The Influence of Flavonoid Dihydroquercetin on the Enzymatic Processes of Dough Ripening and the Antioxidant Properties of Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bread
2.3. Evaluation of the Dihydroquercetin Effect on Fermentation Processes
2.4. Extraction of Bioactive Compounds
2.5. Chemical Composition and Antioxidant Properties of Dough and Breads
2.6. Potential Bioavailability of Dihydroquercetin
2.7. Statistical Analysis
3. Results and Discussion
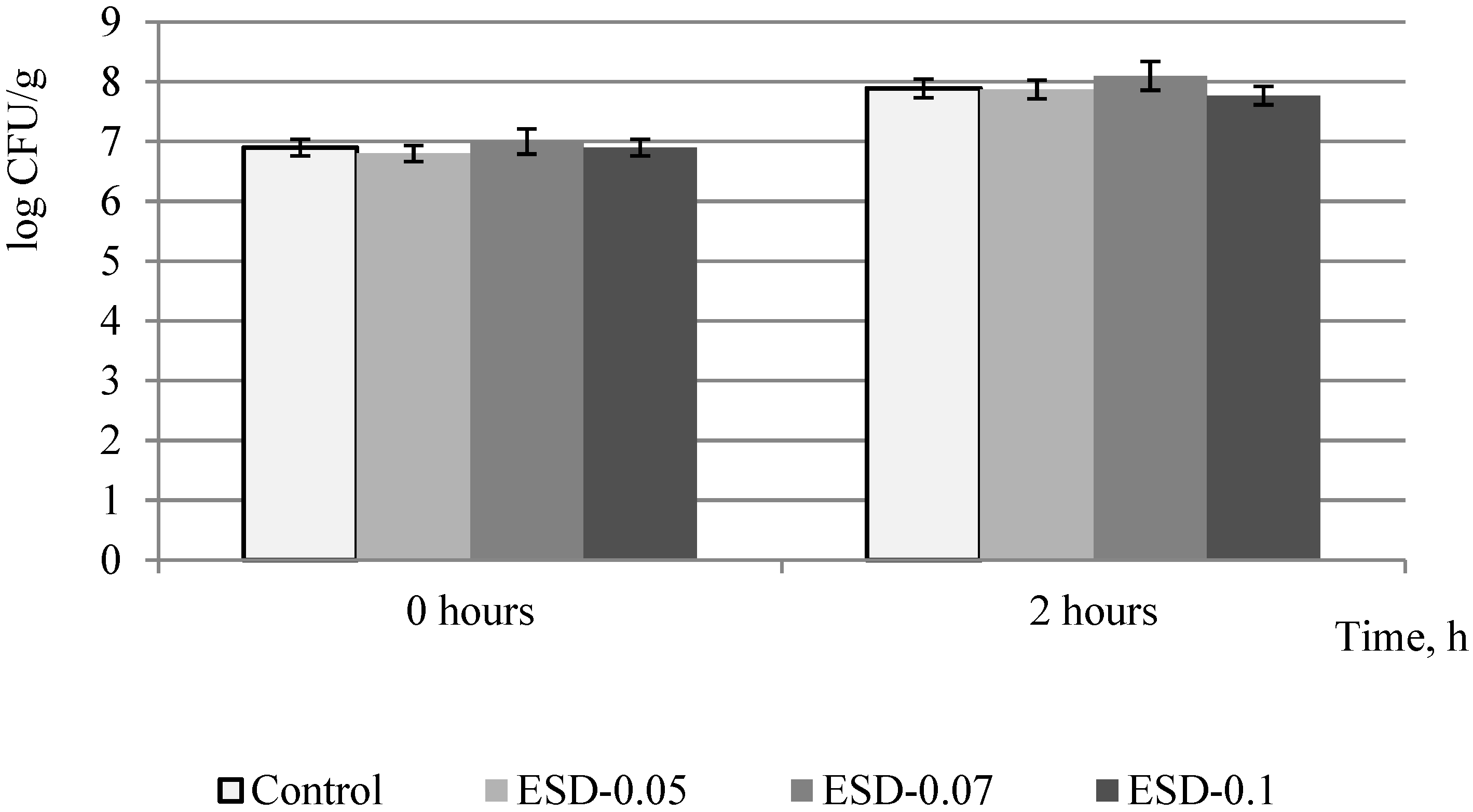
3.1. Evaluation of Dihydroquercetin Effect on Fermentation Processes
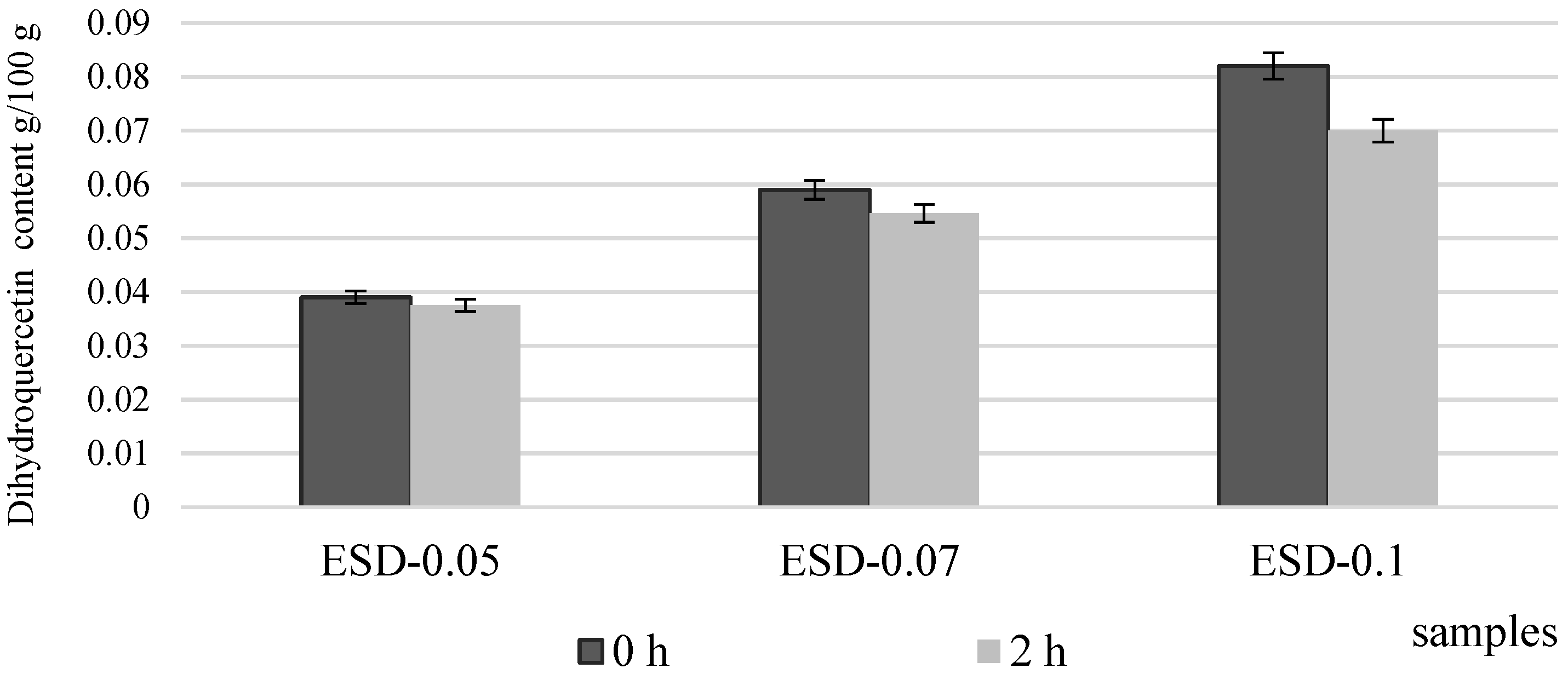
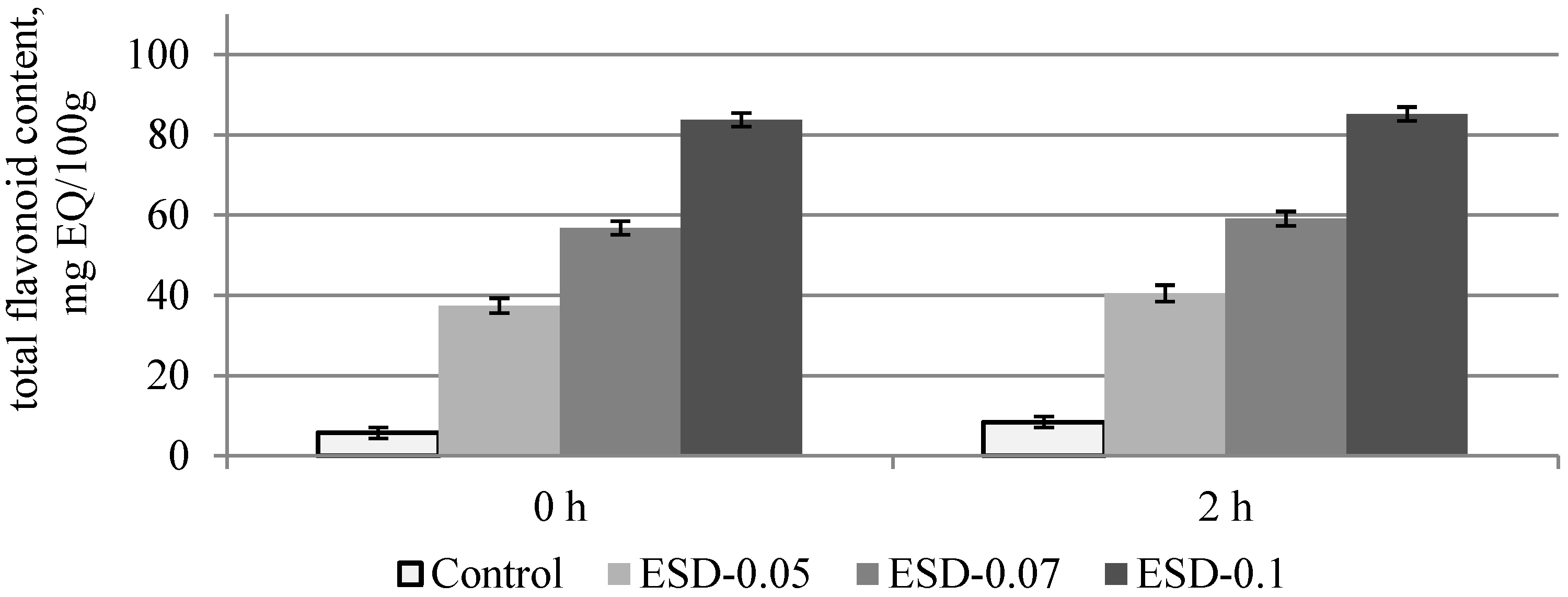
3.2. Chemical Composition and Antioxidant Properties of Dough
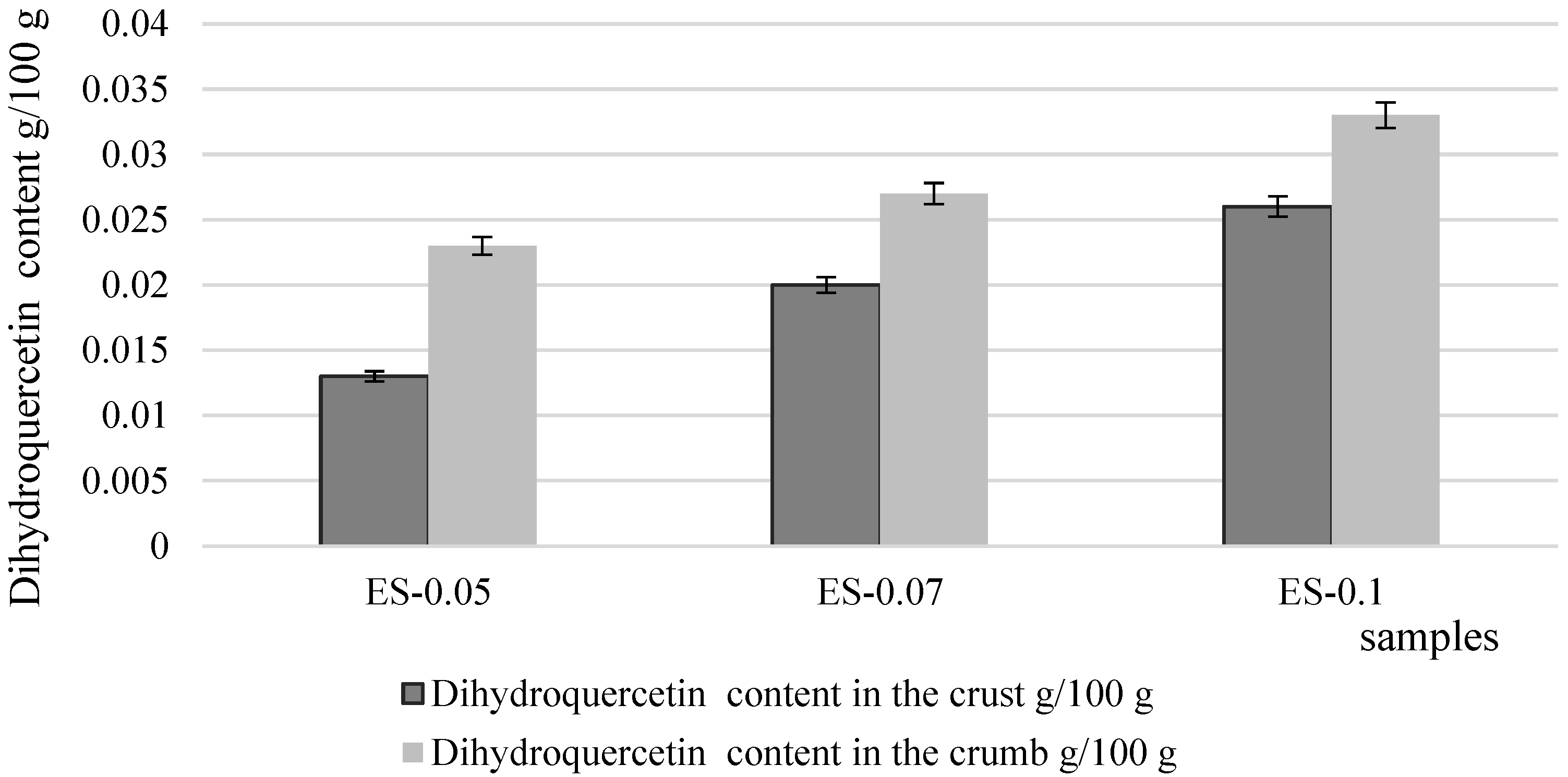
3.3. Chemical Composition and Antioxidant Properties of Bread
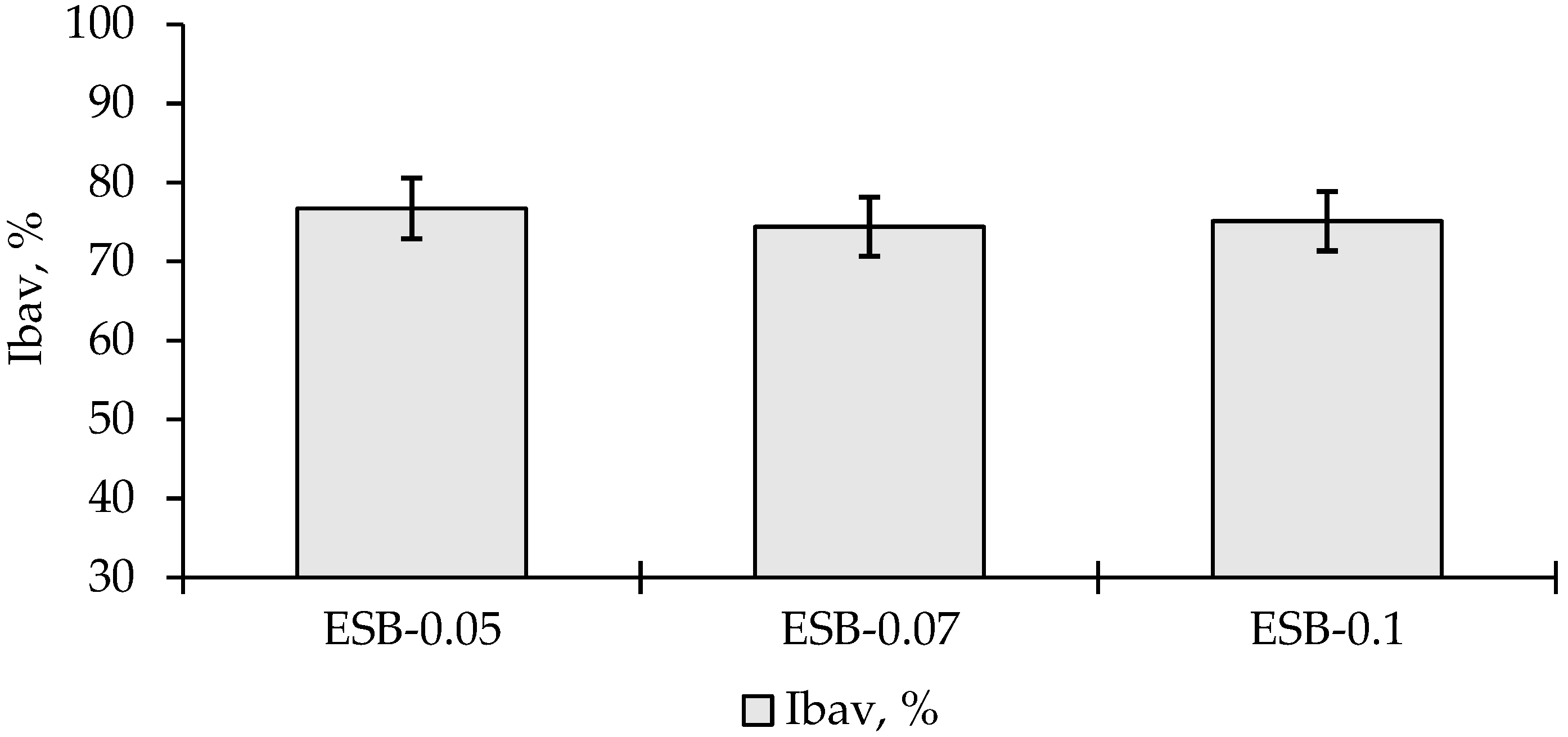
3.4. Potentially Bioaccessible and Bioavailable Dihydroquercetin from Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santetti, G.S.; Dacoreggio, M.V.; Inácio, H.P.; Biduski, B.; Hoff, R.B.; Freire, C.B.F.; Gutkoski, L.C.; Amboni, R.D. The addition of yerba mate leaves on bread dough has influences on fermentation time and the availability of phenolic compounds? LWT 2021, 146, 111442. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, G.; Tilley, M.; Li, Y. Changes in phenolic profiles and antioxidant activities during the whole wheat flour bread-making process. Food Chem. 2021, 345, 128851. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Beta, T. Identification and antioxidant properties of phenolic compounds during production of bread from purple wheat grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef]
- Lin, J.; Teo, L.M.; Leong, L.P.; Zhou, W. In vitro bioaccessibility and bioavailability of quercetin from the quercetin-fortified bread products with reduced glycemic potential. Food Chem. 2019, 286, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ribnicky, D.M.; Roopchand, D.E.; Poulev, A.; Kuhn, P.; Oren, A.; Cefalu, W.T.; Raskin, I. Artemisia dracunculus L. polyphenols complexed to soy protein show enhanced bioavailability and hypoglycemic activity in C57BL/6 mice. Nutrition 2014, 30, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Coțovanu, I.; Stroe, S.-G.; Ursachi, F.; Mironeasa, S. Addition of Amaranth Flour of Different Particle Sizes at Established Doses in Wheat Flour to Achieve a Nutritional Improved Wheat Bread. Foods 2022, 12, 133. [Google Scholar] [CrossRef]
- Raczyk, M.; Kruszewski, B.; Michałowska, D. Effect of Coconut and Chestnut Flour Supplementations on Texture, Nutritional and Sensory Properties of Baked Wheat Based Bread. Molecules 2021, 26, 4641. [Google Scholar] [CrossRef]
- Raczyk, M.; Kruszewski, B.; Zachariasz, E. Effect of Tomato, Beetroot and Carrot Juice Addition on Physicochemical, Antioxidant and Texture Properties of Wheat Bread. Antioxidants 2022, 11, 2178. [Google Scholar] [CrossRef]
- Sivam, A.; Sun-Waterhouse, D.; Perera, C.; Waterhouse, G. Application of FT-IR and Raman spectroscopy for the study of biopolymers in breads fortified with fibre and polyphenols. Food Res. Int. 2011, 50, 574–585. [Google Scholar] [CrossRef]
- Ragaee, S.; Guzar, I.; Dhull, N.; Seetharaman, K. Effects of fiber addition on antioxidant capacity and nutritional quality of wheat bread. LWT 2011, 44, 2147–2153. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals-shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Alarcon-De-La-Lastra, C.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Ravi, G.S.; Charyulu, R.N.; Dubey, A.; Prabhu, P.; Hebbar, S.; Mathias, A.C. Nano-lipid Complex of Rutin: Development, Characterisation and In Vivo Investigation of Hepatoprotective, Antioxidant Activity and Bioavailability Study in Rats. AAPS PharmSciTech 2018, 19, 3631–3649. [Google Scholar] [CrossRef]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenolcatabolites. Mol. Nutr. Food Res. 2011, 55, 35–43. [Google Scholar] [CrossRef]
- Fu, J.-J.; Sun, C.; Tan, Z.-F.; Zhang, G.-Y.; Chen, G.-B.; Song, L. Nanocomplexes of curcumin and glycated bovine serum albumin: The formation mechanism and effect of glycation on their physicochemical properties. Food Chem. 2021, 368, 130651. [Google Scholar] [CrossRef] [PubMed]
- Fatkullin, R.; Kalinina, I.; Vasiliev, A.; Naumenko, E.; Botvinnikova, V. The Effect of Ultrasonic Microstructuring of Biologically Active Substances on the Efficiency of their Encapsulation Process. Bulletin of the South Ural State University. Ser. Food Biotechnol. 2021, 9, 100–107. [Google Scholar]
- Potoroko, I.; Kalinina, I.; Naumenko, N.; Fatkullin, R.; Nenasheva, A.; Uskova, D.; Sonawane, S.; Ivanova, D.; Velyamov, M. Sonochemical micronization of taxifolin aimed at improving its bioavailability in drinks for athletes. Human Sport Med. 2018, 18, 90–100. [Google Scholar] [CrossRef]
- Potoroko, I.; Kalinina, I.; Naumenko, N.; Fatkullin, R.; Shaik, S.; Sonawane, S.; Ivanova, D.; Kiselova-Kaneva, Y.; Tolstykh, O.; Paymulina, A. Possibilities of regulating antioxidant activity of medicinal plant extracts. Human Sport Med. 2017, 17, 77–90. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Zhan, J.; Liu, X.-L.; Wang, Y.; Ji, J.; He, Q.-Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules 2018, 23, 999. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Proskurnina, E.; Demin, E.M.; Matveeva, N.S.; Lubitskiy, O.B.; Novikov, A.A.; Izmailov, D.Y.; Osipov, A.N.; Tikhonov, V.P.; Kagan, V.E. Dihydroquercetin (taxifolin) and other flavonoids as inhibitors of free radical formation at key stages of apoptosis. Biochemistry 2009, 74, 301–307. [Google Scholar] [CrossRef]
- Tyukavkina, N.; Lapteva, K.; Latina, V.; Devyatko, N. Extractive substances of Larix Dahurica. II. Quantitative contents of quercetin and dihydroquercetin. Khimya Prir. Soedin. 1967, 3, 298–301. [Google Scholar]
- Corsetti, A.; Settanni, L.; Braga, T.M.; Lopes, M.D.F.S.; Suzzi, G. An investigation of the bacteriocinogenic potential of lactic acid bacteria associated with wheat (Triticum durum) kernels and non-conventional flours. LWT 2008, 41, 1173–1182. [Google Scholar] [CrossRef]
- Wang, J.; Rosell, C.M.; de Barber, C.B. Effect of the addition of different fibres on wheat dough performance and bread quality. Food Chem. 2002, 79, 221–226. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Li, Y. Dough properties, bread quality, and associated interactions with added phenolic compounds: A review. J. Funct. Foods 2018, 52, 629–639. [Google Scholar] [CrossRef]
- Dergacheva, D.I.; Klein, O.I.; Gessler, N.N.; Isakova, E.P.; Deryabina, Y.I.; Nikolaev, A.V. Influence of Natural Polyphenols on Isolated Yeast Dipodascus magnusii Mitochondria. Dokl. Biochem. Biophys. 2020, 490, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, N.V. Physical Nature FActors in Assuring Quality and Longer Shelf Life of Bread and Bakery Products; Publishing House of SUSU: Chelyabinsk, Russia, 2014; 160p. [Google Scholar]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Papageorgiou, M. Aromatic plants of Lamiaceae family in a traditional bread recipe: Effects on quality and phytochemical content. J. Food Biochem. 2019, 43, e13020. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Saura Calixto, F. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Shafii, Z.A.; Basri, M.; Malek, E.A.; Ismail, M. Phytochemical and antioxidant properties of Manilkara zapota (L.) P Royen fruit extracts and its formulations for cosmceuetical application. Asian J. Plant Sci. Res. 2017, 7, 29–41. [Google Scholar]
- Silva, A.F.; Monteiro, M.; Nunes, R.; Baião, A.; Braga, S.S.; Sarmento, B.; Coimbra, M.A.; Silva, A.M.; Cardoso, S.M. Bread enriched with resveratrol: Influence of the delivery vehicles on its bioactivity. Food Biosci. 2022, 49, 101887. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Roth, M.; Döring, C.; Jekle, M.; Becker, T. Mechanisms Behind Distiller’s Grains Impact on Wheat Dough and Bread Quality. Food Bioprocess Technol. 2015, 9, 274–284. [Google Scholar] [CrossRef]
- Seyer, M.; Gélinas, P. Bran characteristics and wheat performance in whole wheat bread. Int. J. Food Sci. Technol. 2009, 44, 688–693. [Google Scholar] [CrossRef]
- Adeboye, P.T.; Bettiga, M.; Olsson, L. The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. AMB Express 2014, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.J.; Loguinov, A.; Chang, M.; Wintz, H.; Nislow, C.; Arkin, A.P.; Giaever, G.; Vulpe, C.D. Identification of Genes Involved in the Toxic Response of Saccharomyces cerevisiae against Iron and Copper Overload by Parallel Analysis of Deletion Mutants. Toxicol. Sci. 2008, 102, 205. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Smith, B.G.; O’Connor, C.J.; Melton, L.D. Effect of raw and cooked onion dietary fibre on the antioxidant activity of ascorbic acid and quercetin. Food Chem. 2008, 111, 580–585. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, S.; Gao, W. Preparation, physicochemical characterization and in vitro digestibility on solid complex of maize starches with quercetin. LWT-Food Sci. Technol. 2011, 44, 787–792. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Irakli, M.; Katsantonis, D.; Kleisiaris, F. Evaluation of quality attributes, nutraceutical components and antioxidant potential of wheat bread substituted with rice bran. J. Cereal Sci. 2015, 65, 74–80. [Google Scholar] [CrossRef]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.A. Interaction of milk α and β-casein with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein–polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W. Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]

| Samples | pH | TTA | ||
|---|---|---|---|---|
| 0 h | 2 h | 0 h | 2 h | |
| Control | 5.69 ± 0.05 | 5.17 ± 0.02 | 0.90 ± 0.10 | 1.50 ± 0.10 |
| Experimental sample 0.05 | 5.65 ± 0.03 | 5.30 ± 0.03 | 0.80 ± 0.10 | 1.25 ± 0.10 |
| Experimental sample 0.07 | 5.65 ± 0.03 | 5.27 ± 0.02 | 0.80 ± 0.10 | 1.25 ± 0.10 |
| Experimental sample 0.1 | 5.63 ± 0.04 | 5.40 ± 0.02 | 0.75 ± 0.20 | 1.20 ± 0.10 |
| Samples | Total Flavonoid Content, mg EQ/100 g | AOA, % (DPPH) | ||
|---|---|---|---|---|
| Crumb | Crust and the Layer under the Crust | Crumb | Crust and the Layer under the Crust | |
| Control | 26.0 ± 0.5 | 22.4 ± 1.0 | 10.5 ± 0.5 | 12.9 ± 1.0 |
| Experimental sample 0.05 | 53.0 ± 1.0 | 46.7 ± 1.2 | 42.1 ± 0.5 | 46.6 ± 1.0 |
| Experimental sample 0.07 | 61.3 ± 1.0 | 54.1 ± 1.2 | 54.5 ± 1.0 | 58.4 ± 0.5 |
| Experimental sample 0.1 | 69.5 ± 1.3 | 59.5 ± 0.5 | 61.5 ± 0.5 | 64.7 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, I.; Fatkullin, R.; Naumenko, N.; Popova, N.; Stepanova, D. The Influence of Flavonoid Dihydroquercetin on the Enzymatic Processes of Dough Ripening and the Antioxidant Properties of Bread. Fermentation 2023, 9, 263. https://doi.org/10.3390/fermentation9030263
Kalinina I, Fatkullin R, Naumenko N, Popova N, Stepanova D. The Influence of Flavonoid Dihydroquercetin on the Enzymatic Processes of Dough Ripening and the Antioxidant Properties of Bread. Fermentation. 2023; 9(3):263. https://doi.org/10.3390/fermentation9030263
Chicago/Turabian StyleKalinina, Irina, Rinat Fatkullin, Natalya Naumenko, Natalia Popova, and Darya Stepanova. 2023. "The Influence of Flavonoid Dihydroquercetin on the Enzymatic Processes of Dough Ripening and the Antioxidant Properties of Bread" Fermentation 9, no. 3: 263. https://doi.org/10.3390/fermentation9030263
APA StyleKalinina, I., Fatkullin, R., Naumenko, N., Popova, N., & Stepanova, D. (2023). The Influence of Flavonoid Dihydroquercetin on the Enzymatic Processes of Dough Ripening and the Antioxidant Properties of Bread. Fermentation, 9(3), 263. https://doi.org/10.3390/fermentation9030263





