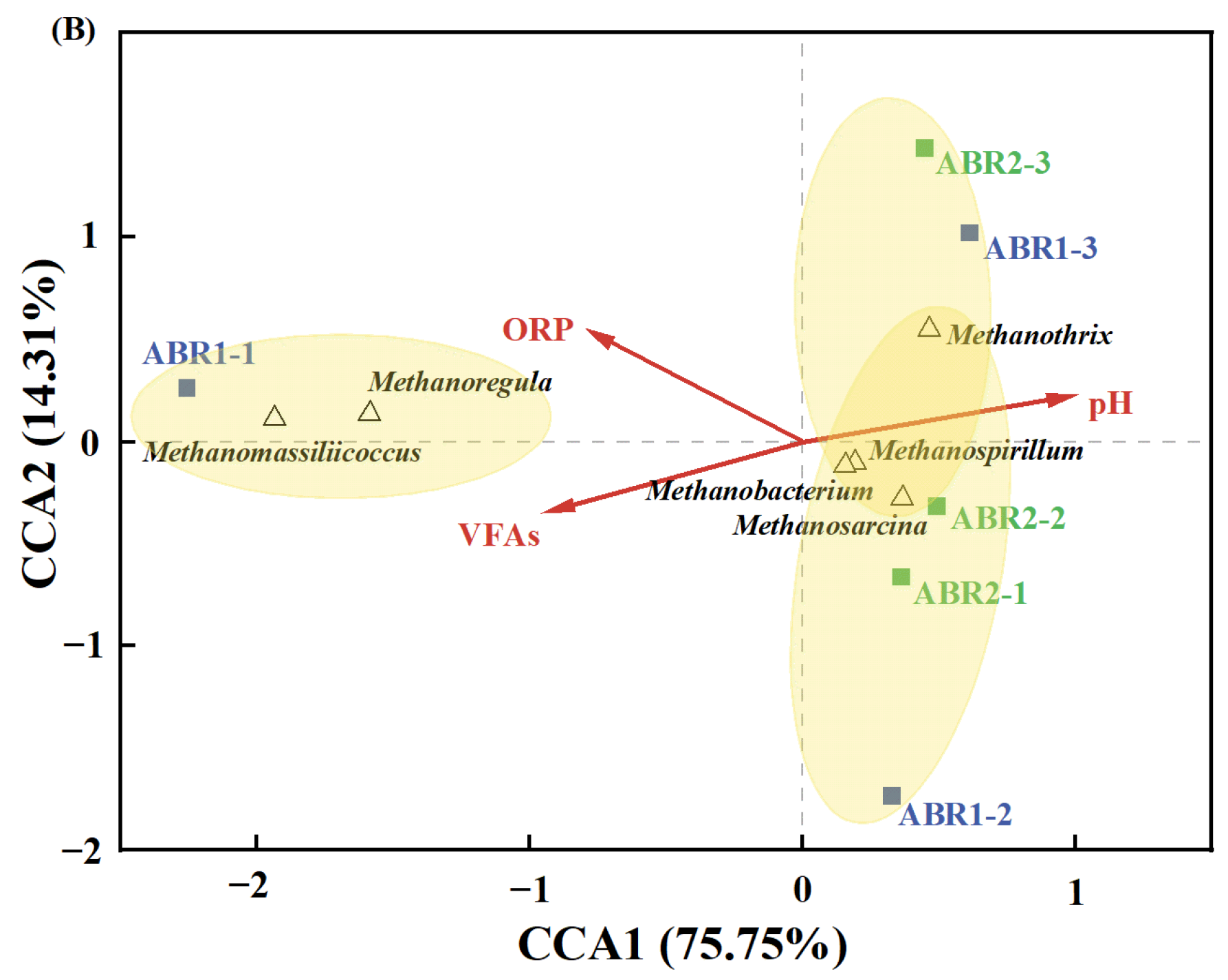
3.4. The Correlation between Environmental Factors and Functional Microbes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arij, Y.; Fatihah, S.; Rakmi, A.R. Performance of pilot scale anaerobic biofilm digester (ABD) for the treatment of leachate from a municipal waste transfer station. Bioresour. Technol. 2018, 260, 213–220. [Google Scholar] [CrossRef]
- Chu, Y.; Fan, J.; Wang, R.; Liu, C.; Zheng, X. Preparation and immobilization of Bi2WO6/BiOI/g-C3N4 nanoparticles for the photocatalytic degradation of tetracycline and municipal waste transfer station leachate. Sep. Purif. Technol. 2022, 300, 121867. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Hu, S.; Diao, Y.; Jin, X.; Jin, P.; Chen, C.; Wu, X.; Wang, X.C. Electro-dissolved ozone flotation (E-DOF) integrated anoxic/oxic membrane reactor for leachate treatment from a waste transfer station. Environ. Sci. Pollut. Res. 2022, 29, 55803–55815. [Google Scholar] [CrossRef]
- Sondh, S.; Upadhyay, D.S.; Patel, S.; Patel, R.N. A strategic review on Municipal Solid Waste (living solid waste) management system focusing on policies, selection criteria and techniques for waste-to-value. J. Clean. Prod. 2022, 356, 131908. [Google Scholar] [CrossRef]
- Wang, X.; Xie, B.; Wu, D.; Hassan, M.; Huang, C. Characteristics and risks of secondary pollutants generation during compression and transfer of municipal solid waste in Shanghai. Waste Manag. 2015, 43, 1–8. [Google Scholar] [CrossRef]
- Thanh, B.X.; Dan, N.P.; Visvanathan, C. Low flux submerged membrane bioreactor treating high strength leachate from a solid waste transfer station. Bioresour. Technol. 2013, 141, 25–28. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.; Qian, G.; Liu, J. Effective anaerobic biodegradation of municipal solid waste fresh leachate using a novel pilot-scale reactor: Comparison under different seeding granular sludge. Bioresour. Technol. 2014, 165, 152–157. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Semicontinuous Temperature-Phased Anaerobic Digestion (TPAD) of Organic Fraction of Municipal Solid Waste (OFMSW). Comparison with single-stage processes. Chem. Eng. J. 2016, 285, 409–416. [Google Scholar] [CrossRef]
- Nabi, M.; Liang, H.; Cheng, L.; Yang, W.; Gao, D. A comprehensive review on the use of conductive materials to improve anaerobic digestion: Focusing on landfill leachate treatment. J. Environ. Manag. 2022, 309, 114540. [Google Scholar] [CrossRef]
- Lv, N.; Zhao, L.; Wang, R.; Ning, J.; Pan, X.; Li, C.; Cai, G.; Zhu, G. Novel strategy for relieving acid accumulation by enriching syntrophic associations of syntrophic fatty acid-oxidation bacteria and H2/formate-scavenging methanogens in anaerobic digestion. Bioresour. Technol. 2020, 313, 123702. [Google Scholar] [CrossRef]
- Dolfing, J. Thermodynamic Constraints on Syntrophic Acetate Oxidation. Appl. Environ. Microbiol. 2014, 80, 1539–1541. [Google Scholar] [CrossRef]
- Amha, Y.M.; Corbett, M.; Smith, A.L. Two-Phase Improves Performance of Anaerobic Membrane Bioreactor Treatment of Food Waste at High Organic Loading Rates. Environ. Sci. Technol. 2019, 53, 9572–9583. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Sun, Y.; Li, Y. Bioaugmentation improves batch psychrophilic anaerobic co-digestion of cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126118. [Google Scholar] [CrossRef]
- Rhee, C.; Park, S.G.; Kim, D.W.; Yu, S.I.; Shin, J.; Hwang, S.; Shin, S.G. Tracking microbial community shifts during recovery process in overloaded anaerobic digesters under biological and non-biological supplementation strategies. Bioresour. Technol. 2021, 340, 125614. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Mccarty, P.L. Environmental Biotechnology Principles & Applications; McGraw-Hill Education: New York, NY, USA, 2014; Volume xiv. [Google Scholar]
- Loesche, W.J. Oxygen sensitivity of various anaerobic bacteria. Appl. Microbiol. 1969, 18, 723. [Google Scholar] [CrossRef]
- Li, J.; Ran, X.; Zhou, M.; Wang, K.; Wang, H.; Wang, Y. Oxidative stress and antioxidant mechanisms of obligate anaerobes involved in biological waste treatment processes: A review. Sci. Total Environ. 2022, 838, 156454. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Gao, L.; Yu, J.; Yuan, X.; Zhu, W.; Wang, X.; Cui, Z. Aerobic deterioration of corn stalk silage and its effect on methane production and microbial community dynamics in anaerobic digestion. Bioresour. Technol. 2018, 250, 828–837. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q.; Li, F.T. Avoiding propionic acid accumulation in the anaerobic process for biohydrogen production. Biomass Bioenergy 2006, 30, 177–182. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; APHA: Washington, DC, USA, 2017. [Google Scholar]
- Guo, Q.; Wang, Y.N.; Qian, J.; Zhang, B.; Hua, M.; Liu, C.; Pan, B. Enhanced production of methane in anaerobic water treatment as mediated by the immobilized fungi. Water Res. 2021, 190, 116761. [Google Scholar] [CrossRef]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a unique biological group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef]
- Heine-Dobbernack, E.; Schoberth, S.M.; Sahm, H. Relationship of Intracellular Coenzyme F420 Content to Growth and Metabolic Activity of Methanobacterium bryantii and Methanosarcina barkeri. Appl. Environ. Microbiol. 1988, 54, 454–459. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, Y.; Zhang, H.; Chen, S.; Zhu, W.; Yuan, X.; Cui, Z. A comparison and evaluation of the effects of biochar on the anaerobic digestion of excess and anaerobic sludge. Sci. Total Environ. 2020, 736, 139159. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wu, B.; Gu, L.; Deng, R. Upgrade the high-load anaerobic digestion and relieve acid stress through the strategy of side-stream micro-aeration: Biochemical performances, microbial response and intrinsic mechanisms. Water Res. 2022, 221, 118850. [Google Scholar] [CrossRef]
- Ali, S.; Hua, B.; Huang, J.J.; Droste, R.L.; Zhou, Q.; Zhao, W.; Chen, L. Effect of different initial low pH conditions on biogas production, composition, and shift in the aceticlastic methanogenic population. Bioresour. Technol. 2019, 289, 121579. [Google Scholar] [CrossRef]
- Lu, F.; Huang, L.; Qian, F.; Jiang, Q.; Khan, S.; Shen, P. Resistance of anaerobic activated sludge acclimated by different feeding patterns: Response to different stress shocks. Water Sci. Technol. 2022, 85, 3023–3035. [Google Scholar] [CrossRef]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Influence of low pH on continuous anaerobic digestion of waste activated sludge. Water Res. 2017, 113, 42–49. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Sawdust-Derived Biochar Much Mitigates VFAs Accumulation and Improves Microbial Activities To Enhance Methane Production in Thermophilic Anaerobic Digestion. ACS Sustain. Chem. Eng. 2019, 7, 2141–2150. [Google Scholar] [CrossRef]
- Yu, H.; Lai, B.; Yang, H.; Rong, H.; Liang, H.; Qu, F. In situ probing methanogenesis in anaerobic wastewater treatment using front-face excitation-emission matrix (FF-EEM) fluorescence. J. Clean. Prod. 2023, 387, 135734. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, S.H.; Lee, C.Y.; Nam, S.Y. Inhibitory effects of long-chain fatty acids on VFA degradation and β-oxidation. Water Sci. Technol. 2003, 47, 139–146. [Google Scholar] [CrossRef]
- Mathai, P.P.; Nicholes, M.S.; Venkiteshwaran, K.; Brown, C.M.; Morris, R.L.; Zitomer, D.H.; Maki, J.S. Dynamic shifts within volatile fatty acid–degrading microbial communities indicate process imbalance in anaerobic digesters. Appl. Microbiol. Biotechnol. 2020, 104, 4563–4575. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Chen, Q.; Meng, J.; Li, J.; Zhang, Y.; Jha, A.K. Performance of anaerobic sludge and the microbial social behaviors induced by quorum sensing in a UASB after a shock loading. Bioresour. Technol. 2021, 330, 124972. [Google Scholar] [CrossRef]
- Nie, S.A.; Lei, X.; Zhao, L.; Wang, Y.; Wang, F.; Li, H.; Yang, W.; Xing, S. Response of activity, abundance, and composition of anammox bacterial community to different fertilization in a paddy soil. Biol. Fertil. Soils 2018, 54, 977–984. [Google Scholar] [CrossRef]
- Duong, C.M.; Lim, T.-T. Optimization and microbial diversity of anaerobic co-digestion of swine manure with waste kitchen oil at high organic loading rates. Waste Manag. 2022, 154, 199–208. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, Z.; Wu, G. Impacts of environmental factors on microbial diversity, distribution patterns and syntrophic correlation in anaerobic processes. Arch. Microbiol. 2019, 201, 603–614. [Google Scholar] [CrossRef]
- Antwi, P.; Li, J.; Boadi, P.O.; Meng, J.; Shi, E.; Xue, C.; Zhang, Y.; Ayivi, F. Functional bacterial and archaeal diversity revealed by 16S rRNA gene pyrosequencing during potato starch processing wastewater treatment in an UASB. Bioresour. Technol. 2017, 235, 348–357. [Google Scholar] [CrossRef]
- Xu, S.; Lu, W.; Liu, Y.; Ming, Z.; Liu, Y.; Meng, R.; Wang, H. Structure and diversity of bacterial communities in two large sanitary landfills in China as revealed by high-throughput sequencing (MiSeq). Waste Manag. 2017, 63, 41–48. [Google Scholar] [CrossRef]
- Sieber, J.R.; McInerney, M.J.; Gunsalus, R.P. Genomic Insights into Syntrophy: The Paradigm for Anaerobic Metabolic Cooperation. Annu. Rev. Microbiol. 2012, 66, 429–452. [Google Scholar] [CrossRef]
- Kulichevskaya, I.S.; Ivanova, A.A.; Suzina, N.E.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S.; Dedysh, S.N. Paludisphaera borealis gen. nov., sp. nov., a hydrolytic planctomycete from northern wetlands, and proposal of Isosphaeraceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 837–844. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Nguyen, A.Q.; Johir, M.A.H.; Guo, W.; Ngo, H.H.; Chaves, A.V.; Nghiem, L.D. Application of rumen and anaerobic sludge microbes for bio harvesting from lignocellulosic biomass. Chemosphere 2019, 228, 702–708. [Google Scholar] [CrossRef]
- Fuentes, L.; Braga, L.; Castelló, E.; Etchebehere, C. Work scheme to isolate the different micro-organisms found in hydrogen-producing reactors: A study of effectiveness by pyrosequencing analysis. J. Appl. Microbiol. 2018, 125, 96–110. [Google Scholar] [CrossRef]
- Amha, Y.M.; Sinha, P.; Lagman, J.; Gregori, M.; Smith, A.L. Elucidating microbial community adaptation to anaerobic co-digestion of fats, oils, and grease and food waste. Water Res. 2017, 123, 277–289. [Google Scholar] [CrossRef]
- Kindaichi, T.; Yamaoka, S.; Uehara, R.; Ozaki, N.; Ohashi, A.; Albertsen, M.; Nielsen, P.H.; Nielsen, J.L. Phylogenetic diversity and ecophysiology of Candidate phylum Saccharibacteria in activated sludge. FEMS Microbiol. Ecol. 2016, 92, fiw078. [Google Scholar] [CrossRef]
- Kuever, J. The Family Syntrophaceae. In The Prokaryotes: Deltaproteobacteria and Epsilonproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 281–288. [Google Scholar] [CrossRef]
- Kim, W.; Shin, S.G.; Han, G.; Cho, K.; Hwang, S. Structures of microbial communities found in anaerobic batch runs that produce methane from propionic acid—Seeded from full-scale anaerobic digesters above a certain threshold. J. Biotechnol. 2015, 214, 192–198. [Google Scholar] [CrossRef]
- Feng, D.; Xia, A.; Huang, Y.; Zhu, X.; Zhu, X.; Show, P.-L.; Liao, Q. Continuous electromethanogenesis of propionate wastewater: Effect of external voltage and hydraulic retention time. Chem. Eng. J. 2023, 454, 140267. [Google Scholar] [CrossRef]
- Zhen, G.; Kobayashi, T.; Lu, X.; Xu, K. Understanding methane bioelectrosynthesis from carbon dioxide in a two-chamber microbial electrolysis cells (MECs) containing a carbon biocathode. Bioresour. Technol. 2015, 186, 141–148. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.-G.; Shin, W.-B.; Tian, D.-J.; Jun, H.-B. Microbial communities change in an anaerobic digestion after application of microbial electrolysis cells. Bioresour. Technol. 2017, 234, 273–280. [Google Scholar] [CrossRef]
- Moestedt, J.; Muller, B.; Westerholm, M.; Schnurer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, H.; Liu, J.; Hazen, T.C.; Huang, V.; He, Q. Unexpected competitiveness of Methanosaeta populations at elevated acetate concentrations in methanogenic treatment of animal wastewater. Appl. Microbiol. Biotechnol. 2017, 101, 1729–1738. [Google Scholar] [CrossRef]
- He, P.; Duan, H.; Han, W.; Liu, Y.; Shao, L.; Lü, F. Responses of Methanosarcina barkeri to acetate stress. Biotechnol. Biofuels 2019, 12, 289. [Google Scholar] [CrossRef]
- Evans, P.N.; Boyd, J.A.; Leu, A.O.; Woodcroft, B.J.; Parks, D.H.; Hugenholtz, P.; Tyson, G.W. An evolving view of methane metabolism in the Archaea. Nat. Rev. Microbiol. 2019, 17, 219–232. [Google Scholar] [CrossRef]
- De Bok, F.A.M.; Stams, A.J.M.; Dijkema, C.; Boone, D.R. Pathway of Propionate Oxidation by a Syntrophic Culture of Smithella propionica and Methanospirillum hungatei. Appl. Environ. Microbiol. 2001, 67, 1800–1804. [Google Scholar] [CrossRef]
- Thomas, M.; William, M. Energy Conservation and Hydrogenase Function in Methanogenic Archaea, in Particular the Genus Methanosarcina. Microbiol. Mol. Biol. Rev. 2019, 83, e00020-19. [Google Scholar] [CrossRef]
- Guo, X.; Yu, H.; Yan, Z.; Gao, H.; Zhang, Y. Tracking variations of fluorescent dissolved organic matter during wastewater treatment by accumulative fluorescence emission spectroscopy combined with principal component, second derivative and canonical correlation analyses. Chemosphere 2018, 194, 463–470. [Google Scholar] [CrossRef]
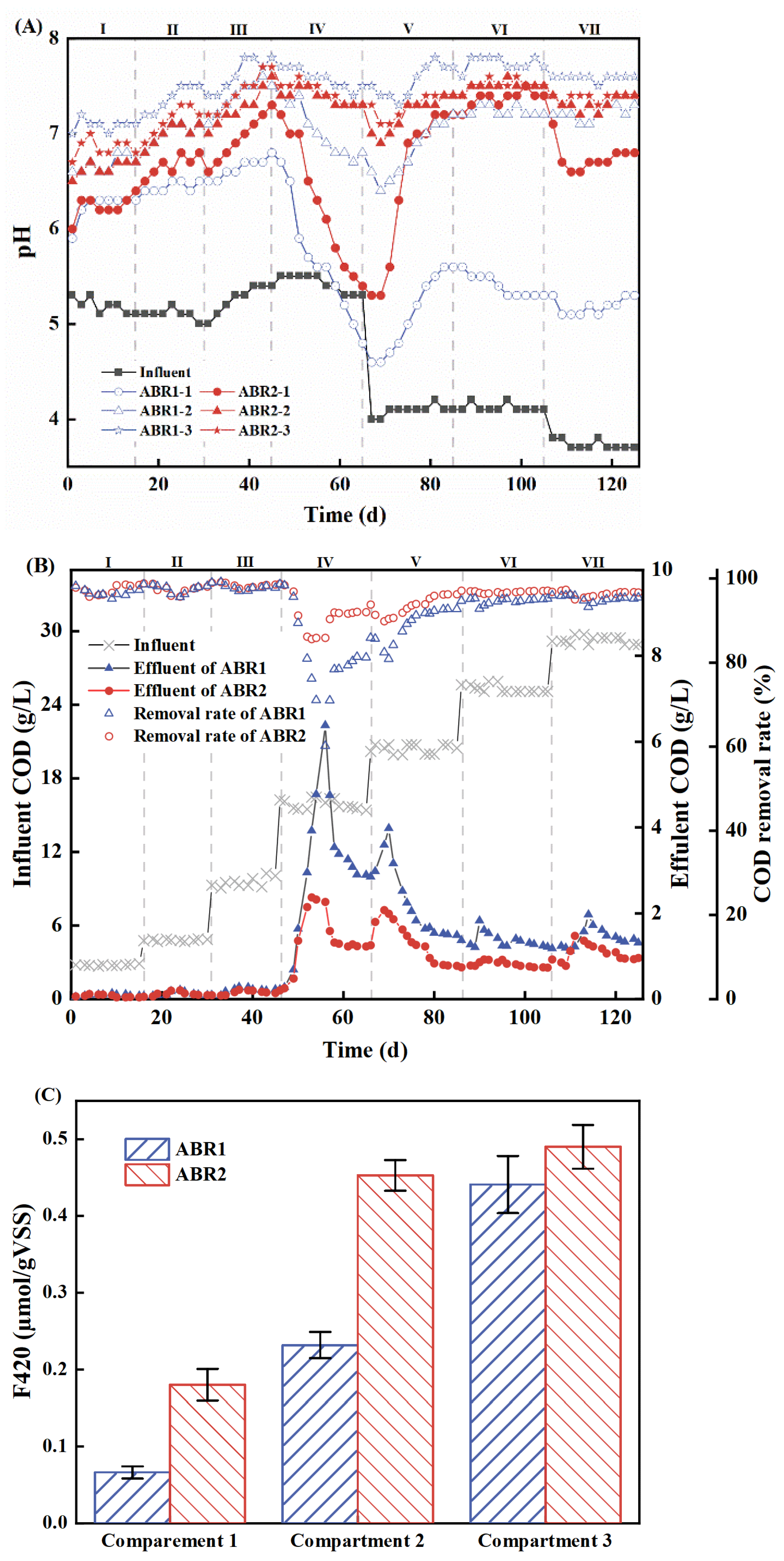
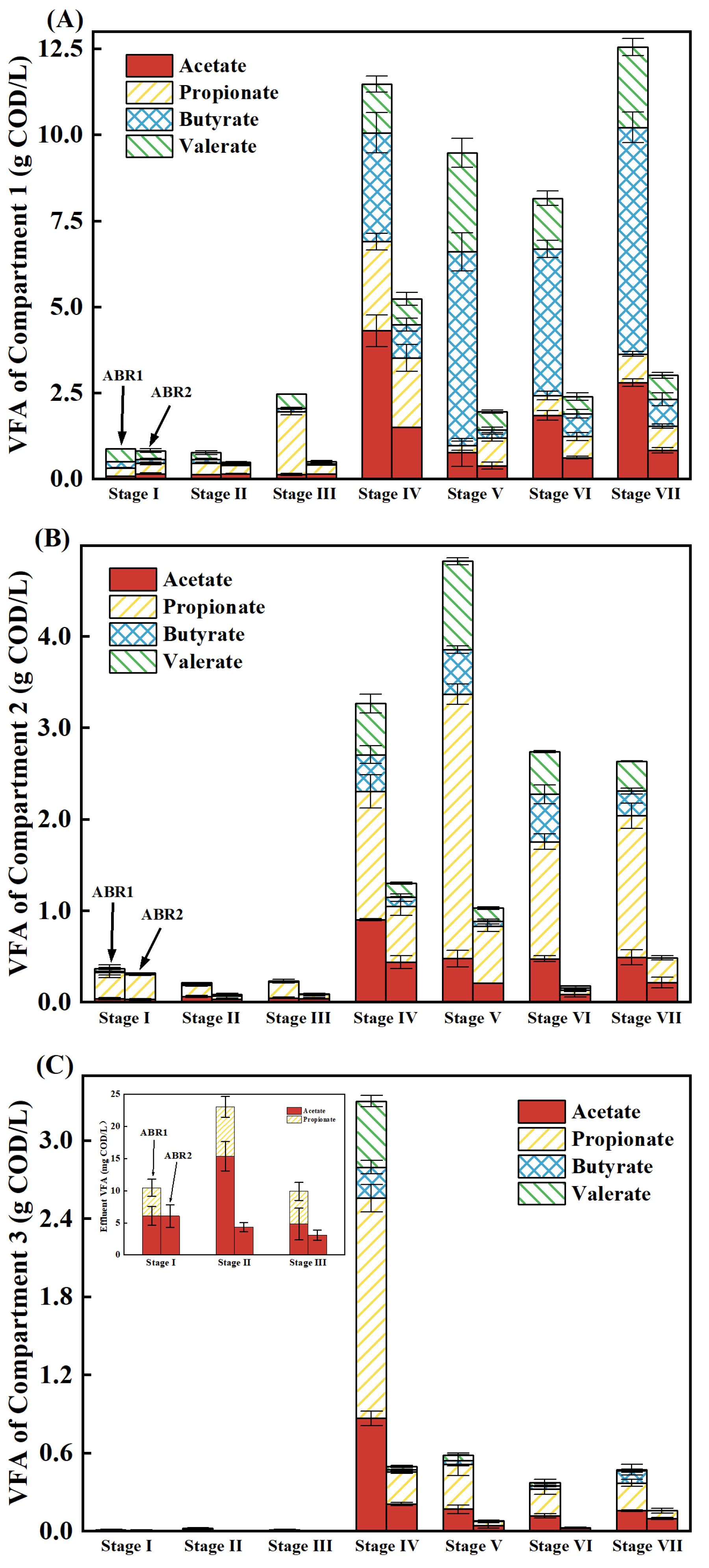
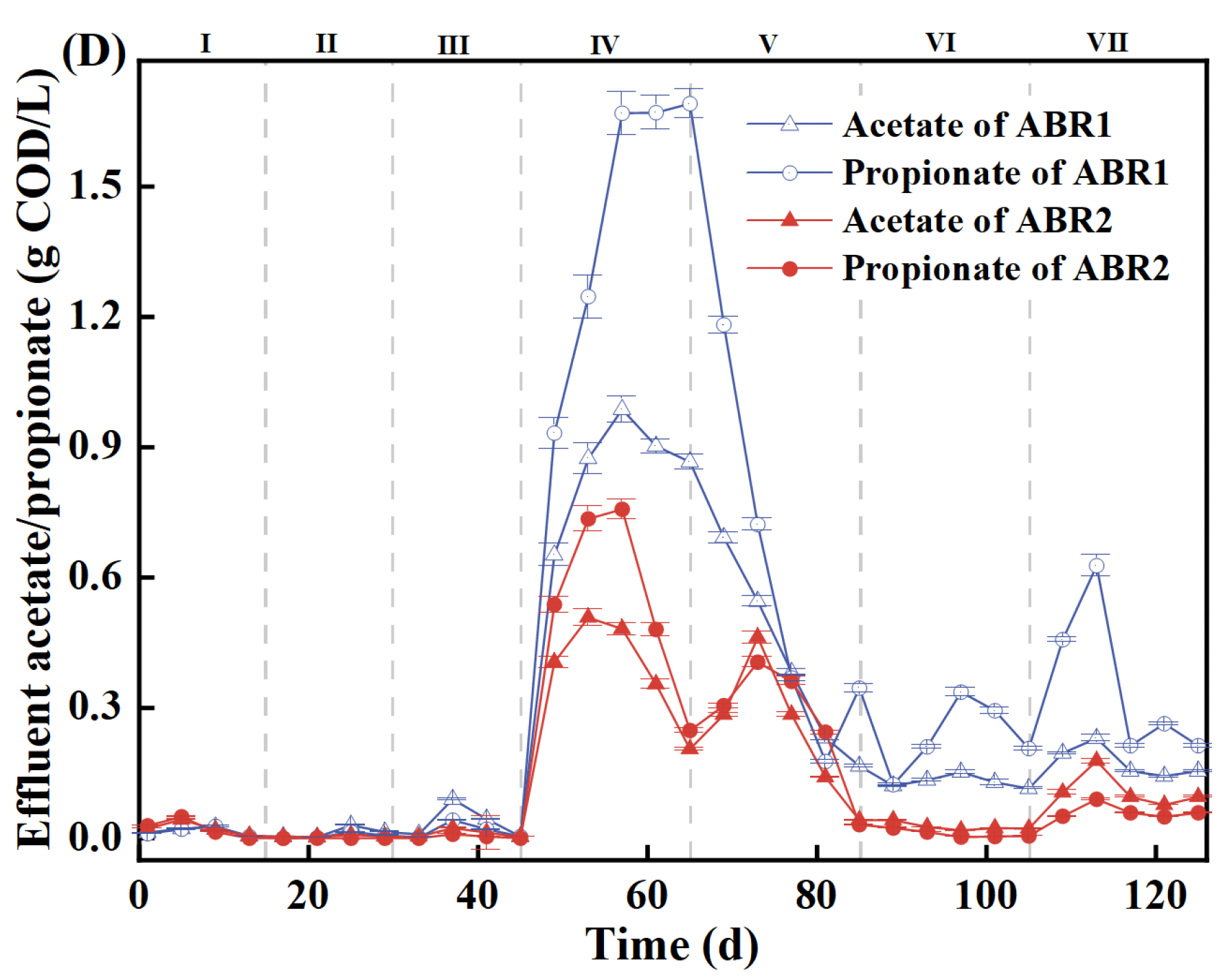
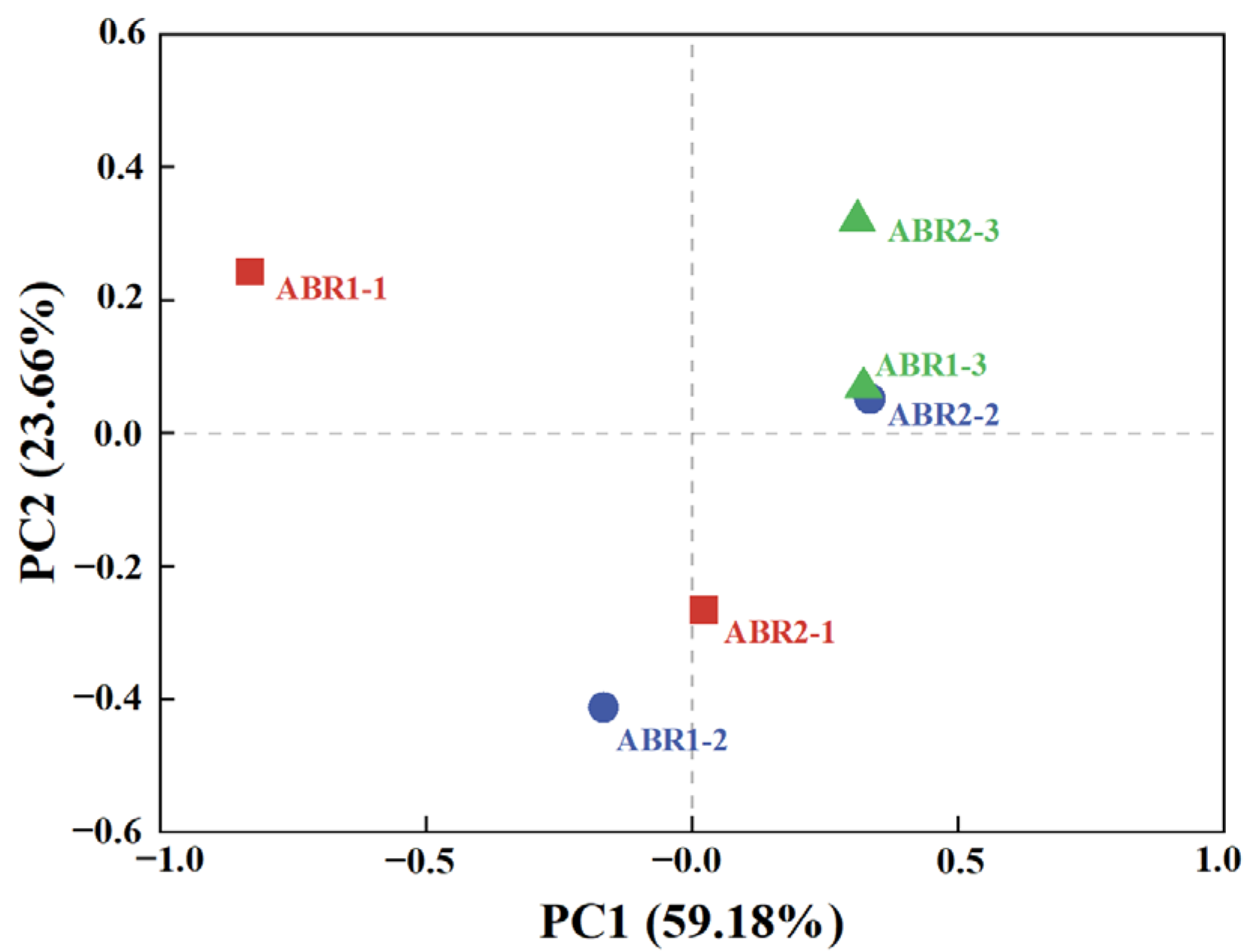

| Stage | Time (d) | Influent COD (g/L) | OLR [kg/(m3·d)] |
|---|---|---|---|
| I | 1 to 15 | 2.98 ± 0.02 | 0.6 |
| II | 16 to 30 | 4.80 ± 0.10 | 1.0 |
| III | 31 to 45 | 9.78 ± 0.26 | 2.0 |
| IV | 46 to 65 | 15.73 ± 0.30 | 3.0 |
| V | 66 to 85 | 20.38 ± 0.36 | 4.0 |
| VI | 86 to 105 | 25.32 ± 0.30 | 5.0 |
| VII | 106 to 125 | 29.26 ± 0.29 | 6.0 |
| Stage | ABR1-1 | ABR2-1 | ABR1-2 | ABR2-2 | ABR1-3 | ABR2-3 | ABR1 | ABR2 |
|---|---|---|---|---|---|---|---|---|
| I | 51.11 ± 5.53 | 57.45 ± 8.86 | 26.03 ± 3.98 | 24.92 ± 7.30 | 19.10 ± 2.82 | 14.79 ± 2.53 | 96.24 ± 0.61 | 97.17 ± 1.08 |
| II | 60.38 ± 5.58 | 73.22 ± 5.25 | 29.45 ± 4.54 | 20.58 ± 4.73 | 7.80 ± 1.74 | 3.89 ± 1.20 | 97.62 ± 0.98 | 97.68 ± 0.92 |
| III | 65.82 ± 8.76 | 76.81 ± 7.71 | 26.35 ± 6.52 | 18.29 ± 6.54 | 5.85 ± 3.56 | 3.31 ± 1.74 | 98.02 ± 0.62 | 98.41 ± 0.48 |
| IV | 18.81 ± 17.44 | 48.91 ± 11.50 | 45.71 ± 10.62 | 33.46 ± 10.41 | 16.83 ± 8.35 | 9.02 ± 4.49 | 81.36 ± 10.22 | 91.39 ± 4.16 |
| V | 16.59 ± 10.73 | 62.30 ± 17.08 | 45.21 ± 13.10 | 21.86 ± 11.51 | 27.10 ± 5.58 | 9.07 ± 5.03 | 88.90 ± 4.19 | 93.24 ± 2.38 |
| VI | 23.79 ± 6.55 | 82.91 ± 4.56 | 48.47 ± 7.26 | 9.68 ± 3.74 | 22.39 ± 2.28 | 4.15 ± 1.93 | 94.65 ± 0.82 | 96.75 ± 0.26 |
| VII | 33.40 ± 4.54 | 66.54 ± 8.53 | 49.67 ± 5.43 | 27.63 ± 7.87 | 11.76 ± 2.38 | 2.32 ± 1.28 | 94.83 ± 1.00 | 96.49 ± 0.67 |
| Sample | Number | OTUs | Shannon | Shannoneven | Chao | Ace |
|---|---|---|---|---|---|---|
| ABR1-1 | 91,757 | 892 | 2.303 | 0.339 | 1063.667 | 1050.813 |
| ABR1-2 | 54,333 | 1083 | 4.395 | 0.629 | 1242.429 | 1242.446 |
| ABR1-3 | 72,997 | 1293 | 4.475 | 0.625 | 1367.930 | 1364.270 |
| ABR2-1 | 56,476 | 1175 | 4.552 | 0.644 | 1349.317 | 1338.694 |
| ABR2-2 | 48,628 | 1257 | 4.501 | 0.631 | 1374.353 | 1364.085 |
| ABR2-3 | 56,070 | 1373 | 5.367 | 0.743 | 1419.902 | 1413.463 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Zhang, C.; Jin, C.; Wu, J.; Li, P. Intermediate Accumulation and Process Stability for Facultative and Obligate Anaerobic Treatment of Leachate from Waste Transfer Stations. Fermentation 2023, 9, 465. https://doi.org/10.3390/fermentation9050465
Li B, Zhang C, Jin C, Wu J, Li P. Intermediate Accumulation and Process Stability for Facultative and Obligate Anaerobic Treatment of Leachate from Waste Transfer Stations. Fermentation. 2023; 9(5):465. https://doi.org/10.3390/fermentation9050465
Chicago/Turabian StyleLi, Bei, Chiqian Zhang, Cong Jin, Jinhua Wu, and Ping Li. 2023. "Intermediate Accumulation and Process Stability for Facultative and Obligate Anaerobic Treatment of Leachate from Waste Transfer Stations" Fermentation 9, no. 5: 465. https://doi.org/10.3390/fermentation9050465
APA StyleLi, B., Zhang, C., Jin, C., Wu, J., & Li, P. (2023). Intermediate Accumulation and Process Stability for Facultative and Obligate Anaerobic Treatment of Leachate from Waste Transfer Stations. Fermentation, 9(5), 465. https://doi.org/10.3390/fermentation9050465








