Effect of the Availability of the Source of Nitrogen and Phosphorus in the Bio-Oxidation of H2S by Sulfolobus metallicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Medium
2.2. Experimental System
2.3. Analytic Techniques
3. Results
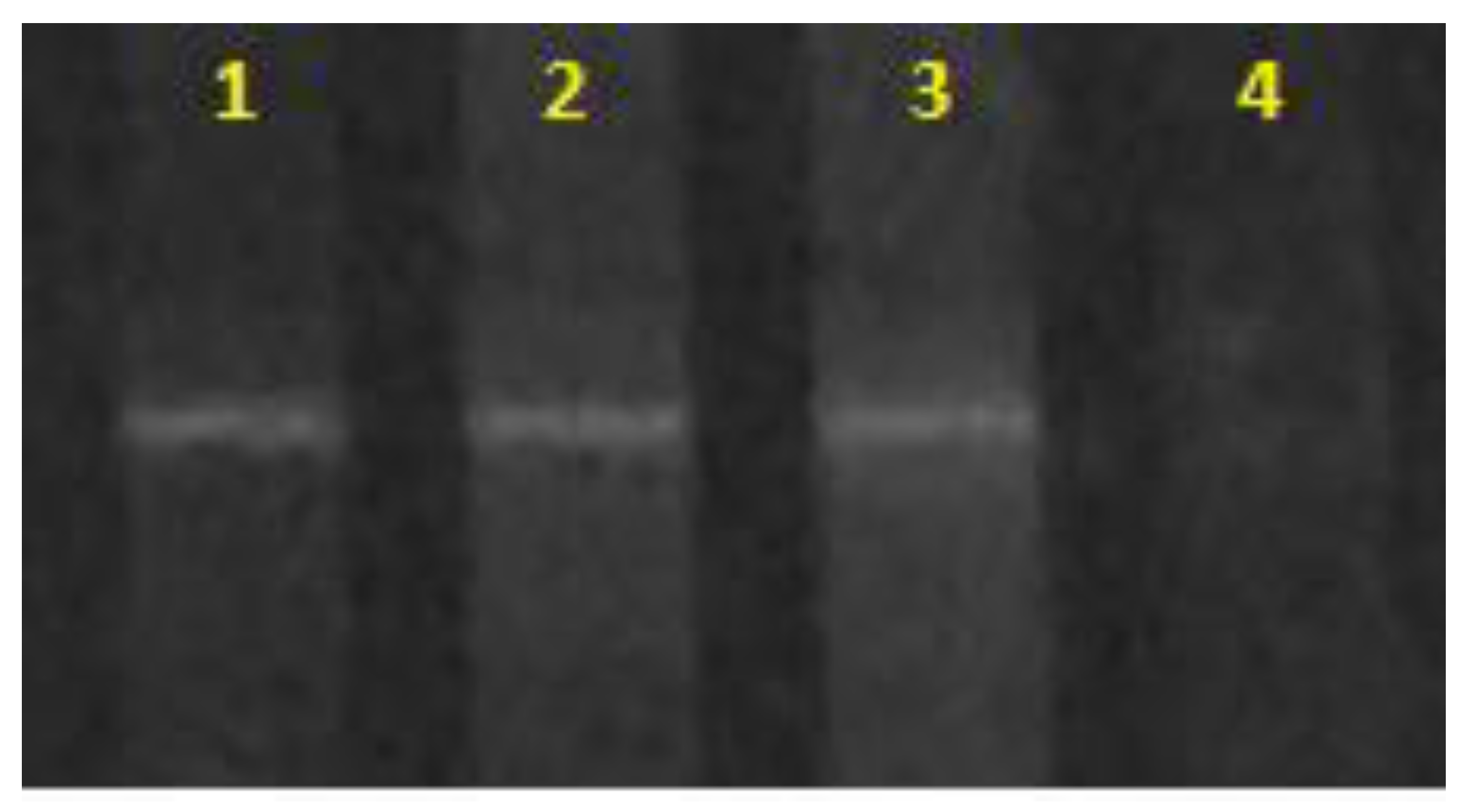
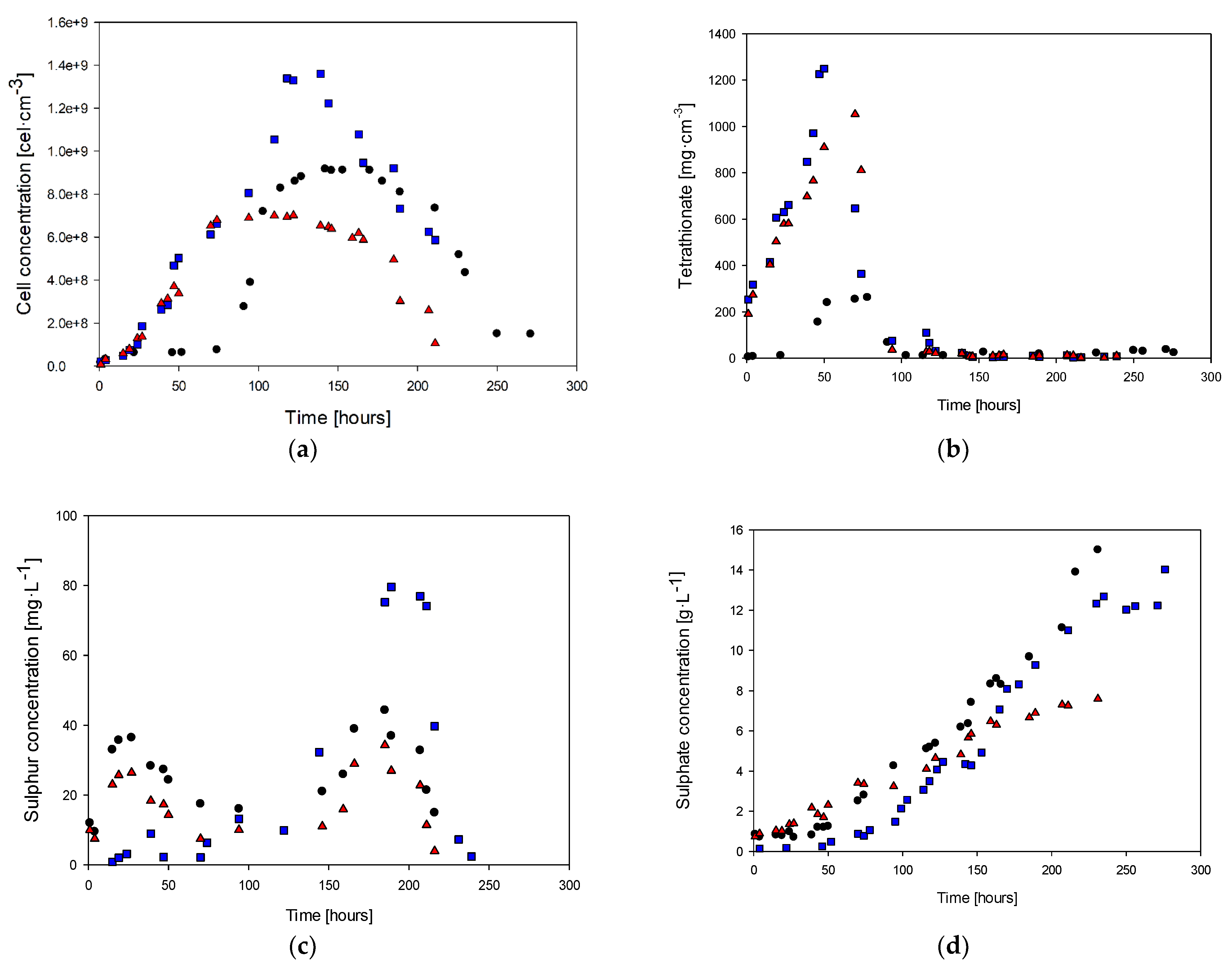
3.1. Study of the Nitrogen Limitation
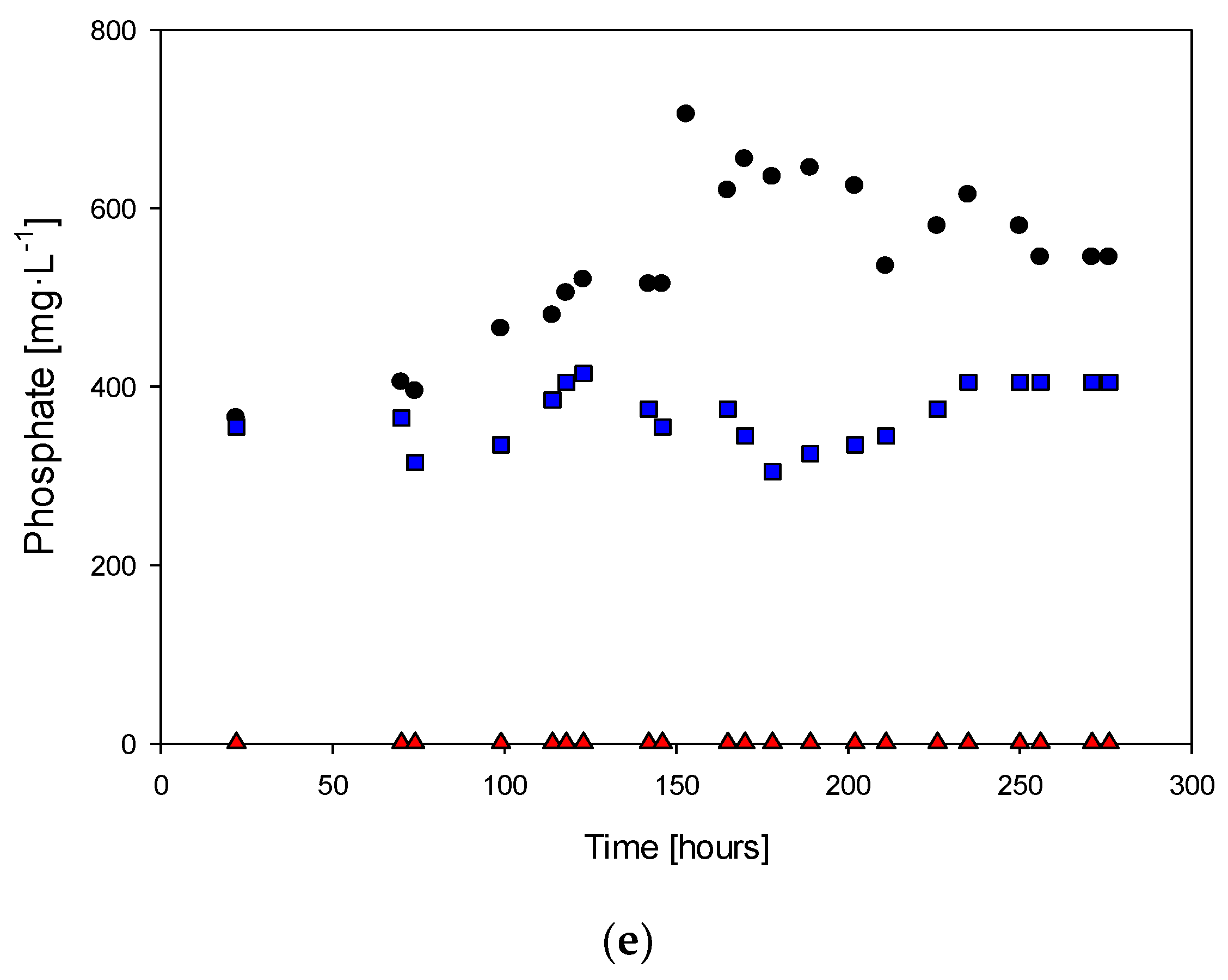
3.2. Study of the Phosphorous Limitation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shu, W.; Huang, L. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Schmerling, C.; Kouril, T.; Snoep, J.; Bräsen, C.; Siebers, B. Enhanced underground metabolism challenges life at high temperature–metabolic thermoadaptation in hyperthermophilic Archaea. Curr. Opin. Syst. Biol. 2022, 30, 100423. [Google Scholar] [CrossRef]
- Caforio, A.; Driessen, A.J.M. Archaeal phospholipids: Structural properties and biosynthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, P.; Rawat, S. Stress response physiology of thermophiles. Arch. Microbiol. 2017, 199, 391–414. [Google Scholar] [CrossRef]
- Leuko, S.; Rettberg, P.; Pontifex, A.L.; Burns, B.P. On the response of halophilic archaea to space conditions. Life 2014, 4, 66–76. [Google Scholar] [CrossRef]
- Lewis, A.M.; Recalde, A.; Bräsen, C.; Counts, J.A.; Nussbaum, P.; Bost, J.; Schocke, L.; Shen, L.; Willard, D.J.; Quax, T.E.F.; et al. The biology of thermoacidophilic archaea from the order Sulfolobales. FEMS Microbiol. Rev. 2021, 45, fuaa063. [Google Scholar] [CrossRef]
- Cobban, A.; Zhang, Y.; Zhou, A.; Weber, Y.; Elling, F.J.; Pearson, A.; Leavitt, W.D. Multiple environmental parameters impact lipid cyclization in Sulfolobus acidocaldarius. Environ. Microbiol. 2020, 22, 4046–4056. [Google Scholar] [CrossRef]
- Bischof, L.F.; Haurat, M.F.; Hoffmann, L.; Albersmeier, A.; Wolf, J.; Neu, A. Early response of Sulfolobus acidocaldarius to nutrient limitation. Front. Microbiol. 2019, 9, 3201. [Google Scholar] [CrossRef]
- Quehenberger, J.; Pittenauer, E.; Allmaier, G.; Spadiut, O. The influence of the specific growth rate on the lipid composition of Sulfolobus acidocaldarius. Extremophiles 2020, 24, 413–420. [Google Scholar] [CrossRef]
- Osorio, G.; Jerez, C.A. Adaptive response of the archaeon Sulfolobus acidocaldarius BC65 to phosphate starvation. Microbiology 1996, 142, 1531–1536. [Google Scholar] [CrossRef]
- Quehenberger, J.; Albersmeier, A.; Glatzel, H.; Hackl, M.; Kalinowski, J.; Spadiut, O. A defined cultivation medium for Sulfolobus acidocaldarius. J. Biotechnol. 2019, 301, 56–67. [Google Scholar] [CrossRef]
- Li, G.; Xu, F.; Yang, T.; Wang, X.; Lyu, T.; Huang, Z. Microbial behavior and influencing factors in the anaerobic digestion of distiller: A comprehensive review. Fermentation 2023, 9, 199. [Google Scholar] [CrossRef]
- Rajpurohit, H.; Eiteman, M.A. Nutrient-limited operational strategies for the microbial production of biochemicals. Microorganisms 2022, 10, 2226. [Google Scholar] [CrossRef]
- Shapiro, B.; Hoehler, T.M.; Jin, Q. Integrating genome-scale metabolic models into the prediction of microbial kinetics in natural environments. Geochim. Cosmochim. Acta 2018, 242, 102–122. [Google Scholar] [CrossRef]
- Biedka, P. Biodegradation kinetics of organic matter in water from sludge dewatering after autothermal thermophilic aerobic digestion. Energies 2023, 16, 203. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kawarabayasi, Y. Experimental confirmation of a whole set of trna molecules in two archaeal species. Int. J. Mol. Sci. 2015, 16, 2187–2203. [Google Scholar] [CrossRef]
- Kawamura, T.; Anraku, R.; Hasegawa, T.; Tomikawa, C.; Hori, H. Transfer RNA Methyltransferases from Thermoplasma acidophilum, a Thermoacidophilic Archaeon. Int. J. Mol. Sci. 2015, 16, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Muloiwa, M.; Nyende-Byakika, S.; Dinka, M. Comparison of unstructured kinetic bacterial growth models. S. Afr. J. Chem. Eng. 2020, 33, 141–150. [Google Scholar] [CrossRef]
- Huber, G.; Stetter, K.O. Sulfolobus metallicus, sp. nov., a novel strictly chemolithoautotrophic thermophilic archaeal species of metal-mobilizers. Syst. Appl. Microbiol. 1991, 14, 372–378. [Google Scholar] [CrossRef]
- Astudillo, C.; Acevedo, F. Adaptation of Sulfolobus metallicus to high pulp densities in the biooxidation of a flotation gold concentrate. Hydrometallurgy 2008, 92, 11–15. [Google Scholar] [CrossRef]
- Valdebenito-Rolack, E.; Ruiz-Tagle, N.; Abarzúa, L.; Aroca, G.; Urrutia, H. Characterization of a hyperthermophilic sulphur-oxidizing biofilm produced by archaea isolated from a hot spring. Electron. J. Biotechnol. 2017, 25, 58–63. [Google Scholar] [CrossRef]
- Morales, M.; Arancibia, J.; Lemus, M.; Silva, J.; Gentina, J.C.; Aroca, G. Bio-oxidation of H2S by Sulfolobus metallicus. Biotechnol. Lett. 2011, 33, 2141–2145. [Google Scholar] [CrossRef]
- Morales, M.; Silva, J.; Morales, P.; Gentina, J.C.; Aroca, G. Biofiltration of hydrogen sulfide by Sulfolobus metallicus at high temperatures. Water Sci. Technol. 2012, 66, 1958–1961. [Google Scholar] [CrossRef]
- Nishihara, A.; Matsuura, K.; Tank, M.; McGlynn, S.E.; Thiel, V.; Haruta, S. Nitrogenase activity in thermophilic chemolithoautotrophic bacteria in the phylum aquificae isolated under nitrogen-fixing conditions from Nakabusa hot springs. Microbes Environ. 2018, 33, 394–401. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, H.; Xu, Z.; Zhao, D.; Zhang, C. Microbial diversity in ultra-high-pressure rocks and fluids from the Chinese continental scientific drilling project in China. Appl. Environ. Microbiol. 2005, 71, 3213–3227. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Koonce, E.; Howells, A.; Havig, J.R.; Jewell, T.; de la Torre, J.R.; Peters, J.W.; Boyd, E.S. Competition for ammonia influences the structure of chemotrophic communities in geothermal springs. Appl. Environ. Microbiol. 2014, 80, 653–661. [Google Scholar] [CrossRef]
- Lübben, M.; Güldenhaupt, J.; Zoltner, M.; Deigweiher, K.; Haebel, P.; Urbanke, C.; Scheidig, A.J. Sulfate acts as phosphate analog on the monomeric catalytic fragment of the CPx-ATPase CopB from Sulfolobus solfataricus. J. Mol. Biol. 2007, 369, 368–385. [Google Scholar] [CrossRef] [PubMed]
- Reimann, J.; Esser, D.; Orell, A.; Amman, F.; Pham, T.K.; Noirel, J.; Lindås, A.C.; Bernander, R.; Wright, P.C.; Siebers, B.; et al. Archaeal signal transduction: Impact of protein phosphatase deletions on cell size, motility, and energy metabolism in Sulfolobus acidocaldarius. Mol. Cell. Proteom. 2013, 12, 3908–3923. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Jiang, Z.; Wang, P.; Qin, Y.L.; Xu, W.; Wang, Y.; Liu, S.J.; Jiang, C.Y. Physiology, taxonomy, and sulfur metabolism of the Sulfolobales, an order of thermoacidophilic archaea. Front. Microbiol. 2021, 12, 768283. [Google Scholar] [CrossRef]
- Counts, J.A.; Vitko, N.P.; Kelly, R.M. Genome sequences of five type strain members of the archaeal family Sulfolobaceae, Acidianus ambivalens, Acidianus infernus, Stygiolobus azoricus, Sulfuracidifex metallicus, and Sulfurisphaera ohwakuensis. Microbiol. Resour. Announc. 2020, 9, e01490-19. [Google Scholar] [CrossRef]
- Page, S.C.; Immormino, R.M.; Miller, T.H.; Bourret, R.B. Experimental analysis of functional variation within protein families: Receiver domain autodephosphorylation kinetics. J. Bacteriol. 2016, 198, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Rastädter, K.; Wurm, D.J.; Spadiut, O.; Quehenberger, J. Physiological characterization of Sulfolobus acidocaldarius in a controlled bioreactor environment. Int. J. Environ. Res. Public Health 2021, 18, 5532. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Campbell, K.; Pereira, R.; Björkeroth, J.; Qi, Q.; Vorontsov, E.; Sihlbom, C.; Nielsen, J. Nitrogen limitation reveals large reserves in metabolic and translational capacities of yeast. Nat. Commun. 2020, 11, 1881. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Marinari, E.; De Martino, A. A yield-cost tradeoff governs Escherichia coli’s decision between fermentation and respiration in carbon-limited growth. NPJ Syst. Biol. Appl. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Čapek, P.; Kotas, P.; Manzoni, S.; Šantrůčková, H. Drivers of phosphorus limitation across soil microbial communities. Funct. Ecol. 2016, 30, 1705–1713. [Google Scholar] [CrossRef]
- Li, S.H.J.; Li, Z.; Park, J.O.; King, C.G.; Rabinowitz, J.D.; Wingreen, N.S.; Gitai, Z. Escherichia coli translation strategies differ across carbon, nitrogen and phosphorus limitation conditions. Nat. Microbiol. 2018, 3, 939–947. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Y.; Zhang, L.; Lu, W. Transcriptome analysis reveals the molecular mechanism of Saccharomyces cerevisiae in response to nitrogen limitation. Microb. Cell Factories 2021, 20, 142. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, Y.; Chen, Y. Metabolomics analysis reveals global acetoin stress response of Bacillus licheniformis. Metabolomics 2019, 15, 25. [Google Scholar] [CrossRef]
- Salo-Zieman, V.; Sivonen, T.; Plumb, J.; Haddad, C.; Laukkanen, K.; Kinnunen, P.; Kaksonen, A.; Franzmann, P.; Puhakka, J. Characterization of a thermophilic sulfur oxidizing enrichment culture dominated by a Sulfolobus sp. obtained from an underground hot spring for use in extreme bioleaching conditions. J. Ind. Microbiol. Biotechnol. 2006, 33, 984–994. [Google Scholar] [CrossRef]
- Norris, P.R.; Burton, N.P.; Clark, D.A. Mineral sulfide concentrate leaching in high temperature bioreactors. Miner. Eng. 2013, 48, 10–19. [Google Scholar] [CrossRef]
- Oyarzún, P.; Arancibia, F.; Canales, C.; Aroca, G.E. Biofiltration of high concentration of hydrogen sulphide using Thiobacillus thioparus. Process Biochem. 2003, 39, 165–170. [Google Scholar] [CrossRef]
- Kaplan, A.; Chaney, A.L.; Lynch, R.L.; Meites, S. Urea nitrogen and urinary ammonia. Stand. Methods Clin. Chem. 1965, 5, 245–256. [Google Scholar] [CrossRef]
- Wei, F.S. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Water Works Association (AWWA, WEF and APHA): Denver, CO, USA, 2017; ISBN-13 978-0875532875. [Google Scholar]
- Kelly, D.P.; Chambers, L.A.; Trudinger, P.A. Cyanolysis and spectrophotometric estimation of trithionate in mixture with thiosulfate and tetrathionate. Anal Chem. 1969, 41, 898–901. [Google Scholar] [CrossRef]
- Bartlett, J.K.; Skoog, D.A. Colorimetric determination of elemental sulfur in hydrocarbons. Anal. Chem. 1954, 26, 1008–1011. [Google Scholar] [CrossRef]
- Banerjee, R.; Chaudhari, N.M.; Lahiri, A.; Gautam, A.; Bhowmik, D.; Dutta, C.; Chattopadhyay, S.; Huson, D.H.; Paul, S. Interplay of various evolutionary modes in genome diversification and adaptive evolution of the family Sulfolobaceae. Front. Microbiol. 2021, 12, 639995. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ulloa, A.; Sánchez-Andrea, I.; Buisman, C.; Weijma, J. Sulfur reduction at hyperthermoacidophilic conditions with mesophilic anaerobic sludge as the inoculum. Environ. Sci. Technol. 2020, 54, 14656–14663. [Google Scholar] [CrossRef]
- Yao, S.; Li, S.; Zhan, Y.; Wan, C. Proteome-wide analysis of stress response to temperature in Sulfolobus Isl. J. Proteom. 2022, 266, 104681. [Google Scholar] [CrossRef]
- Counts, J.A.; Willard, D.J.; Kelly, R.M. Life in hot acid: A genome-based reassessment of the archaeal order Sulfolobales. Environ. Microbiol. 2021, 23, 3568–3584. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Do, P.T.; Pham, Y.B.; Doan, T.O.; Nguyen, X.C.; Lee, W.K.; Nguyen, D.D.; Vadiveloo, A.; Um, M.-J.; Ngo, H.H. Roles, mechanism of action, and potential applications of sulfur-oxidizing bacteria for environmental bioremediation. Sci. Total Environ. 2022, 852, 158203. [Google Scholar] [CrossRef]
- Zeldes, B.M.; Loder, A.J.; Counts, J.A.; Haque, M.; Widney, K.A.; Keller, L.M.; Albers, S.-V.; Kelly, R.M. Determinants of sulphur chemolithoautotrophy in the extremely thermoacidophilic Sulfolobales. Environ. Microbiol. 2019, 21, 3696–3710. [Google Scholar] [CrossRef]
- Nemati, M.; Lowenadler, J.; Harrison, S. Particle size effects in bioleaching of pyrite by acidophilic thermophile Sulfolobus metallicus (BC). Appl. Microbial. Biotechnol. 2000, 53, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ford, B.A.; Sullivan, G.J.; Moore, L.; Varkey, D.; Zhu, H.; Ostrowski, M.; Mabbutt, B.C.; Paulsen, I.T.; Shah, B.S. Functional characterisation of substrate-binding proteins to address nutrient uptake in marine picocyanobacteria. Biochem. Soc. Trans. 2021, 49, 2465–2481. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.; Singh, S.; Kaushik, M.S.; Mishra, A.K. Regulation of organophosphate metabolism in cyanobacteria. A review. Microbiology 2015, 84, 291–302. [Google Scholar] [CrossRef]
- Nandy, P. The role of sigma factor competition in bacterial adaptation under prolonged starvation. Microbiology 2022, 168, 001195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Q.; Li, Y. A review on sulfur transformation during anaerobic digestion of organic solid waste: Mechanisms, influencing factors and resource recovery. Sci. Total Environ. 2023, 865, 161193. [Google Scholar] [CrossRef] [PubMed]




| Ammonia in the Culture Medium | |||
|---|---|---|---|
| Couple | 0.4–0.2 g·L−1 | 0.2–0 g·L−1 | 0.4–0 g·L−1 |
| F | 1.53 | 3.16 | 2.08 |
| F-critic | 2.04 | 1.97 | 1.98 |
| p-value (F) | 0.16 | 0.01 | 0.04 |
| Statistic t | −1.10 | 2.11 | 0.98 |
| t-critic | 2.01 | 2.03 | 2.02 |
| p-value (t-test) | 0.28 | 0.04 | 0.33 |
| Ammonia in the Culture Medium | |||
|---|---|---|---|
| Growth Parameter | 0.4 g·L−1 | 0.2 g·L−1 | 0 g·L−1 |
| µ [h−1] | 0.068 ± 0.012 | 0.052 ± 0.011 | 0.048 ± 0.007 |
| YX/S [gcel·gS−1] * | 0.221 ± 0.005 | 0.161 ± 0.006 | 0.102 ± 0.009 |
| YX/S 1 [gcel·gS−1] ** | 0.191 ± 0.009 | 0.092 ± 0.004 | 0.070 ± 0.006 |
| Phosphorus in the Culture Medium | |||
|---|---|---|---|
| Couple | 0.2–0.1 g·L−1 | 0.1–0 g·L−1 | 0.2–0 g·L−1 |
| F | 1.80 | 1.61 | 2.89 |
| F-critic | 2.04 | 2.05 | 2.05 |
| p-value (F-test) | 0.09 | 0.14 | 0.01 |
| Statistic t | 0.24 | 0.10 | 0.34 |
| t critic | 2.01 | 2.02 | 2.03 |
| p-value (t-test) | 0.81 | 0.92 | 0.73 |
| Phosphate in the Culture Medium | |||
|---|---|---|---|
| Growth Parameter | 0.2 g·L−1 | 0.1 g·L−1 | 0 g·L−1 |
| µ1 [h−1] | 0.052 ± 0.016 | 0.035 ± 0.002 | 0.017 ± 0.004 |
| YX/S [gcel·gS−1] * | 0.221 ± 0.013 | 0.38 ± 0.005 | 0.072 ± 0.012 |
| YX/S 1 [gcel·gS−1] ** | 0.196 ± 0.012 | 0.30 ± 0.012 | 0.059 ± 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.; Ortiz-Soto, R.; Morales, M.; Aroca, G. Effect of the Availability of the Source of Nitrogen and Phosphorus in the Bio-Oxidation of H2S by Sulfolobus metallicus. Fermentation 2023, 9, 406. https://doi.org/10.3390/fermentation9050406
Silva J, Ortiz-Soto R, Morales M, Aroca G. Effect of the Availability of the Source of Nitrogen and Phosphorus in the Bio-Oxidation of H2S by Sulfolobus metallicus. Fermentation. 2023; 9(5):406. https://doi.org/10.3390/fermentation9050406
Chicago/Turabian StyleSilva, Javier, Rodrigo Ortiz-Soto, Marjorie Morales, and Germán Aroca. 2023. "Effect of the Availability of the Source of Nitrogen and Phosphorus in the Bio-Oxidation of H2S by Sulfolobus metallicus" Fermentation 9, no. 5: 406. https://doi.org/10.3390/fermentation9050406
APA StyleSilva, J., Ortiz-Soto, R., Morales, M., & Aroca, G. (2023). Effect of the Availability of the Source of Nitrogen and Phosphorus in the Bio-Oxidation of H2S by Sulfolobus metallicus. Fermentation, 9(5), 406. https://doi.org/10.3390/fermentation9050406







