Optimization of the Operational Conditions to Produce Extracellular and Cell-Bound Biosurfactants by Aneurinibacillus aneurinilyticus Using Corn Steep Liquor as a Unique Source of Nutrients
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strain and Inoculum Preparation
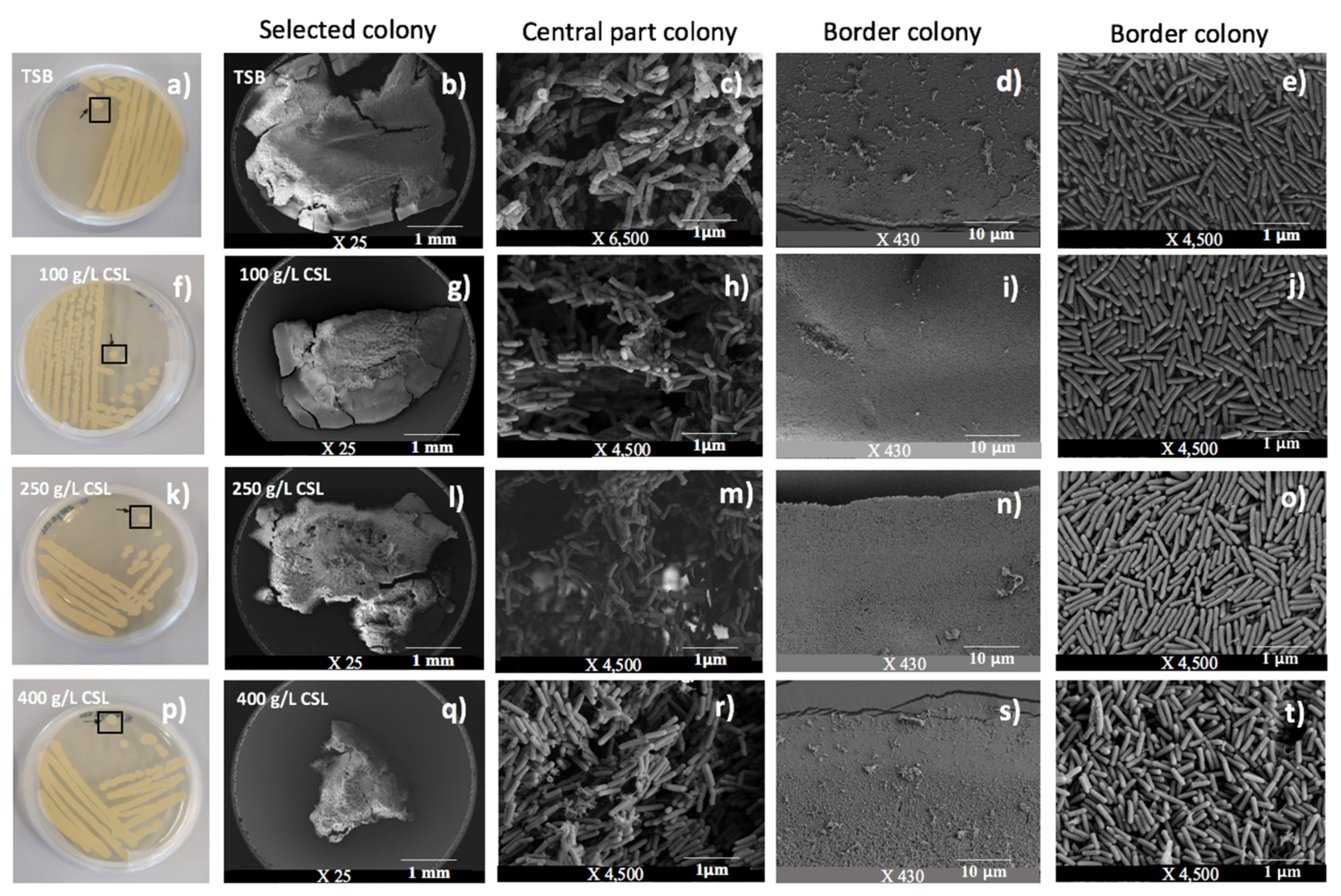
2.2. Phenotypic Characterization of A. aneurinilyticus after Fermentation
2.3. Preparation of CSL Medium and Fermentation
2.4. Experimental Design
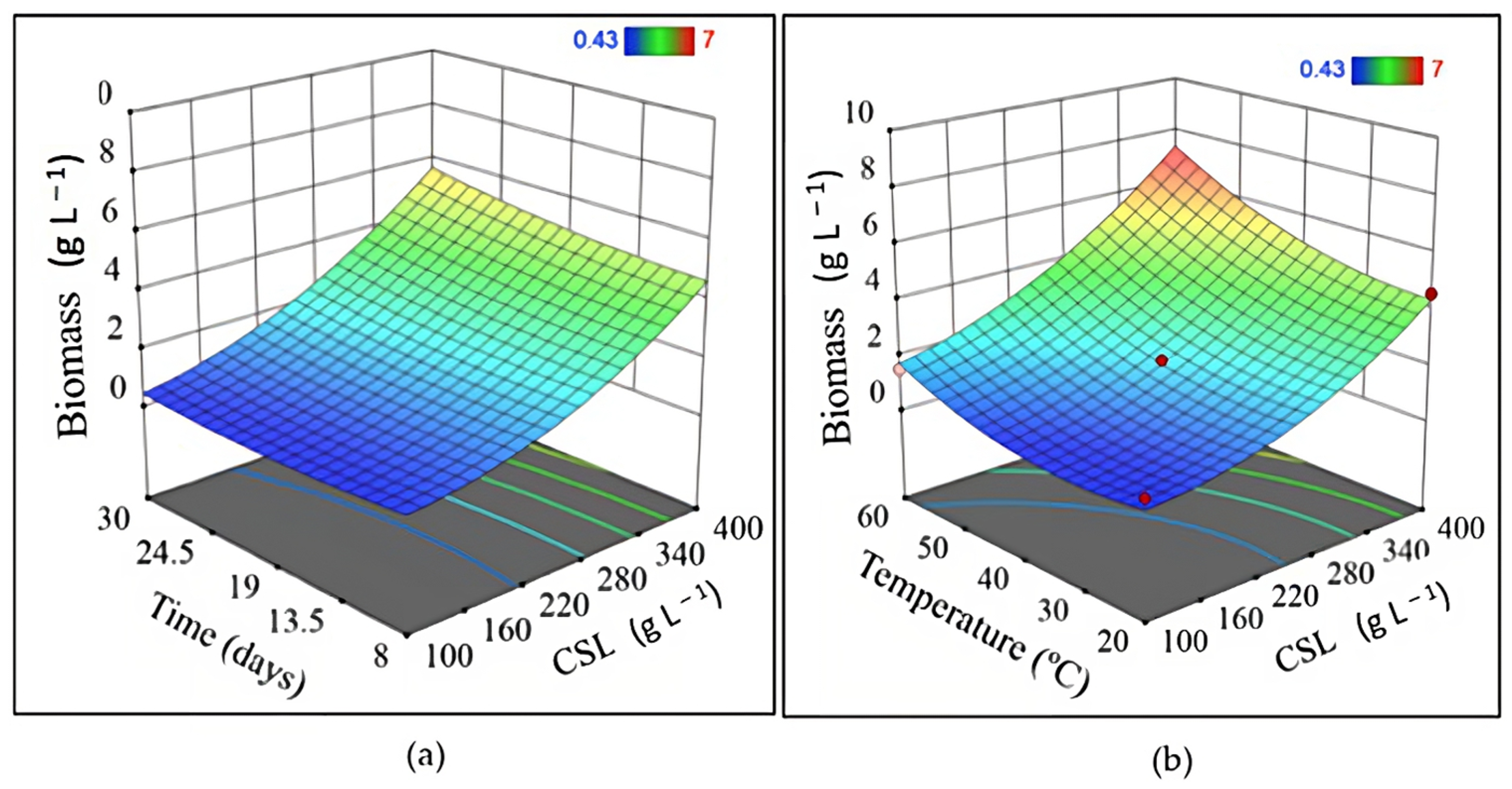
2.5. Quantification of Biomass
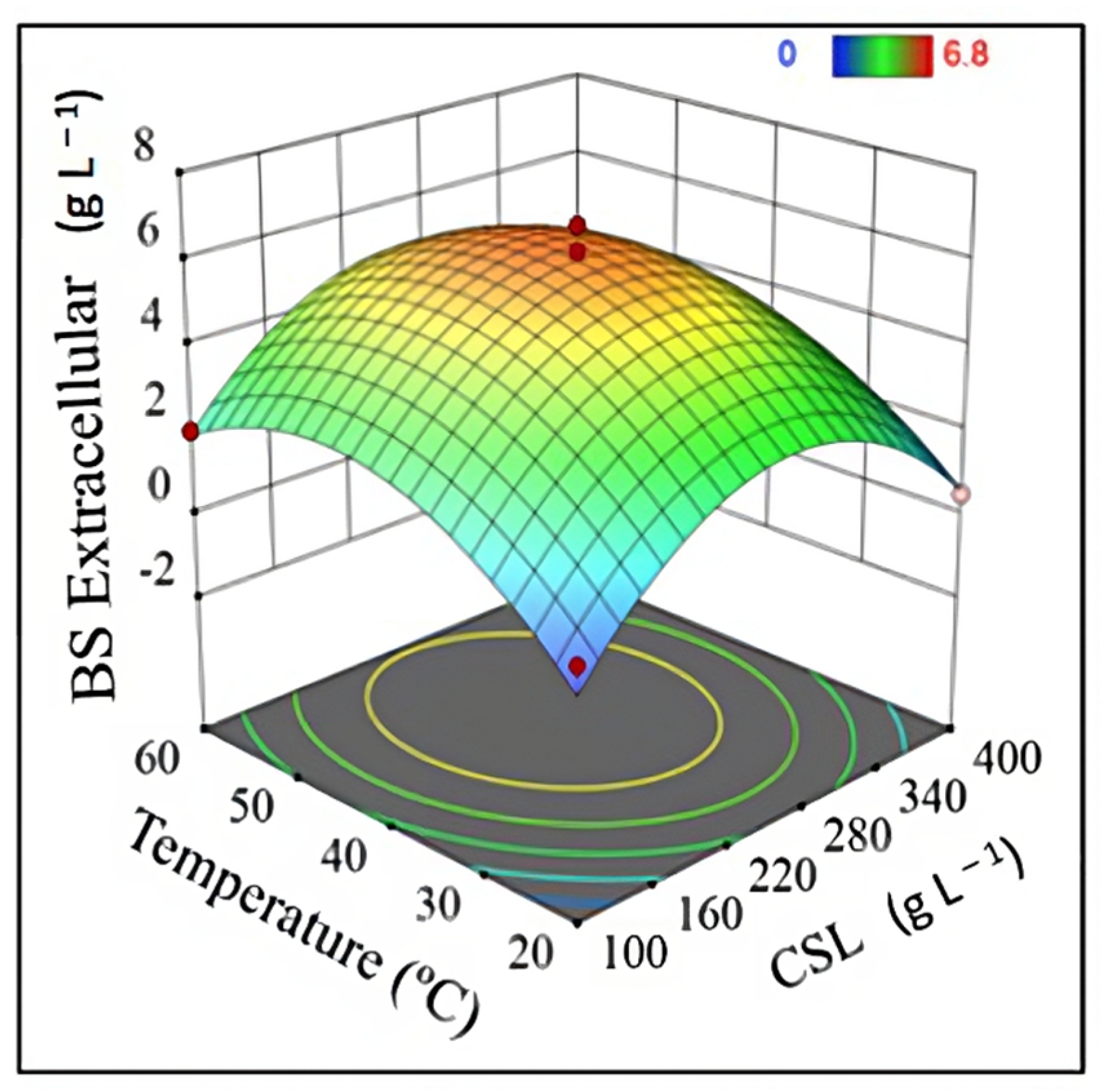
2.6. Quantification of Extracellular Biosurfactants
2.7. Extraction and Analysis of Cell-Bound Biosurfactants
2.8. Determination of ST and CMC
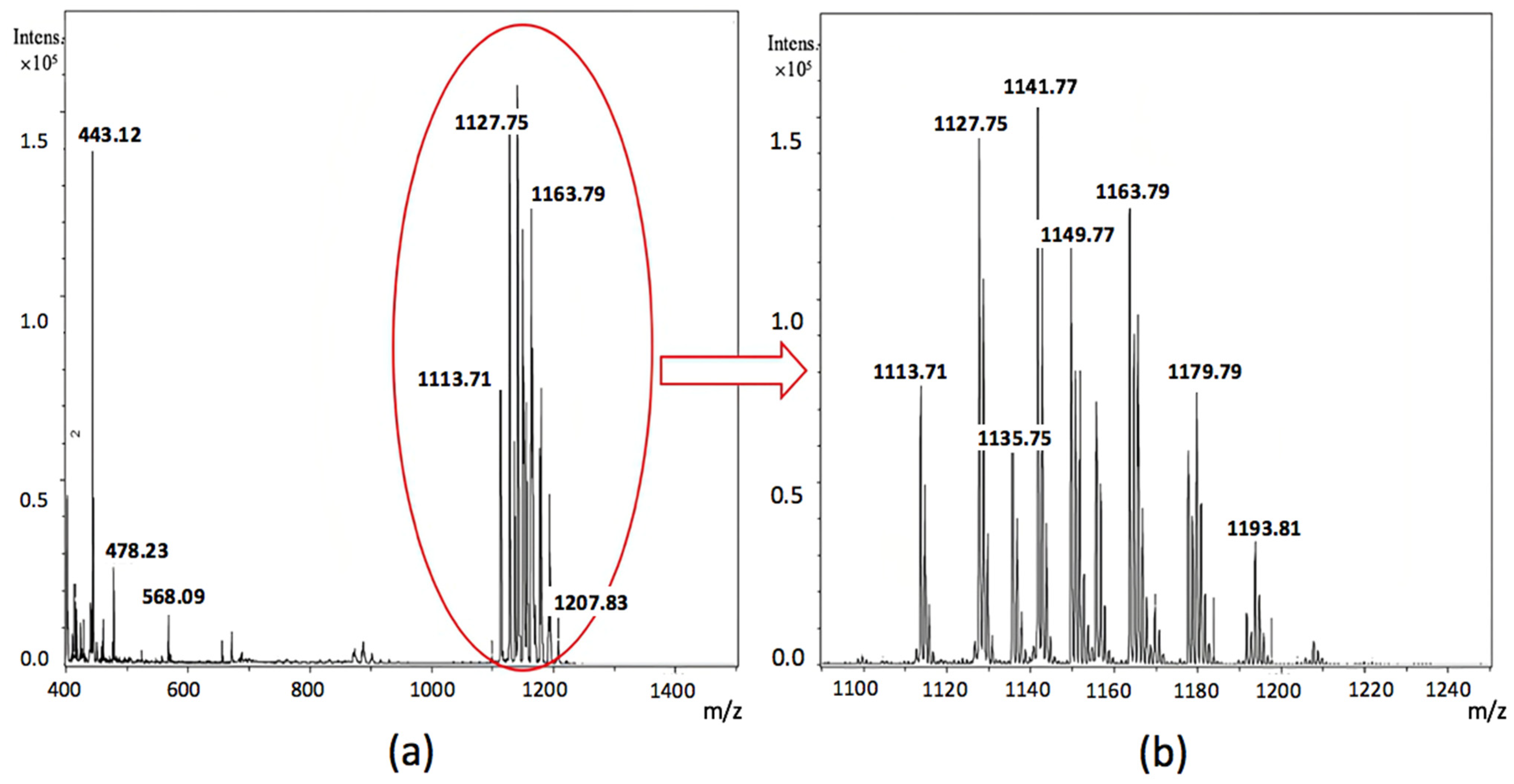
2.9. Analysis of Cell-Bound Biosurfactans by Matrix Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry
2.10. Elemental Analysis of Cell-Bound Biosurfactant Extract
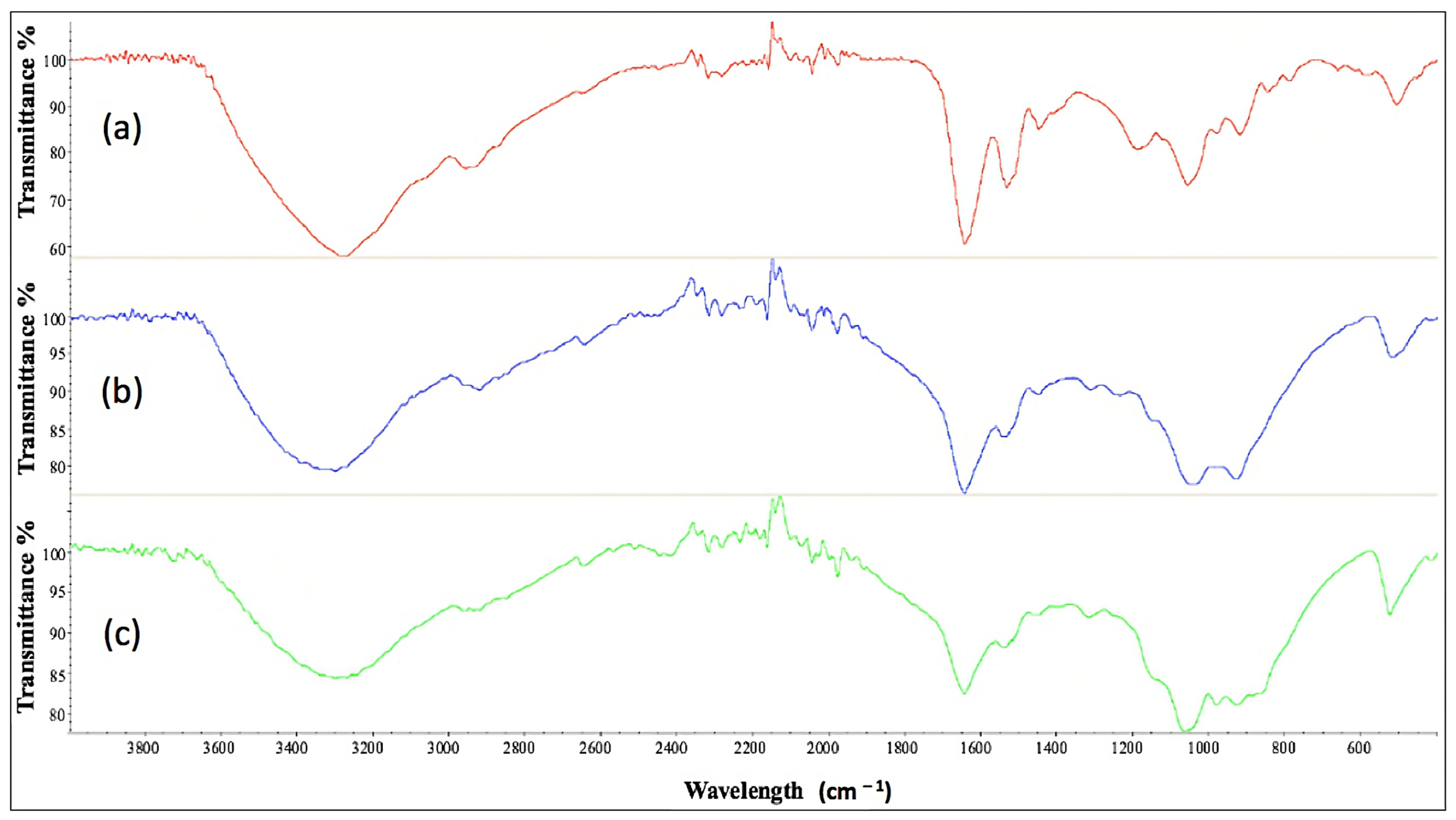
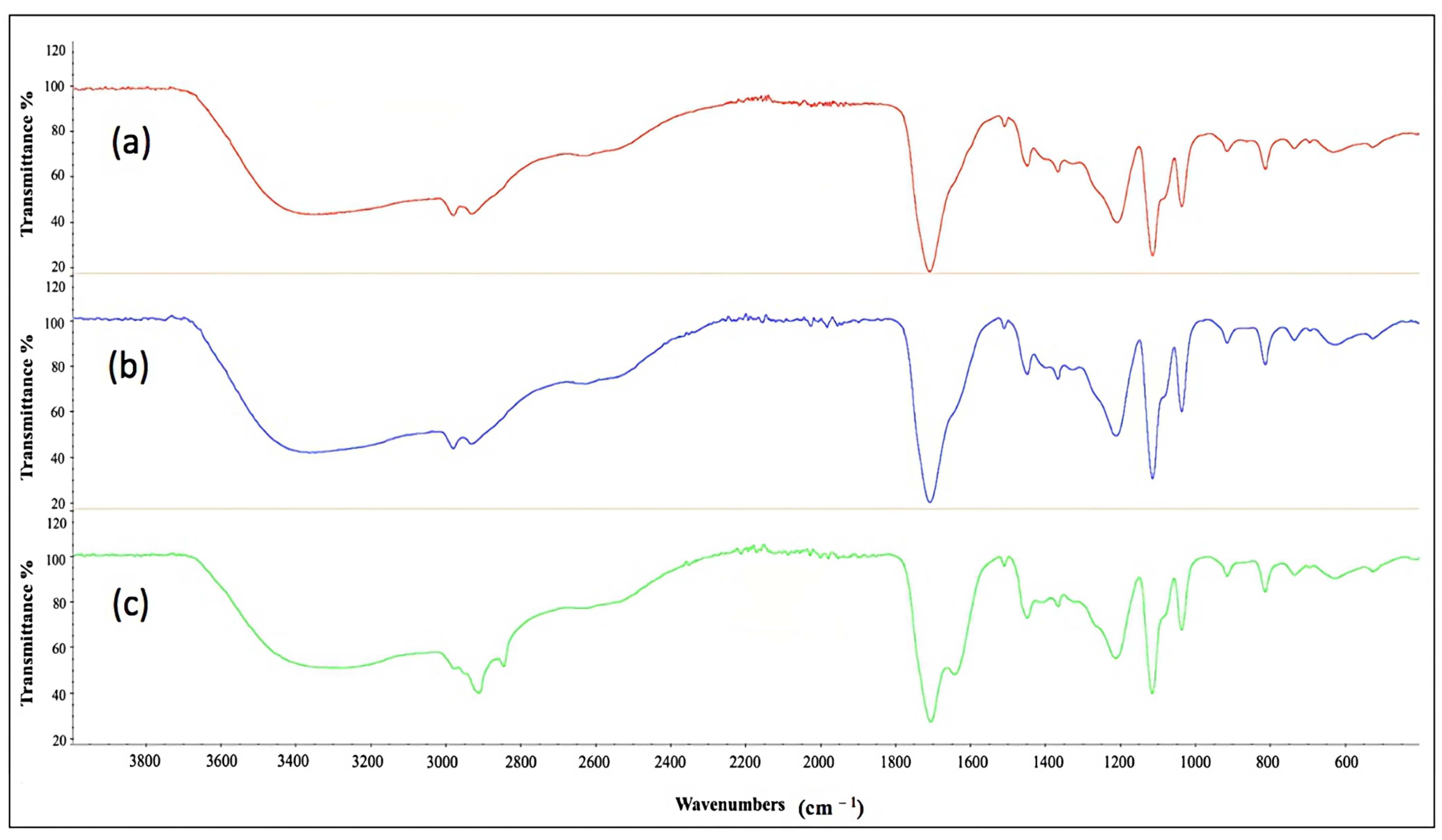
2.11. Characterization of Cell-Bound Biosurfactants from CSL and TSB Medium by Fourier Transform Infrared Spectroscopy
2.12. Statistical Treatment Data
2.13. Production of Biosurfactant Extracts from Different Brands of CSL
3. Results and Discussion
3.1. Evaluation of A. aneurinilyticus Capacity to Produce Biosurfactants
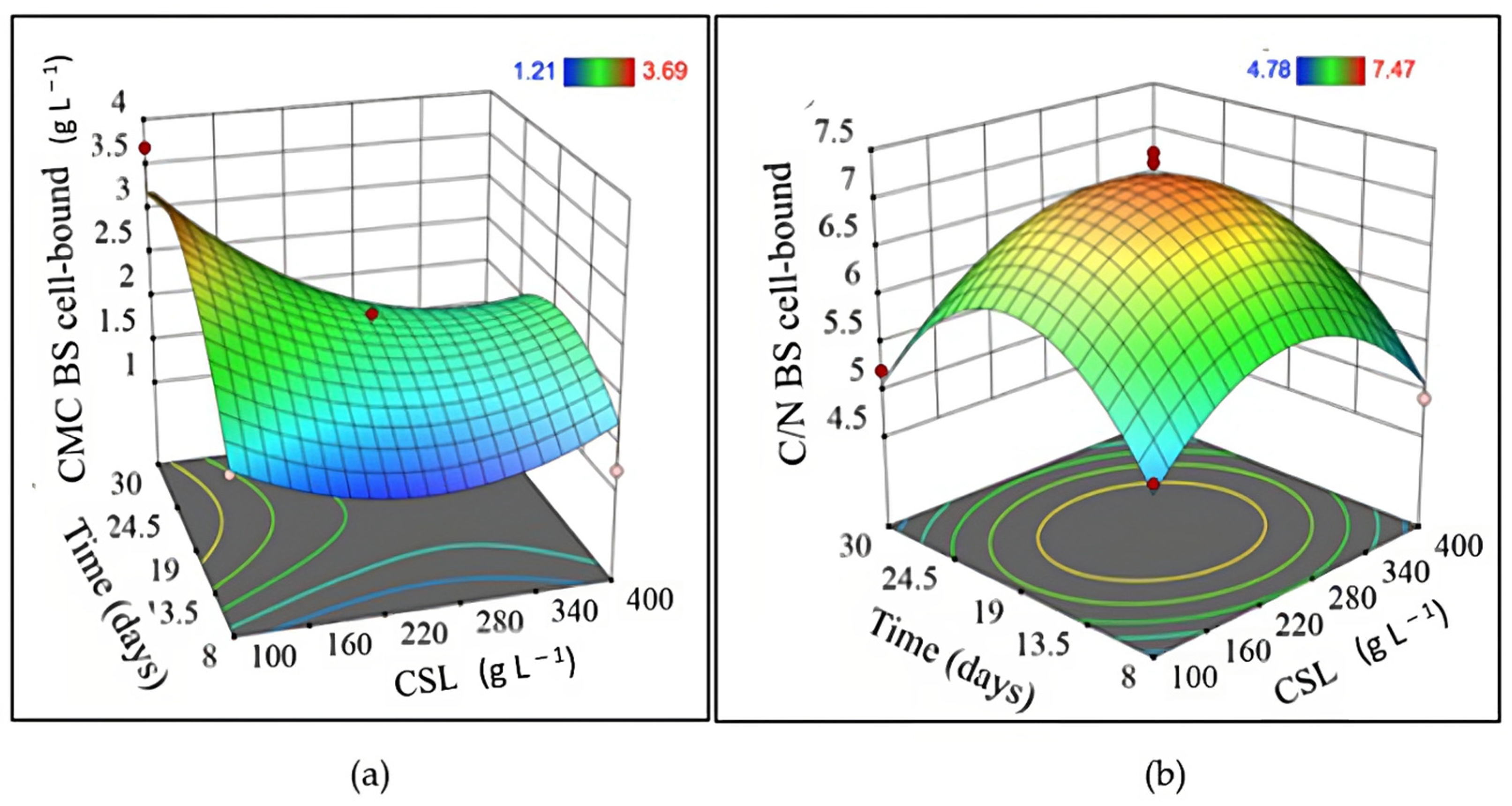
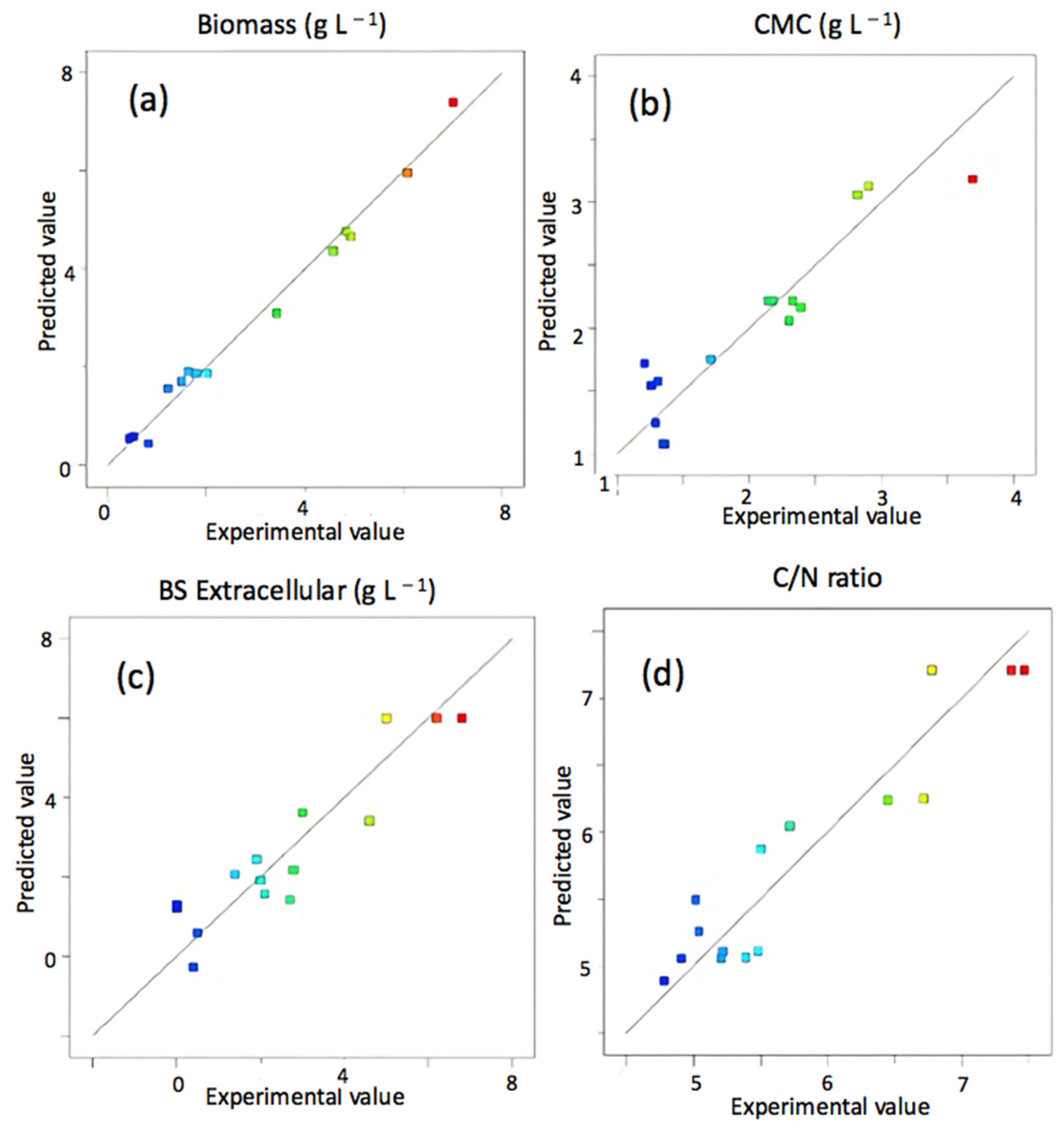
3.2. CSL as a Unique Source of Nutrients in Fermentation Processes
3.3. Reproducibility of CSL as a Source for Biosurfactant Production
3.4. Phenotypic Characterization of A. aneurinilyticus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.K.; Nakhla, G.; Yanful, E.K. Biokinetics of biodegradation of surfactants under aerobic, anoxic and anaerobic conditions. Water Res. 2006, 40, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N. Recent advances in the environmental applications of biosurfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 372–378. [Google Scholar] [CrossRef]
- Das, K.; Mukherjee, A.K. Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid state fermentation systems using a cheap carbon source: Some industrial applications of biosurfactants. Process Biochem. 2007, 42, 1191–1199. [Google Scholar] [CrossRef]
- Rufino, R.D.; Porto, A.; Sarubbo, L.; Sobrinho, H.B.S.; Luna, J.M.; Lúcia, A. Biosurfactants: Classification, properties and environmental applications. Biotechnology 2014, 11, 1–29. [Google Scholar]
- Radzuan, M.N.; Banat, I.M.; Winterburn, J. Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Bioresour. Technol. 2017, 225, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019, 126, 2–13. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S. Utilization of molasses for biosurfactant production by two Bacillus strains at thermophilic conditions. J. Am. Oil Chem. Soc. 1997, 74, 887–889. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S. An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl. Microbiol. Biotechnol. 2002, 58, 428–434. [Google Scholar]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar]
- Thompson, D.N.; Fox, S.L.; Bala, G.A. Biosurfactants from potato process effluents. Appl. Biochem. Biotechnol. 2000, 84–86, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Roukas, T.; Biliaderis, C.G.; Vaikousi, H. Characterization of pullulan produced from beet molasses by Aureobasidium pullulans in a stirred tank reactor under varying agitation. Enzym. Microb. Technol. 2002, 31, 122–132. [Google Scholar] [CrossRef]
- Sobrinho, H.B.S.; Rufino, R.D.; Luna, J.M.; Salgueiro, A.A.; Leite, F.C.; Sarubbo, L.A.; Campos-takaki, G.M. Utilization of two agroindustrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochem. 2008, 43, 912–917. [Google Scholar] [CrossRef]
- Joshi, S.; Bharucha, C.; Jha, S.; Yadav, S.; Nerurkar, A.; Desai, A.J. Biosurfactant production using molasses and whey under thermophilic conditions. Bioresour. Technol. 2008, 99, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.S. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Factories 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Kumar, A.P.; Janardhan, A.; Viswanath, B.; Monika, K.; Jung, J.Y.; Narasimha, G. Evaluation of orange peel for biosurfactant production by Bacillus licheniformis and their ability to degrade naphthalene and crude oil. 3 Biotech 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, J.; Sengupta, B. Production of surfactin from rice mill polishing residue by submerged fermentation using Bacillus subtilis MTCC 2423. Bioresour. Technol. 2015, 189, 243–249. [Google Scholar] [CrossRef]
- Vecino, X.; Barbosa-Pereira, L.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Study of the surfactant properties of aqueous stream from the corn milling industry. J. Agric. Food Chem. 2014, 62, 5451–5457. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A. Ionic behavior assessment of surface-active compounds from corn steep liquor by exchange resins. J. Surfactants Deterg. 2017, 20, 207–217. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; López-Prieto, A.; Lopez-Álvarez, M.; Pérez-Davila, S.; Serra, J.; González, P. Characterization and cytotoxic effect of biosurfactants obtained from different sources. ACS Omega 2020, 5, 31381–31390. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Extraction, separation and characterization of lipopeptides and phospholipids from corn steep water. Sep. Purif. Technol. 2020, 248, 117076. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.K.; Rathore, D. Analysis of biosurfactants produced by bacteria growing on textile sludge and their toxicity evaluation for environmental application. J. Dispers. Sci. Technol. 2020, 41, 510–522. [Google Scholar] [CrossRef]
- López-Prieto, A.; Rodríguez-López, L.; Rincón-Fontán, M.; Cruz, J.M.; Moldes, A.B. Characterization of extracellular and cell-bound biosurfactants produced by Aneurinibacillus aneurinilyticus isolated from commercial corn steep liquor. Microbiol. Res. 2021, 242, 126614. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Dhillon, O. Characterization, screening, and application of bacteria with probiotic properties isolated from the gut of Labeo calbasu (Hamilton). Arch. Pol. Fish. 2019, 27, 178–189. [Google Scholar] [CrossRef]
- Fernand, S.C. Use of Probiotic Bacterial Strains and Cell Extracts to Inhibit Acidosis and Liver Abscesses in Cattle. U.S. Patent Application No. 16/344,968, 5 September 2019. [Google Scholar]
- Alenezi, F.N.; Rekik, I.; Bouket, A.C.; Luptakova, L.; Weitz, H.J.; Rateb, M.E. Increased biological activity of Aneurinibacillus migulanus strains correlates with the production of new gramicidin secondary metabolites. Front. Microbiol. 2017, 8, 517. [Google Scholar] [CrossRef]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: Purification, characterization and its biological evaluation. Microbiol. Res. 2017, 194, 1–9. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, J.; Ma, Y.; Cai, L.; Zheng, L.; Gong, W.; Liu, Q.-A. Corn steep liquor: Green biological resources for bioindustry. Appl. Biochem. Biotechnol. 2022, 194, 3280–3295. [Google Scholar] [CrossRef]
- Hull, S.R.; Yang, B.Y.; Venzke, D.; Kulhavy, K.; Montgomery, R. Composition of corn steep water during steeping. J. Agric. Food Chem. 1996, 44, 1857–1863. [Google Scholar] [CrossRef]
- Martínez-Arcos, A.; Moldes, A.B.; Vecino, X. Adding value to secondary streams of corn wet milling industry. CYTA-J. Food 2021, 19, 675–681. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour. Technol. 2017, 232, 389–397. [Google Scholar] [CrossRef]
- López-Prieto, A.; Martínez-Padrón, H.; Rodríguez-López, L.; Moldes, A.B.; Cruz, J.M. Isolation and characterization of a microorganism that produces biosurfactants in corn steep water. CYTA-J. Food 2019, 17, 509–516. [Google Scholar] [CrossRef]
- Bustos, G.; Arcos, U.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Recycled Lactobacillus pentosus biomass can regenerate biosurfactants after various fermentative and extractive cycles. Biochem. Eng. J. 2018, 132, 191–195. [Google Scholar] [CrossRef]
- López-Prieto, A.; Vecino, X.; Rodríguez-López, L.; Moldes, A.B.; Cruz, J.M. A multifunctional biosurfactant extract obtained from corn steep water as bactericide for agrifood industry. Foods 2019, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Rincón-Fontán, M.; Rodríguez-López, L.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Influence of micelle formation on the adsorption capacity of a biosurfactant extracted from corn on dyed hair. RSC Adv. 2017, 7, 16444–16452. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2019, 48, 177–184. [Google Scholar] [CrossRef]
- López-Prieto, A.; Moldes, A.B.; Cruz, J.M.; Pérez Cid, B. Towards more ecofriendly pesticides: Use of biosurfactants obtained from the corn milling industry as solubilizing agent of copper oxychloride. J. Surfactants Deterg. 2020, 23, 1055–1066. [Google Scholar] [CrossRef]
- Dong, H.; Zheng, A.; He, Y.; Wang, X.; Li, Y.; Yu, G.; Gu, Y.; Banat, I.M.; Sun, S.; She, Y.; et al. Optimization and characterization of biosurfactant produced by indigenous: Brevibacillus borstelensis isolated from a low permeability reservoir for application in MEOR. RSC Adv. 2022, 12, 2036–2047. [Google Scholar] [CrossRef]
- Goyal, S.; Singh, J. Bioprocess optimization for glycopeptide biosurfactant production by means of Lactobacillus delbrueckii: Design expert laden approach. J. Food Process. Preserv. 2022, e17195. [Google Scholar] [CrossRef]
- Liu, J.H.; Chen, Y.T.; Li, H.; Jia, Y.P.; Xu, R.D.; Wang, J. Optimization of fermentation conditions for biosurfactant production by Bacillus subtilis strains CCTCC M201162 from oilfield wastewater. Environ. Prog. Sustain. Energy 2015, 34, ep12013. [Google Scholar]
- Mnif, I.; Ellouze-Chaabouni, S.; Ghribi, D. Optimization of inocula conditions for enhanced biosurfactant production by Bacillus subtilis SPB1, in submerged culture, using Box-Behnken design. Probiotics Antimicrob. Proteins 2013, 5, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.H.; Wang, L.C.; Chen, W.C.; Chen, S.Y. Production and characterization of fengycin by indigenous Bacillus Subtilis F29-3 originating from a potato farm. Int. J. Mol. Sci. 2010, 11, 426–4538. [Google Scholar] [CrossRef]
- Freitas de Oliveira, D.W.; Lima França, Í.W.; Nogueira Félix, A.K.; Lima Martins, J.J.; Aparecida Giro, M.E.; Melo, V.M.M.; Gonçalves, L.R.B. Kinetic study of biosurfactant production by Bacillus subtilis LAMI005 grown in clarified cashew apple juice. Colloids Surf. B Biointerfaces 2013, 101, 34–43. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Peng, H.; Wang, S.; Zhang, J.; Li, X.; Ma, C.; Zhao, X. High-density fermentation of Bacillus subtilis with corn steep liquor as an alternative substrate. J. Agric. Vet. Sci. 2020, 13, 12–17. [Google Scholar]
- Berditsch, M.; Trapp, M.; Afonin, S.; Weber, C.; Misiewicz, J.; Turkson, J.; Ulrich, A.S. Antimicrobial peptide gramicidin S is accumulated in granules of producer cells for storage of bacterial phosphagens. Sci. Rep. 2017, 7, 44324. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Fernandes, E.C.; Rodrigues, A.I.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 2015, 6, 59. [Google Scholar] [PubMed]
- Ramos, P.R.; Kamimura, E.S.; Pires, N.A.M.; Maldonado, R.R.; Oliveira, A.L.D. Esterification reaction in SC-CO2 catalyzed by lipase produced with corn steep liquor and Minas Frescal cheese whey. Bioresour. Technol. Rep. 2021, 14, 100670. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Z.; Li, S.; Zhang, J.; Duan, R. Study on extraction process of corn steep liquor protein by response surface methodology and single cell protein fermentation. Sci. Technol. Food Ind. 2022, 43, 231–237. [Google Scholar]
- Hartmann, D. Pattern formation in cultures of Bacillus subtilis. J. Biol. Syst. 2004, 12, 179–199. [Google Scholar] [CrossRef]
| Factor | Units | Range | Code |
|---|---|---|---|
| Independent variable | |||
| Temperature | °C | 20–60 | x1 |
| CSL concentration | g L−1 | 100–400 | x2 |
| Fermentation time | days | 8–30 | x3 |
| Dependent variable | |||
| Biomass | g L−1 | y1 | |
| Concentration of extracellular biosurfactant | g L−1 | y2 | |
| CMC of cell-bound biosurfactant | g L−1 | y3 | |
| C/N ratio of cell-bound biosurfactant | - | y4 | |
| Media | CMC (g L−1) | FTIR Spectrometer |
|---|---|---|
| TSB (a) | 0.58 | 1071 cm−1; 1537 cm−1; 1634 cm−1; 2934 cm−1; 3275 cm−1 |
| CSL, experiment 12 (b) | 1.29 | 1051 cm−1; 1539 cm−1; 1648 cm−1; 2925 cm−1; 3305 cm−1 |
| CSL, experiment 14 (c) | 2.33 | 1068 cm−1; 1540 cm−1; 1648 cm−1; 2924 cm−1; 3306 cm−1 |
| Experiment | Independent Variable | Dependent Variable | |||||
|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | y1 | y2 | y3 | y4 | |
| 1 | 20 | 250 | 8 | 1.63 | 2.79 | 1.35 | 5.48 |
| 2 | 60 | 250 | 8 | 3.43 | 4.56 | 1.36 | 5.72 |
| 3 | 20 | 250 | 30 | 1.23 | 0.00 | 1.26 | 5.39 |
| 4 | 60 | 250 | 30 | 4.93 | 3.04 | 1.31 | 5.50 |
| 5 | 20 | 100 | 19 | 0.83 | 0.38 | 2.90 | 5.02 |
| 6 | 60 | 100 | 19 | 1.50 | 1.98 | 2.82 | 6.45 |
| 7 | 20 | 400 | 19 | 4.57 | 0.51 | 2.30 | 5.04 |
| 8 | 60 | 400 | 19 | 7.00 | 1.35 | 2.39 | 6.72 |
| 9 | 40 | 100 | 8 | 0.43 | 0.00 | 1.71 | 5.22 |
| 10 | 40 | 100 | 30 | 0.53 | 2.11 | 3.69 | 5.21 |
| 11 | 40 | 400 | 8 | 4.83 | 1.94 | 1.21 | 4.91 |
| 12 | 40 | 400 | 30 | 6.07 | 2.70 | 1.29 | 4.78 |
| 13 | 40 | 250 | 19 | 1.81 | 6.20 | 2.18 | 7.37 |
| 14 | 40 | 250 | 19 | 1.77 | 5.00 | 2.33 | 7.47 |
| 15 | 40 | 250 | 19 | 2.01 | 6.80 | 2.14 | 6.78 |
| Dependent Variable | ||||||||
|---|---|---|---|---|---|---|---|---|
| y1 | Py1 | y2 | Py2 | y3 | Py3 | y4 | Py4 | |
| 1.8633 | 6 | 2.2167 | 7.2067 | |||||
| 2.3975 | <0.0001 * | 0.2500 | 0.6402 | −0.4913 | 0.0296 * | −0.0563 | 0.7731 | |
| 1.0750 | 0.0007 * | 0.9138 | 0.1289 | 0.0088 | 0.9593 | 0.4325 | 0.0664 ** | |
| 0.3050 | 0.0866 ** | −0.1863 | 0.7263 | 0.2400 | 0.2008 | −0.0563 | 0.7731 | |
| 0.4400 | 0.0820 ** | −0.1750 | 0.8154 | 0.0425 | 0.8610 | 0.0625 | 0.8205 | |
| 0.2850 | 0.2187 | −0.3250 | 0.6668 | −0.4750 | 0.0943 ** | −0.0300 | 0.9131 | |
| 0.4750 | 0.0661 ** | 0.2975 | 0.6931 | 0.0100 | 0.9671 | −0.0325 | 0.9059 | |
| 0.8858 | 0.0085 * | −2.9238 | 0.0109 * | 0.5204 | 0.0822 ** | −0.9458 | 0.0177 * | |
| 0.7258 | 0.0184 * | −2.0013 | 0.0426 * | −0.1346 | 0.5990 | −0.4533 | 0.1565 | |
| 0.2158 | 0.3532 | −1.4013 | 0.1169 | −0.7621 | 0.0246 * | −1.2308 | 0.0063 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lvova, K.; Martínez-Arcos, A.; López-Prieto, A.; Vecino, X.; Moldes, A.B.; Cruz, J.M. Optimization of the Operational Conditions to Produce Extracellular and Cell-Bound Biosurfactants by Aneurinibacillus aneurinilyticus Using Corn Steep Liquor as a Unique Source of Nutrients. Fermentation 2023, 9, 351. https://doi.org/10.3390/fermentation9040351
Lvova K, Martínez-Arcos A, López-Prieto A, Vecino X, Moldes AB, Cruz JM. Optimization of the Operational Conditions to Produce Extracellular and Cell-Bound Biosurfactants by Aneurinibacillus aneurinilyticus Using Corn Steep Liquor as a Unique Source of Nutrients. Fermentation. 2023; 9(4):351. https://doi.org/10.3390/fermentation9040351
Chicago/Turabian StyleLvova, Ksenia, Andrea Martínez-Arcos, Alejandro López-Prieto, Xanel Vecino, Ana Belén Moldes, and José Manuel Cruz. 2023. "Optimization of the Operational Conditions to Produce Extracellular and Cell-Bound Biosurfactants by Aneurinibacillus aneurinilyticus Using Corn Steep Liquor as a Unique Source of Nutrients" Fermentation 9, no. 4: 351. https://doi.org/10.3390/fermentation9040351
APA StyleLvova, K., Martínez-Arcos, A., López-Prieto, A., Vecino, X., Moldes, A. B., & Cruz, J. M. (2023). Optimization of the Operational Conditions to Produce Extracellular and Cell-Bound Biosurfactants by Aneurinibacillus aneurinilyticus Using Corn Steep Liquor as a Unique Source of Nutrients. Fermentation, 9(4), 351. https://doi.org/10.3390/fermentation9040351








