Butyrate Properties in Immune-Related Diseases: Friend or Foe?
Abstract
1. Introduction
2. Butyrate Properties
2.1. What Is Butyrate?
2.2. Internal Butyrate Biosynthesis

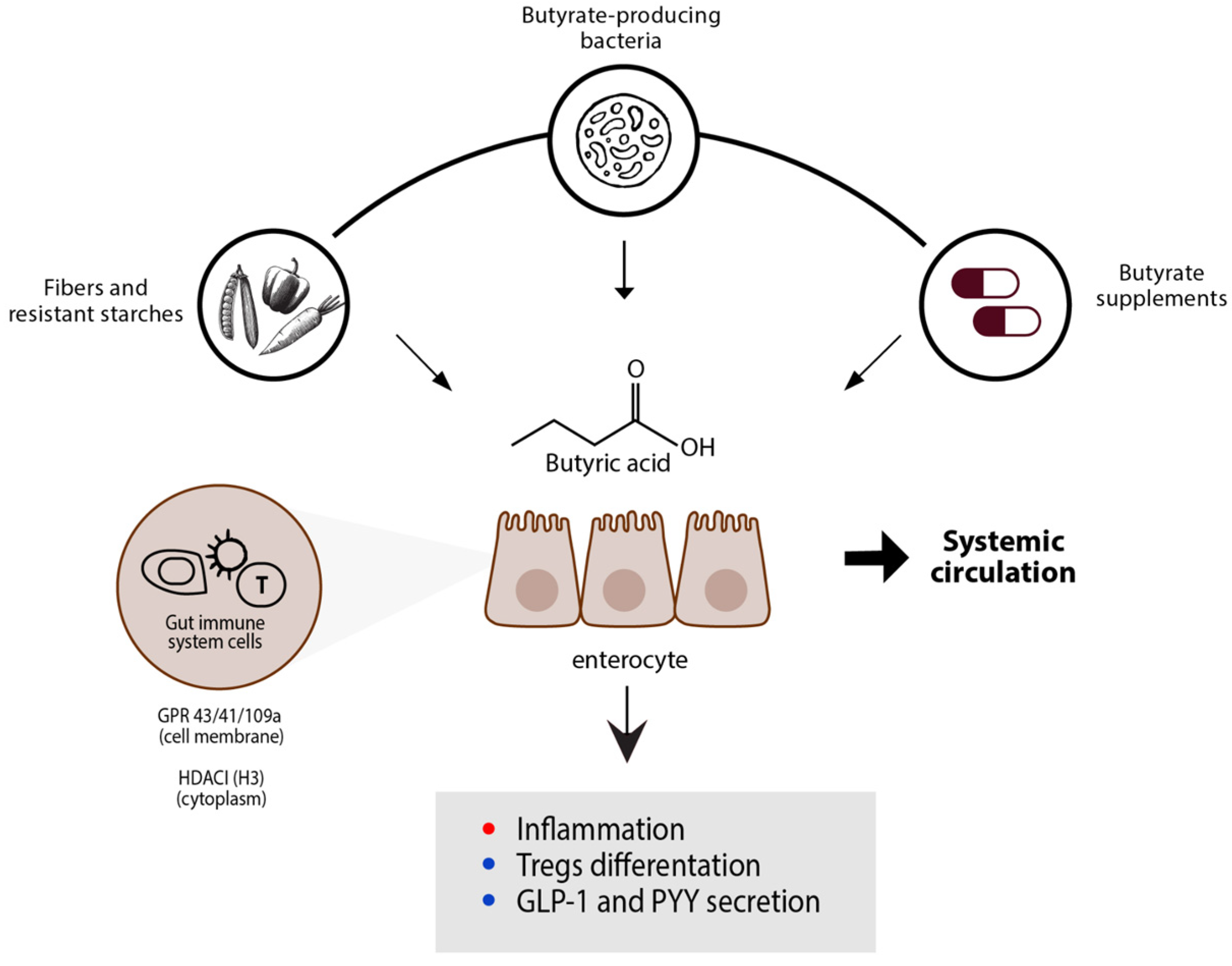
2.3. External Sources of Butyrate
2.4. Butyrate-Producing Bacteria
2.5. Butyrate Effect on Immune System
3. Butyrate as Prevention and Treatment
3.1. Overactive Immune Disease
3.1.1. Systemic Lupus Erythematosus
3.1.2. Chronic Inflammatory Skin Diseases
- a.
- Atopic Dermatitis
- b.
- Psoriasis
3.2. Immunodeficiency
3.2.1. Human Immunodeficiency Virus (HIV) Patients
3.2.2. Cancer
4. Butyrate in Immune-Related Special Condition
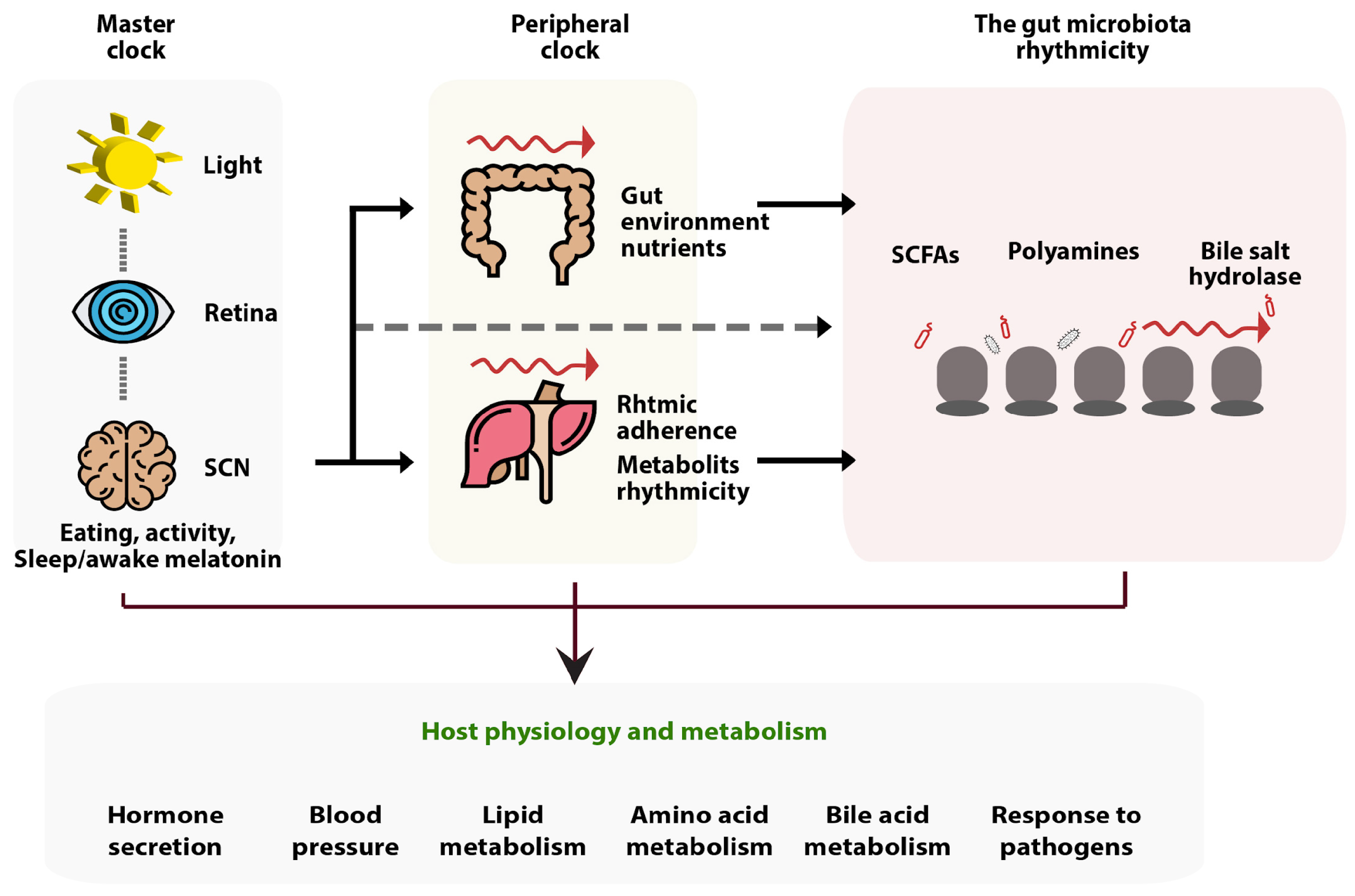
4.1. The Relation of Butyrate to the Sleep Cycle, Day and Night Rhythms, and Sleep Hormones
4.2. Butyrate in Infection
5. Disadvantages of Butyrate
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune. Netw. 2020, 20, e15. [Google Scholar] [CrossRef]
- Mokhtari, Z.; Gibson, D.L.; Hekmatdoost, A. Nonalcoholic Fatty Liver Disease, the Gut Microbiome, and Diet. Adv. Nutr. 2017, 8, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, C.; Colombo, J.P.; Berüter, J. Short chain fatty acids in plasma and brain: Quantitative determination by gas chromatography. Clin. Chim. Acta 1979, 92, 153–159. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Marano, L.; Merola, E.; Roviello, F.; Połom, K. Sodium butyrate in both prevention and supportive treatment of colorectal cancer. Front. Cell. Infect. Microbiol. 2022, 12, 1023806. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Hosseini, S.A.; Ghaffari, S.; Tutunchi, H.; Ghaffari, S.; Mosharkesh, E.; Asghari, S.; Roshanravan, N. Role of Butyrate, a Gut Microbiota Derived Metabolite, in Cardiovascular Diseases: A comprehensive narrative review. Front. Pharmacol. 2021, 12, 837509. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Zhu, L.; Yang, X.; He, F.; Wang, T.; Bao, T.; Lu, H.; Wang, H.; Yang, S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharmacol. 2020, 78, 106062. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Canani, R.B.; Calignano, A.; Perretti, M.; et al. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 2017, 174, 1484–1496. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Deroanne, C.F.; Bonjean, K.; Servotte, S.; Devy, L.; Colige, A.; Clausse, N.; Blacher, S.; Verdin, E.; Foidart, J.M.; Nusgens, B.V.; et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene 2002, 21, 427–436. [Google Scholar] [CrossRef]
- Liang, D.; Kong, X.; Sang, N. Effects of histone deacetylase inhibitors on HIF-1. Cell Cycle 2006, 5, 2430–2435. [Google Scholar] [CrossRef]
- Coppola, S.; Avagliano, C.; Sacchi, A.; Laneri, S.; Calignano, A.; Voto, L.; Luzzetti, A.; Canani, R.B. Potential Clinical Applications of the Postbiotic Butyrate in Human Skin Diseases. Molecules 2022, 27, 1849. [Google Scholar] [CrossRef]
- Pituch, A.; Walkowiak, J.; Banaszkiewicz, A. Butyric acid in functional constipation. Prz. Gastroenterol. 2013, 8, 295–298. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. 1), S130–S152. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Flint, H.J.; Louis, P.; Scott, K.P.; Duncan, S.H. Commensal bacteria in health and disease. Virulence Mech. Bact. Pathog. 2007, 101–115. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Peña, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. mSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Kotrba, P.; Inui, M.; Yukawa, H. Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of carbon metabolism. J. Biosci. Bioeng. 2001, 92, 502–517. [Google Scholar] [CrossRef]
- Hawkes, F.R.; Dinsdale, R.; Hawkes, D.L.; Hussy, I. Sustainable fermentative hydrogen production: Challenges for process optimisation. Int. J. Hydrog. Energy 2002, 27, 1339–1347. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.W.; Lengeler, J.W.; Jacobson, G.R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 1993, 57, 543–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, K. Production of mesaconate in Escherichia coli by engineered glutamate mutase pathway. Metab. Eng. 2015, 30, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W. Energy Conservation in Fermentations of Anaerobic Bacteria. Front. Microbiol. 2021, 12, 703525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Friedrich, P.; Pierik, A.J.; Martins, B.M.; Buckel, W. Substrate-induced radical formation in 4-hydroxybutyryl coenzyme A dehydratase from Clostridium aminobutyricum. Appl. Environ. Microbiol. 2015, 81, 1071–1084. [Google Scholar] [CrossRef]
- Brennenstuhl, H.; Didiasova, M.; Assmann, B.; Bertoldi, M.; Molla, G.; Jung-Klawitter, S.; Hübschmann, O.K.; Schröter, J.; Opladen, T.; Tikkanen, R. Succinic Semialdehyde Dehydrogenase Deficiency: In Vitro and In Silico Characterization of a Novel Pathogenic Missense Variant and Analysis of the Mutational Spectrum of ALDH5A1. Int. J. Mol. Sci. 2020, 21, 8578. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.S. Encyclopedia of the Human Brain; Col-Mem; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Yang, X.; Schnackenberg, L.K.; Shi, Q.; Salminen, W.F. Hepatic toxicity biomarkers. In Biomarkers in Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 241–259. [Google Scholar]
- Heldt, H.W.; Piechulla, B. Nitrate assimilation is essential for the synthesis of organic matter. Plant Biochem. 2005, 275–308. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch-A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Duncan, S.H.; Louis, P.; Flint, H.J. Phylogeny, culturing, and metagenomics of the human gut microbiota. Trends Microbiol. 2014, 22, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Detman, A.; Mielecki, D.; Chojnacka, A.; Salamon, A.; Blaszczyk, M.K.; Sikora, A. Cell factories converting lactate and acetate to butyrate: Clostridium butyricum and microbial communities from dark fermentation bioreactors. Microb. Cell Fact. 2019, 18, 36. [Google Scholar] [CrossRef]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Scott, K.P.; Antoine, J.M.; Midtvedt, T.; van Hemert, S. Manipulating the gut microbiota to maintain health and treat disease. Microb. Ecol. Health Dis. 2015, 26, 25877. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, T.; He, Y.; Xie, Y.; Xu, Y.; Huang, W. The role and mechanism of butyrate in the prevention and treatment of diabetic kidney disease. Front. Microbiol. 2022, 13, 961536. [Google Scholar] [CrossRef] [PubMed]
- Hazem, S.H.; Hamed, M.F.; Saad, M.A.; Gameil, N.M. Comparison of lactate and beta-hydroxybutyrate in the treatment of concanavalin-A induced hepatitis. Int. Immunopharmacol. 2018, 61, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, H.; Lin, F.H.; Gong, R.; Xie, F.; Peng, Y.; Feng, J.; Hu, C.H. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2018, 24, 40–46. [Google Scholar] [CrossRef]
- Diakos, C.; Prieschl, E.E.; Saemann, M.D.; Bohmig, G.A.; Csonga, R.; Sobanov, Y.; Baumruker, T.; Zlabinger, G.J. n-Butyrate inhibits Jun NH(2)-terminal kinase activation and cytokine transcription in mast cells. Biochem. Biophys. Res. Commun. 2006, 349, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Millard, A.L.; Mertes, P.M.; Ittelet, D.; Villard, F.; Jeannesson, P.; Bernard, J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin. Exp. Immunol. 2002, 130, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L.; Min, J.; Wang, J.; Wu, H.; Zeng, Y.; Chen, S.; Chu, Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell. Immunol. 2012, 277, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Rocamora-Reverte, L.; Melzer, F.L.; Wurzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2020, 11, 616949. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Investig. Dermatol. 2017, 137, 855–864. [Google Scholar] [CrossRef]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef]
- Vernia, P.; Monteleone, G.; Grandinetti, G.; Villotti, G.; Di Giulio, E.; Frieri, G.; Marcheggiano, A.; Pallone, F.; Caprilli, R.; Torsoli, A. Combined oral sodium butyrate and mesalazine treatment compared to oral mesalazine alone in ulcerative colitis: Randomized, double-blind, placebo-controlled pilot study. Dig. Dis. Sci. 2000, 45, 976–981. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef]
- Silverman, G.J.; Azzouz, D.F.; Alekseyenko, A.V. Systemic Lupus Erythematosus and dysbiosis in the microbiome: Cause or effect or both? Curr. Opin. Immunol. 2019, 61, 80–85. [Google Scholar] [CrossRef]
- Ma, L.; Morel, L. Loss of gut barrier integrity in lupus. Front. Immunol. 2022, 13, 919792. [Google Scholar] [CrossRef] [PubMed]
- Effendi, R.M.R.A.; Anshory, M.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Pardo, L.M.; Nijsten, T.E.C.; Thio, H.B. Akkermansia muciniphila and Faecalibacterium prausnitzii in Immune-Related Diseases. Microorganisms 2022, 10, 2382. [Google Scholar] [CrossRef] [PubMed]
- Toumi, E.; Goutorbe, B.; Plauzolles, A.; Bonnet, M.; Mezouar, S.; Militello, M.; Mege, J.L.; Chiche, L.; Halfon, P. Gut microbiota in systemic lupus erythematosus patients and lupus mouse model: A cross species comparative analysis for biomarker discovery. Front. Immunol. 2022, 13, 943241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.F.; Li, X.; Li, H.X.; Zhang, Q.; Zhou, H.W.; He, Y.; Li, P.; Fu, C.; Zhang, X.H.; et al. Disordered intestinal microbes are associated with the activity of Systemic Lupus Erythematosus. Clin. Sci. 2019, 133, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Hevia, A.; Milani, C.; Lopez, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 2014, 5, e01548-14. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shao, T.; Li, H.; Xie, Z.; Wen, C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 2016, 8, 64. [Google Scholar] [CrossRef]
- Vieira, J.R.P.; Rezende, A.T.O.; Fernandes, M.R.; da Silva, N.A. Intestinal microbiota and active systemic lupus erythematosus: A systematic review. Adv. Rheumatol. 2021, 61, 42. [Google Scholar] [CrossRef]
- Luo, X.M.; Edwards, M.R.; Mu, Q.; Yu, Y.; Vieson, M.D.; Reilly, C.M.; Ahmed, S.A.; Bankole, A.A. Gut Microbiota in Human Systemic Lupus Erythematosus and a Mouse Model of Lupus. Appl. Environ. Microbiol. 2018, 84, e02288-17. [Google Scholar] [CrossRef]
- Pan, Q.; Guo, F.; Huang, Y.; Li, A.; Chen, S.; Chen, J.; Liu, H.F.; Pan, Q. Gut Microbiota Dysbiosis in Systemic Lupus Erythematosus: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Front. Immunol. 2021, 12, 799788. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, H.; Xu, J.; Zhao, H.; Lin, Q.; Zhou, Y.; Nie, Y. Sodium Butyrate Ameliorates Gut Microbiota Dysbiosis in Lupus-Like Mice. Front. Nutr. 2020, 7, 604283. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y.; et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef]
- Fernando, M.R.; Saxena, A.; Reyes, J.L.; McKay, D.M. Butyrate enhances antibacterial effects while suppressing other features of alternative activation in IL-4-induced macrophages. Am. J. Physiol. Gastrointest Liver Physiol. 2016, 310, G822–G831. [Google Scholar] [CrossRef]
- Xin, M.L.; Michael, R.E.; Christopher, M.R.; Qinghui, M.; Ahmed, S.A. Diet and Microbes in the Pathogenesis of Lupus. In Lupus; Wahid Ali, K., Ed.; IntechOpen: Rijeka, Croatia, 2017; p. Ch. 8. [Google Scholar] [CrossRef]
- Panther, E.J.; Ren, J.; Cabana-Puig, X.; Abdelhamid, L.; Swartwout, B.; Luo, X.M.; Reilly, C.M. The Effect of Dietary Fiber Intake on Systemic Lupus Erythematosus (SLE) Disease in NZB/W Lupus Mice. J. Clin. Cell. Immunol. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Belkaid, Y.; Naik, S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 2013, 14, 646–653. [Google Scholar] [CrossRef]
- Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güell, M.; Paetzold, B. Skin microbiome transplantation and manipulation: Current state of the art. Comput. Struct. Biotechnol. J. 2021, 19, 624–631. [Google Scholar] [CrossRef]
- Mantziari, A.; Salminen, S.; Szajewska, H.; Malagón-Rojas, J.N. Postbiotics against Pathogens Commonly Involved in Pediatric Infectious Diseases. Microorganisms 2020, 8, 1510. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Santarcangelo, C.; Badolati, N.; Sommella, E.; De Filippis, A.; Dacrema, M.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. In vivo bioavailability and in vitro toxicological evaluation of the new butyric acid releaser N-(1-carbamoyl-2-phenyl-ethyl) butyramide. Biomed. Pharmacother. 2021, 137, 111385. [Google Scholar] [CrossRef]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef]
- Lakhdari, O.; Tap, J.; Béguet-Crespel, F.; Le Roux, K.; de Wouters, T.; Cultrone, A.; Nepelska, M.; Lefèvre, F.; Doré, J.; Blottière, H.M. Identification of NF-κB modulation capabilities within human intestinal commensal bacteria. J. Biomed. Biotechnol. 2011, 2011, 282356. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef]
- Alesa, D.I.; Alshamrani, H.M.; Alzahrani, Y.A.; Alamssi, D.N.; Alzahrani, N.S.; Almohammadi, M.E. The role of gut microbiome in the pathogenesis of psoriasis and the therapeutic effects of probiotics. J. Family Med. Prim. Care. 2019, 8, 3496–3503. [Google Scholar]
- Tan, L.; Zhao, S.; Zhu, W.; Wu, L.; Li, J.; Shen, M.; Lei, L.; Chen, X.; Peng, C. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp. Dermatol. 2018, 27, 144–149. [Google Scholar] [CrossRef]
- Eppinga, H.; Sperna Weiland, C.J.; Thio, H.B.; van der Woude, C.J.; Nijsten, T.E.C.; Peppelenbosch, M.P.; Konstantinov, S.R. Similar Depletion of Protective Faecalibacterium prausnitzii in Psoriasis and Inflammatory Bowel Disease, but not in Hidradenitis Suppurativa. J. Crohn’s Colitis 2016, 10, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; Cohen, N.A.; Shalev, V.; Uzan, A.; Koren, O.; Maharshak, N. Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J. Dermatol. 2019, 46, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Czerwińska, J.; Placek, W. The role of regulatory T cells and anti-inflammatory cytokines in psoriasis. Acta Derm. Alp Pannonica Adriat 2018, 27, 17–23. [Google Scholar] [CrossRef]
- Stockenhuber, K.; Hegazy, A.N.; West, N.R.; Ilott, N.E.; Stockenhuber, A.; Bullers, S.J.; Thornton, E.E.; Arnold, I.C.; Tucci, A.; Waldmann, H.; et al. Foxp3(+) T reg cells control psoriasiform inflammation by restraining an IFN-I-driven CD8(+) T cell response. J. Exp. Med. 2018, 215, 1987–1998. [Google Scholar] [CrossRef]
- Schwarz, A.; Philippsen, R.; Schwarz, T. Induction of Regulatory T Cells and Correction of Cytokine Disbalance by Short-Chain Fatty Acids: Implications for Psoriasis Therapy. J. Investig. Dermatol. 2021, 141, 95–104.e2. [Google Scholar] [CrossRef]
- Dillon, S.M.; Kibbie, J.; Lee, E.J.; Guo, K.; Santiago, M.L.; Austin, G.L.; Gianella, S.; Landay, A.L.; Donovan, A.M.; Frank, D.N.; et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS 2017, 31, 511–521. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Vazquez-Castellanos, J.F.; Vallejo, A.; Latorre, A.; Sainz, T.; Ferrando-Martinez, S.; Rojo, D.; Martínez-Botas, J.; del Romero, J.; Madrid, N.; et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 2017, 10, 1279–1293. [Google Scholar] [CrossRef]
- Williams, B. Gut Microbiome in HIV Infection: Overcoming Barriers? Dig. Dis. Sci. 2019, 64, 1725–1727. [Google Scholar] [CrossRef]
- Ortiz, A.M.; Simpson, J.; Langner, C.A.; Baker, P.J.; Aguilar, C.; Brooks, K.; Flynn, J.K.; Vinton, C.L.; Rahmberg, A.R.; Hickman, H.D.; et al. Butyrate administration is not sufficient to improve immune reconstitution in antiretroviral-treated SIV-infected macaques. Sci. Rep. 2022, 12, 7491. [Google Scholar] [CrossRef]
- Lopez, C.A.; Kingsbury, D.D.; Velazquez, E.M.; Baumler, A.J. Collateral damage: Microbiota-derived metabolites and immune function in the antibiotic era. Cell Host Microbe 2014, 16, 156–163. [Google Scholar] [CrossRef]
- Koh, A.; Backhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.L.; Connors, B.M.; Stevenson, D.M.; Hromada, S.E.; Hamilton, J.J.; Amador-Noguez, D.; Venturelli, O.S. Design of synthetic human gut microbiome assembly and butyrate production. Nat. Commun. 2021, 12, 3254. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. The Human Microbiota and Skin Cancer. Int. J. Mol. Sci. 2022, 23, 1813. [Google Scholar] [CrossRef]
- González-Sánchez, P.; DeNicola, G.M. The microbiome(s) and cancer: Know thy neighbor(s). J. Pathol. 2021, 254, 332–343. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Marks, P.A.; Richon, V.M.; Miller, T.; Kelly, W.K. Histone Deacetylase Inhibitors. Adv. Cancer Res. 2004, 91, 137–168. [Google Scholar]
- Dashwood, R.H.; Ho, E. Dietary histone deacetylase inhibitors: From cells to mice to man. Semin Cancer Biol. 2007, 17, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Gao, Z.; Marks, P.A.; Jiang, X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2004, 101, 18030–18035. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Jouniaux, J.M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Akbari, S.K.A.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef] [PubMed]
- Rangan, P.; Mondino, A. Microbial short-chain fatty acids: A strategy to tune adoptive T cell therapy. J. Immunother. Cancer 2022, 10, e004147. [Google Scholar] [CrossRef] [PubMed]
- Teichman, E.M.; O’Riordan, K.J.; Gahan, C.G.M.; Dinan, T.G.; Cryan, J.F. When Rhythms Meet the Blues: Circadian Interactions with the Microbiota-Gut-Brain Axis. Cell Metabolism 2020, 31, 448–471. [Google Scholar] [CrossRef]
- Mehling, A.; Fluhr, J.W. Chronobiology: Biological clocks and rhythms of the skin. Skin Pharmacol. Physiol. 2006, 19, 182–189. [Google Scholar] [CrossRef]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Su, Y. New Insights into the Diurnal Rhythmicity of Gut Microbiota and Its Crosstalk with Host Circadian Rhythm. Animals 2022, 12, 1677. [Google Scholar] [CrossRef] [PubMed]
- Otasowie, C.O.; Tanner, R.; Ray, D.W.; Austyn, J.M.; Coventry, B.J. Chronovaccination: Harnessing circadian rhythms to optimize immunisation strategies. Front. Immunol. 2022, 13, 5588. [Google Scholar] [CrossRef] [PubMed]
- Langlois, P.H.; Smolensky, M.H.; Glezen, W.P.; Keitel, W.A. Diurnal Variation in Responses to Influenza Vaccine. Chronobiol. Int. 1995, 12, 28–36. [Google Scholar] [CrossRef]
- Lu, D.; Zhao, M.; Chen, M.; Wu, B. Circadian Clock-Controlled Drug Metabolism: Implications for Chronotherapeutics. Drug Metab. Dispos. 2020, 48, 395–406. [Google Scholar] [CrossRef]
- Chen, X.; Eslamfam, S.; Fang, L.; Qiao, S.; Ma, X. Maintenance of Gastrointestinal Glucose Homeostasis by the Gut-Brain Axis. Curr. Protein Pept. Sci. 2017, 18, 541–547. [Google Scholar] [CrossRef]
- Szentirmai, É.; Millican, N.S.; Massie, A.R.; Kapás, L. Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci. Rep. 2019, 9, 7035. [Google Scholar] [CrossRef]
- Pearson, J.A.; Wong, F.S.; Wen, L. Crosstalk between circadian rhythms and the microbiota. Immunology 2020, 161, 278–290. [Google Scholar] [CrossRef]
- Du, K.; Bereswill, S.; Heimesaat, M.M. A literature survey on antimicrobial and immune-modulatory effects of butyrate revealing non-antibiotic approaches to tackle bacterial infections. Eur. J. Microbiol. Immunol. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 1427–1452. [Google Scholar] [CrossRef]
- Chemudupati, M.; Kenney, A.D.; Smith, A.C.; Fillinger, R.J.; Zhang, L.; Zani, A.; Liu, S.L.; Anderson, M.Z.; Sharma, A.; Yount, J.S. Butyrate Reprograms Expression of Specific Interferon-Stimulated Genes. J. Virol. 2020, 94, e00326-20. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, H.; Delgado-Morales, R.; Sanz-Garcia, A.; Armario, A. High doses of the histone deacetylase inhibitor sodium butyrate trigger a stress-like response. Neuropharmacology 2014, 79, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 167, 1137. [Google Scholar] [CrossRef]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease—A pilot study. J. Crohn’s Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef]
- Zumbrun, S.D.; Melton-Celsa, A.R.; Smith, M.A.; Gilbreath, J.J.; Merrell, D.S.; O’Brien, A.D. Dietary choice affects Shiga toxin-producing Escherichia coli (STEC) O157:H7 colonization and disease. Proc. Natl. Acad. Sci. USA 2013, 110, E2126–E2133. [Google Scholar] [CrossRef] [PubMed]
| Intestinal Effect | Systemic Effect | Immune System Effect |
|---|---|---|
| Ion absorption | Increases insulin sensitivity | Inhibits HDAC 3 activity |
| Improves gut microbial diversity | Cholesterol synthesis | Agonist for GPR41, GPR43, or GPR109A 4 |
| Promotes production of AMPs 1 by intestinal epithelial cells | Decreases atherosclerotic and ischemic lesions | Stimulating anti-inflammatory cells like T-reg and M2 macrophages |
| Intestinal cell proliferation and differentiation | Increases energy expenditure | Inhibits pro-inflammatory cytokines |
| Energy substrate for colonic cells | Ammonia scavenger | Reduces NF-κB 5 activation and TNF-α 6 secretion |
| Intestinal barrier function | Antioxidant effects | Increases PGE2 7 production |
| Regulation of fluid and electrolyte uptake | Stimulation of β-oxidation of very long-chain fatty acids and peroxisome proliferation | Reduces mTOR 8 activity, which results in decreased TNF-α, IL-6 9, IL-12 production and increased IL-10 production |
| Immune-regulation | CFTR 2 function | Promotes the development of effector T cells such as Th1 10 and Th17 cells |
| Anti-inflammatory effect | Neurogenesis | Inhibits neutrophils and M1 macrophages |
| Oxidative stress | Induces cancer cells apoptosis | Activation of PPAR-γ 11 |
| Intestinal motility | Anti-metastatic and antiangiogenic properties | Reduces mast cell degranulation |
| Visceral perception and rectal compliance | S. aureus bactericidal activity | Stimulates DC 12 |
| Increases digestive secretions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.C.; Nouwen, J.L.; Thio, H.B. Butyrate Properties in Immune-Related Diseases: Friend or Foe? Fermentation 2023, 9, 205. https://doi.org/10.3390/fermentation9030205
Anshory M, Effendi RMRA, Kalim H, Dwiyana RF, Suwarsa O, Nijsten TEC, Nouwen JL, Thio HB. Butyrate Properties in Immune-Related Diseases: Friend or Foe? Fermentation. 2023; 9(3):205. https://doi.org/10.3390/fermentation9030205
Chicago/Turabian StyleAnshory, Muhammad, Raden Mohamad Rendy Ariezal Effendi, Handono Kalim, Reiva Farah Dwiyana, Oki Suwarsa, Tamar E. C. Nijsten, Jan L. Nouwen, and Hok Bing Thio. 2023. "Butyrate Properties in Immune-Related Diseases: Friend or Foe?" Fermentation 9, no. 3: 205. https://doi.org/10.3390/fermentation9030205
APA StyleAnshory, M., Effendi, R. M. R. A., Kalim, H., Dwiyana, R. F., Suwarsa, O., Nijsten, T. E. C., Nouwen, J. L., & Thio, H. B. (2023). Butyrate Properties in Immune-Related Diseases: Friend or Foe? Fermentation, 9(3), 205. https://doi.org/10.3390/fermentation9030205







