Optimal Fermentation of Artemisia annua Residues and Its Effects on Production Performance of Laying Hens
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of AAR and Microbial Inoculum
2.2. Optimization of Microbial Fermentation Conditions
2.3. Chemical Compound Composition Analysis
2.4. Observation of Surface Morphology
2.5. Analysis of Microbiota Composition
2.6. Animal Experiment
2.7. Determination of Laying Performance and Egg Quality
2.8. Statistical Analysis
3. Results
3.1. Optimization of Microbial Fermentation Conditions
3.2. Chemical Compound Composition and Surface Morphology of Fermented AAR
3.3. Microbiota Composition of Fermented AAR
3.4. Laying Performance and Egg Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelli, N.; Sola-Oriol, D.; Perez, J.F. Phytogenic feed additives in poultry: Achievements, prospective and challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- El-Sabrout, K.; Khalifah, A.; Mishra, B. Application of botanical products as nutraceutical feed additives for improving poultry health and production. Vet. World 2023, 16, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Kim, I.H. Efficacy of phytogenic feed additive on performance, production and health status of monogastric animals—A review. Ann. Anim. Sci. 2017, 17, 929–948. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Barkat, R.A.; Gabr, A.A.; Foda, M.A.; Noreldin, A.E.; Khafaga, A.F.; El-Sabrout, K.; et al. Potential role of important nutraceuticals in poultry performance and health—A comprehensive review. Res. Vet. Sci. 2021, 137, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- De Almeida, G.F.; Horsted, K.; Thamsborg, S.M.; Kyvsgaard, N.C.; Ferreira, J.F.; Hermansen, J.E. Use of Artemisia annua as a natural coccidiostat in free-range broilers and its effects on infection dynamics and performance. Vet. Parasitol. 2012, 186, 178–187. [Google Scholar] [CrossRef]
- Wan, X.L.; Niu, Y.; Zheng, X.C.; Huang, Q.; Su, W.P.; Zhang, J.F.; Zhang, L.L.; Wang, T. Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers. Anim. Feed Sci. Tech. 2016, 221, 27–34. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, K.; Zhang, L.; Wang, T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 2017, 69, 184–190. [Google Scholar] [CrossRef]
- Yang, C.; Ye, P.; Huo, J.; Moller, A.P.; Liang, W.; Feeney, W.E. Sparrows use a medicinal herb to defend against parasites and increase offspring condition. Curr. Biol. 2020, 30, R1391–R1412. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Xiao, F.X.; Lin, L.; Chen, K.; Wang, Z.H.; Tian, J.; Song, J.P.; Wang, Q. Optimization of extraction process for total polysaccharides from Artemisiae annuae herba residue by response surface methodology and evaluation of its antioxidant activity. Chin. J. Exp. Tradit. Med. Form. 2015, 21, 8–11. [Google Scholar] [CrossRef]
- Zengin, M.; Sur, A.; Ilhan, Z.; Azman, M.A.; Tavsanli, H.; Esen, S.; Bacaksiz, O.K.; Demir, E. Effects of fermented distillers grains with solubles, partially replaced with soybean meal, on performance, blood parameters, meat quality, intestinal flora, and immune response in broiler. Res. Vet. Sci. 2022, 150, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mat, K.; Ishigaki, G.; Akashi, R. A review of okara (soybean curd residue) utilization as animal feed: Nutritive value and animal performance aspects. Anim. Sci. J. 2021, 92, e13594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ye, Y.; Wang, L.; Tan, B. Nutrition and sensory evaluation of solid-state fermented brown rice based on cluster and principal component analysis. Foods 2022, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y. Study on Extraction Technology and Determination Method of Artemisinin from Artemisia annua L. Master’s Thesis, Southwest University, Chongqing, China, 2013. Available online: http://kreader.cnki.net/Kreader/CatalogViewPage.aspx?dbCode=cdmd&filename=1013268708.nh&tablename=CMFD201302&compose=&first=1&uid= (accessed on 1 March 2023).
- Li, H.; Li, T.T.; Yao, M.J.; Li, J.B.; Zhang, S.H.; Wirth, S.; Cao, W.D.; Lin, Q.; Li, X.Z. Diet diversity is associated with beta but not alpha diversity of pika gut microbiota. Front. Microbiol. 2016, 7, 1269. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in traditional medicine and current state of knowledge on the chemistry, biological activity and possible applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Chen, Q.L.; Fu, L.Z.; Fu, W.J.; Zheng, H.; Tang, H.M.; Zhai, S.Q.; Chen, C.L. Effect of Qing Chang oral liquid on the treatment of artificially infected chicken coccidiosis and the cellular immunity. Vet. Med. Sci. 2022, 8, 2504–2510. [Google Scholar] [CrossRef]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Therapeut. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Baghban-Kanani, P.; Hosseintabar-Ghasemabad, B.; Azimi-Youvalari, S.; Seidavi, A.; Ragni, M.; Laudadio, V.; Tufarelli, V. Effects of using Artemisia annua leaves, probiotic blend, and organic acids on performance, egg quality, blood biochemistry, and antioxidant status of laying hens. Jap. Poult. Sci. Assoc. 2019, 56, 120–127. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Liu, W.; Wang, T.; De Sanctis, F.; Zhu, L.; Zhang, G.; Cheng, J.; Cao, Q.; Zhou, J.; et al. Targeting inhibition of accumulation and function of myeloid-derived suppressor cells by artemisinin via PI3K/AKT, mTOR, and MAPK pathways enhances anti-PD-L1 immunotherapy in melanoma and liver tumors. J. Immunol. Res. 2022, 2022, 2253436. [Google Scholar] [CrossRef]
- Banerjee, R.; Hks, K.; Banerjee, M. Medicinal significance of furan derivatives: A review. Int. J. Life Cycle Ass. 2012, 2, 7–16. [Google Scholar]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Baptista, R.; Fazakerley, D.; Whatley, K.E.; Hoffmann, K.F.; Shen, J.; Mur, L.A.J. The anti-mycobacterial activity of Artemisia annua L. is based on deoxyartemisinin and artemisinic acid. BioRxiv 2020. [Google Scholar] [CrossRef]
- Ganesan, T.; Subban, M.; Leslee, C.D.B.; Kuppannan, S.B.; Seedevi, P. Structural characterization of n-hexadecanoic acid from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activities. Biomass Convers. Bior. 2022, 1–12. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Shill, C.M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Mojarab-Mahboubkar, M.; Sendi, J.J. Chemical composition, insecticidal and physiological effect of methanol extract of sweet wormwood (Artemisia annua L.) on Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Toxin Rev. 2016, 35, 106–115. [Google Scholar] [CrossRef]
- Wan, X.; Jiang, L.; Zhong, H.; Lu, Y.; Zhang, L.; Wang, T. Effects of enzymatically treated Artemisia annua L. on growth performance and some blood parameters of broilers exposed to heat stress. Anim. Sci. J. 2017, 88, 1239–1246. [Google Scholar] [CrossRef]
- Husseiny, S.; Dishisha, T.; Soliman, H.A.; Adeleke, R.; Raslan, M. Characterization of growth promoting bacterial endophytes isolated from Artemisia annua L. S. Afr. J. Bot. 2021, 143, 238–247. [Google Scholar] [CrossRef]
- Yi, S.Y.; Azad, M.A.K.; Ji, Y.J.; Liu, Y.; Dou, M.Y.; Kong, X. Microbial and protease fermentation of Mao-Tai lees alters nutritional composition and promotes in vitro intestinal proteolysis. Agriculture 2023, 13, 64. [Google Scholar] [CrossRef]
- Yang, D.; Li, C.; Li, L.; Wang, Y.; Wu, Y.; Chen, S.; Zhao, Y.; Wei, Y.; Wang, D. Novel insight into the formation mechanism of umami peptides based on microbial metabolism in Chouguiyu, a traditional Chinese fermented fish. Food Res. Int. 2022, 157, 111211. [Google Scholar] [CrossRef]
- Liu, C.; Gong, X.; Zhao, G.; Soe Htet, M.N.; Jia, Z.; Yan, Z.; Liu, L.; Zhai, Q.; Huang, T.; Deng, X.; et al. Liquor flavour is associated with the physicochemical property and microbial diversity of fermented grains in waxy and non-waxy sorghum (Sorghum bicolor) during fermentation. Front. Microbiol. 2021, 12, 618458. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, X.; Hadiatullah, H.; Zhang, J.; Zhao, G. Determination of microbial diversities and aroma characteristics of Beitang shrimp paste. Food Chem. 2021, 344, 128695. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Sun, H.; Wu, J.; Zhao, C.; Li, T.; Huang, H.; Fang, Q.; Mu, E.; Wu, R. Investigating the core microbiota and its influencing factors in traditional Chinese pickles. Food Res. Int. 2021, 147, 110543. [Google Scholar] [CrossRef] [PubMed]
- Tarakhovskaya, E.; Zuy, E.; Yanshin, N.; Islamova, R. Concise review of the genus Vertebrata S.F. Gray (Rhodophyta: Ceramiales). J. Appl. Phycol. 2022, 34, 2225–2242. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Sun, J.; Ni, L. The dynamics of volatile compounds and their correlation with the microbial succession during the traditional solid-state fermentation of Gutian Hong Qu glutinous rice wine. Food Microbiol. 2020, 86, 103347. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Liang, Z.C.; Lin, X.Z.; He, Z.G.; Ren, X.Y.; Li, W.X.; Molnar, I. Fungal community diversity and fermentation characteristics in regional varieties of traditional fermentation starters for Hong Qu glutinous rice wine. Food Res. Int. 2021, 141, 110146. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, X.; Wu, B. Evaluation of anti-microbial activities of extracts of endophytic fungi from Artemisia annua. Bangl. J. Pharmacol. 2012, 7, 120–123. [Google Scholar] [CrossRef]
- Demirci, H.; Kurt-Gur, G.; Ordu, E. Microbiota profiling and screening of the lipase active halotolerant yeasts of the olive brine. World J. Microb. Biot. 2021, 37, 23. [Google Scholar] [CrossRef]
- Brisibe, E.A.; Umoren, U.E.; Owai, P.U.; Brisibe, F. Dietary inclusion of dried Artemisia annua leaves for management of coccidiosis and growth enhancement in chickens. Afr. J. Biotechnol. 2008, 7, 4083–4092. [Google Scholar] [CrossRef]
- Lee, A.R.; Niu, K.M.; Lee, W.D.; Kothari, D.; Kim, S.K. Comparison of the dietary supplementation of Lactobacillus plantarum, and fermented and non-fermented Artemisia annua on the performance, egg quality, serum cholesterol, and eggyolk-oxidative stability during storage in laying hens. Braz. J. Poult. Sci. 2019, 21. [Google Scholar] [CrossRef]
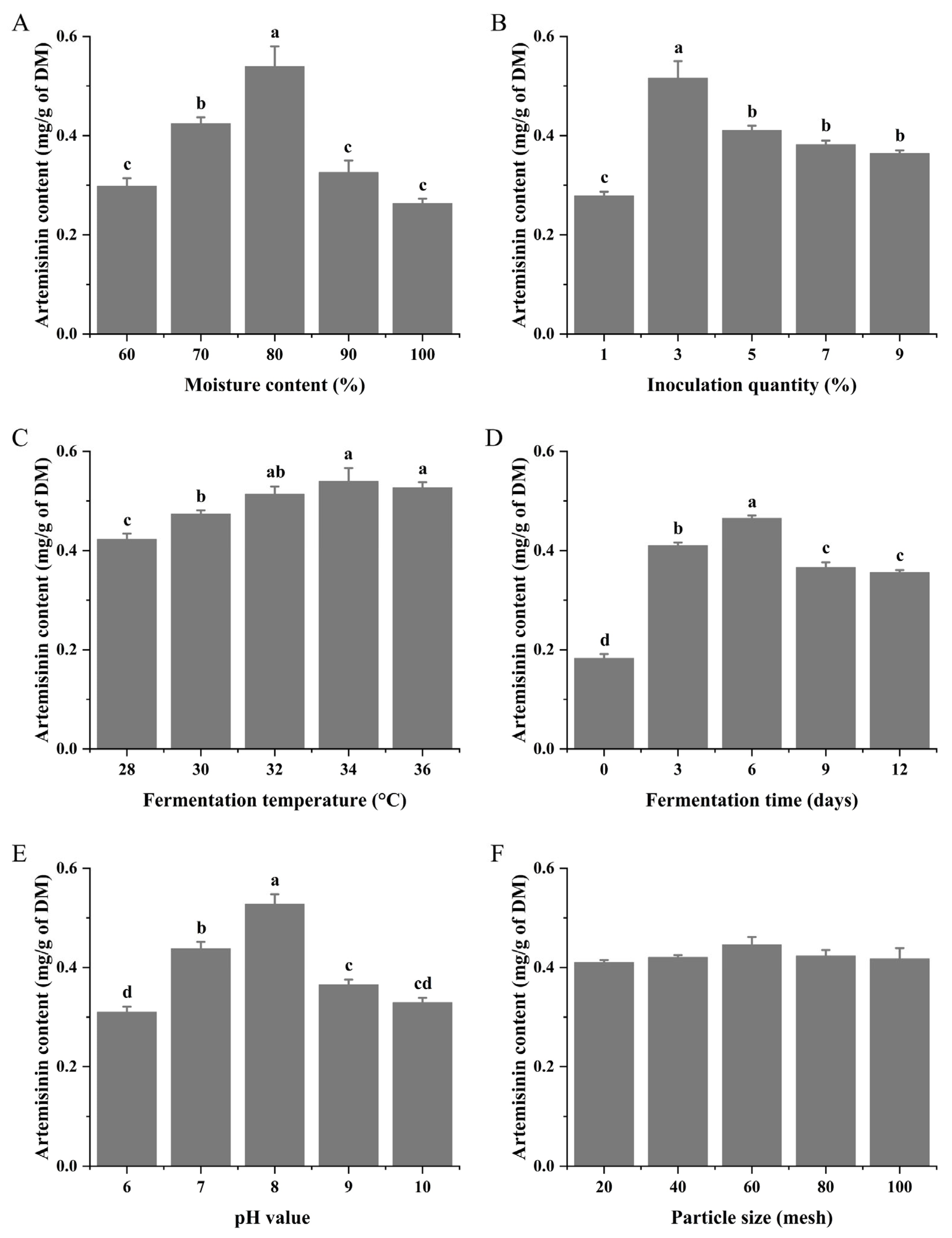
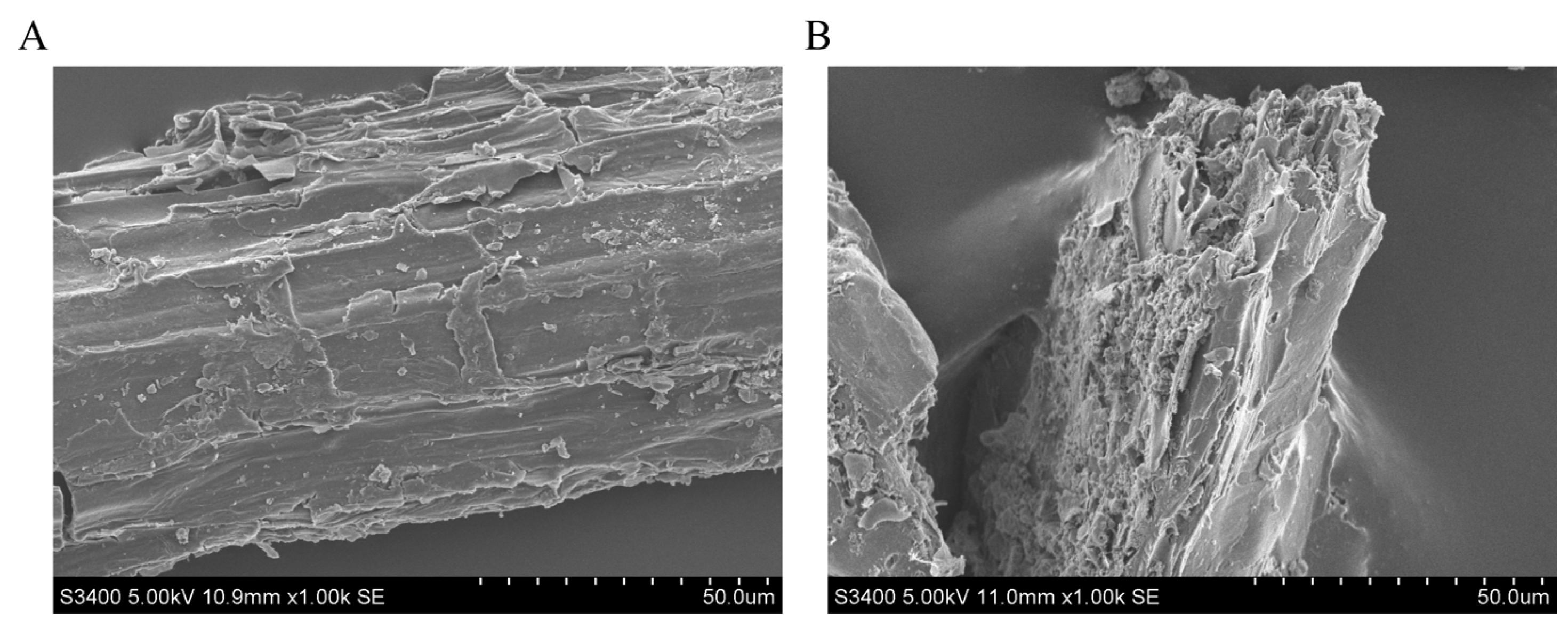
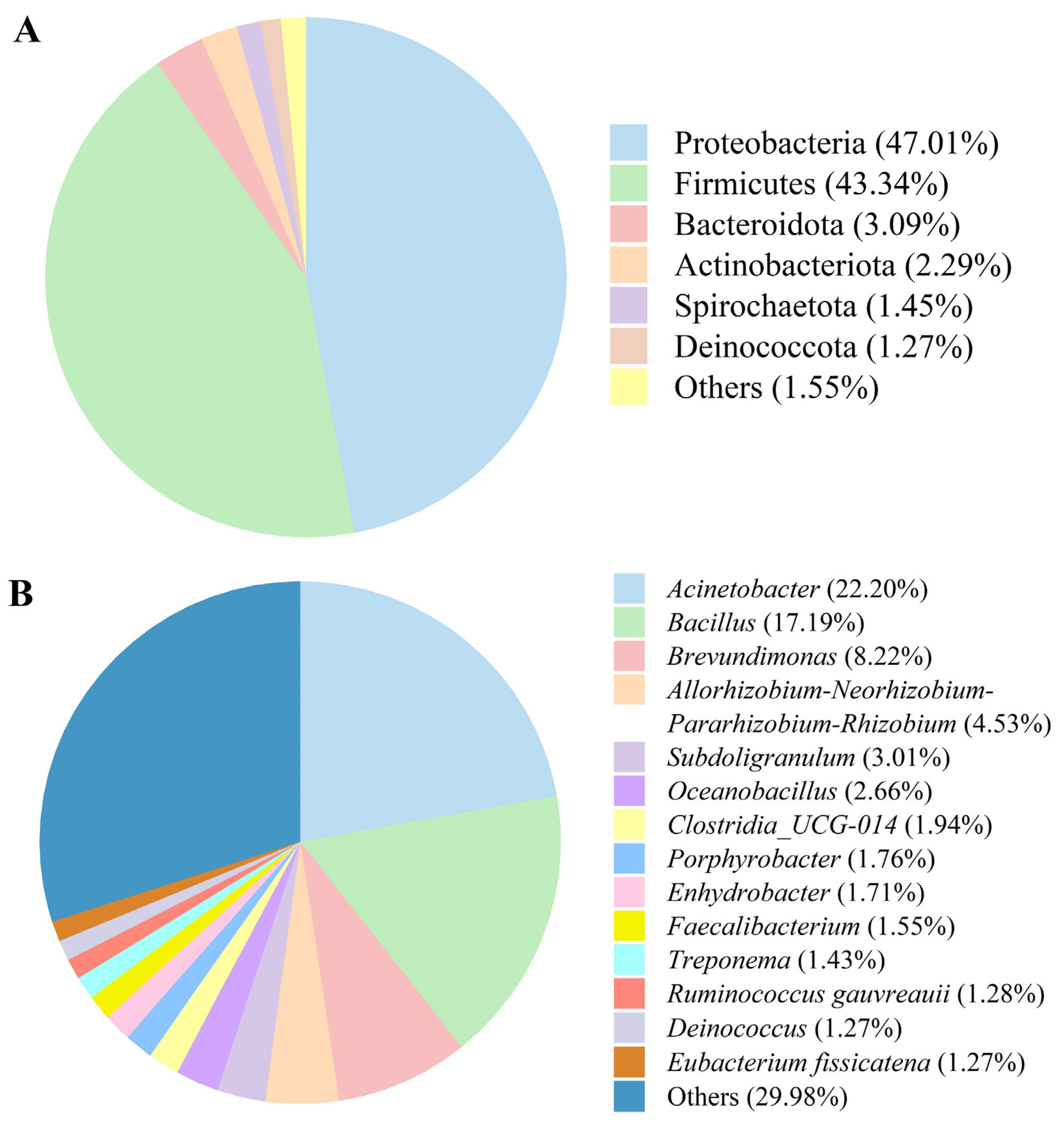
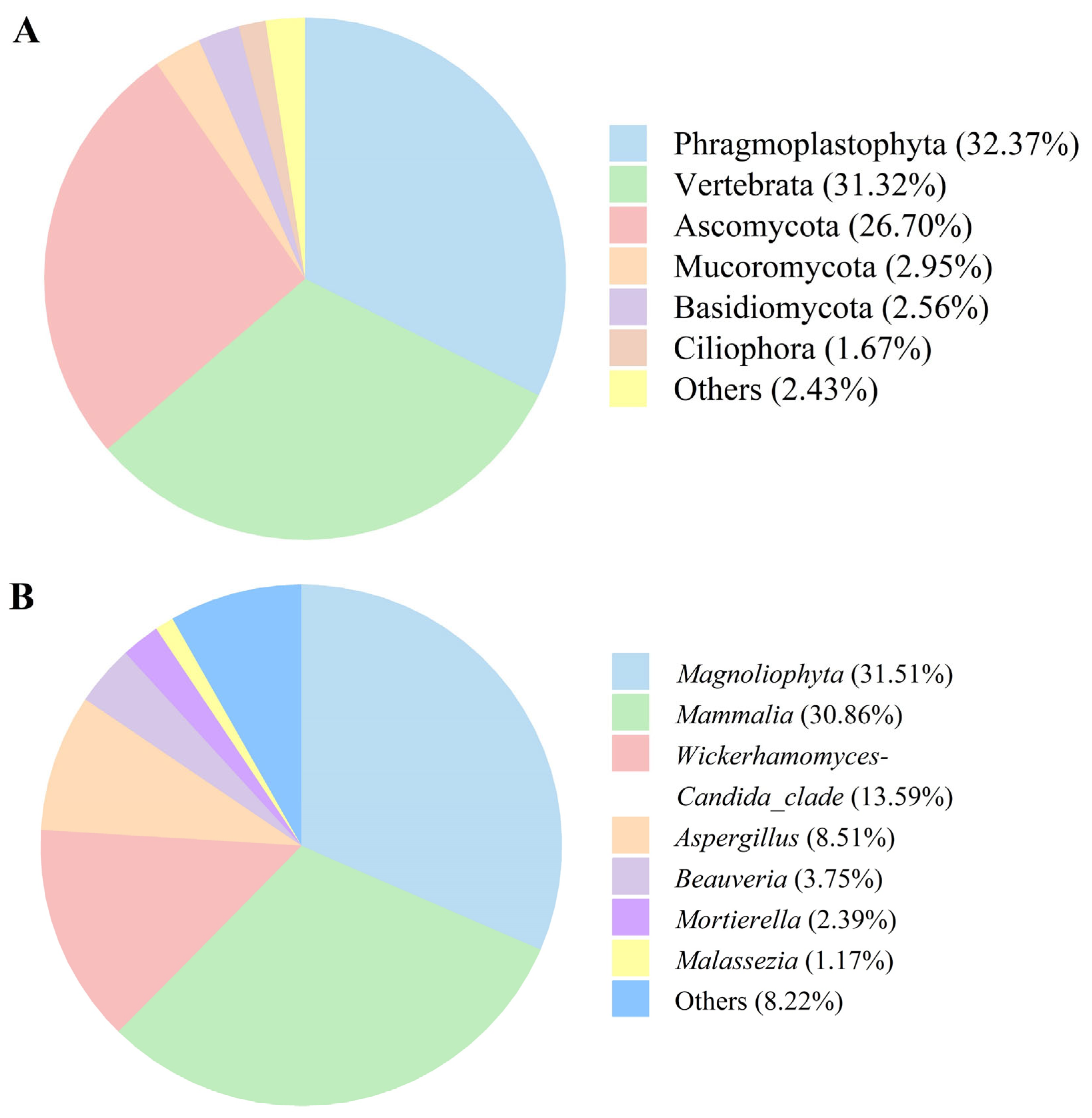
| Item | Value |
|---|---|
| Ingredient, g/kg | |
| Corn | 645.08 |
| Soybean meal | 225.50 |
| Limestone powder | 88.90 |
| DL-methionine | 0.52 |
| Premix a | 40.00 |
| Nutrient levels b, g/kg | |
| Available phosphorus | 3.30 |
| Calcium | 32.30 |
| Crude protein | 168.0 |
| Metabolizable energy, MJ/kg | 11.54 |
| Lysine | 6.70 |
| Methionine | 3.10 |
| Total phosphorus | 4.60 |
| Item | Value |
|---|---|
| Artemisinin (mg/g of DM) | 0.88 |
| Chemical compound composition (% of total compounds) | |
| Alloaromadendrene | 1.41 |
| Aromandendrene | 3.71 |
| 5-butyl-6-hexyloctahydro-1H-indene | 1.05 |
| Calarene | 3.51 |
| Caparratriene | 1.56 |
| Caryophyllene oxide | 3.20 |
| Decamethylcyclopentasiloxane | 1.79 |
| Deoxyartemisinin | 9.38 |
| 3a9-dimethyldodecahydrocyclohepta[d]inden-3-one | 1.84 |
| Dodecamethylcyclohexasiloxane | 1.32 |
| 2-furfuryl-5-methylfuran | 12.13 |
| n-hexadecanoic acid | 5.24 |
| 5,6,8,9,10,11-hexahydrobenz[a]anthracene | 2.53 |
| 4-isopropenyl-4,7-dimethyl-1-oxaspiro[2.5]octane | 1.92 |
| cis-jasmone | 1.01 |
| Octadecamethylcyclononasiloxan | 1.31 |
| Octamethyl cyclotetrasiloxane | 2.90 |
| Phytol | 7.21 |
| Tetradecamethylcycloheptasiloxane | 1.22 |
| 1,5,5-trimethyl-6-methylene-cyclohexene | 2.14 |
| Others | 33.61 |
| Item | Fermented AAR Levels in Diet, DM Basis | SEM | p-Values | |||
|---|---|---|---|---|---|---|
| 0% | 1% | 2% | 4% | |||
| Laying performance | ||||||
| ADFI (g/d) | 91.61 c | 103.70 b | 112.58 a | 110.31 a | 1.642 | <0.001 |
| Egg weight (g) | 50.50 c | 53.68 b | 57.45 a | 55.58 ab | 0.599 | <0.001 |
| Feed-to-egg ratio | 2.05 a | 1.93 ab | 1.81 b | 1.92 ab | 0.026 | 0.009 |
| Laying rate (%) | 71.63 b | 74.41 b | 84.22 a | 81.85 a | 1.512 | 0.004 |
| Egg quality | ||||||
| Albumen height (mm) | 4.29 b | 4.87 a | 5.11 a | 4.95 a | 0.081 | <0.001 |
| Egg shape index | 1.35 | 1.35 | 1.33 | 1.34 | 0.006 | 0.626 |
| Eggshell thickness (mm) | 0.36 | 0.37 | 0.38 | 0.38 | 0.003 | 0.051 |
| Haugh unit | 69.06 b | 72.12 ab | 75.88 a | 72.85 ab | 0.838 | 0.031 |
| Yolk color | 4.17 | 4.56 | 4.81 | 4.53 | 0.118 | 0.291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, S.; He, F.; Azad, M.A.K.; Zhu, Q.; Zhang, M.; Xu, X.; Cui, Y.; Lan, W.; Li, F.; Kong, X. Optimal Fermentation of Artemisia annua Residues and Its Effects on Production Performance of Laying Hens. Fermentation 2023, 9, 456. https://doi.org/10.3390/fermentation9050456
Yi S, He F, Azad MAK, Zhu Q, Zhang M, Xu X, Cui Y, Lan W, Li F, Kong X. Optimal Fermentation of Artemisia annua Residues and Its Effects on Production Performance of Laying Hens. Fermentation. 2023; 9(5):456. https://doi.org/10.3390/fermentation9050456
Chicago/Turabian StyleYi, Siyu, Fumeng He, Md. Abul Kalam Azad, Qian Zhu, Minghui Zhang, Xiaojie Xu, Yadong Cui, Wei Lan, Fenglan Li, and Xiangfeng Kong. 2023. "Optimal Fermentation of Artemisia annua Residues and Its Effects on Production Performance of Laying Hens" Fermentation 9, no. 5: 456. https://doi.org/10.3390/fermentation9050456
APA StyleYi, S., He, F., Azad, M. A. K., Zhu, Q., Zhang, M., Xu, X., Cui, Y., Lan, W., Li, F., & Kong, X. (2023). Optimal Fermentation of Artemisia annua Residues and Its Effects on Production Performance of Laying Hens. Fermentation, 9(5), 456. https://doi.org/10.3390/fermentation9050456






