Evaluation of Fermentative Xylitol Production Potential of Adapted Strains of Meyerozyma caribbica and Candida tropicalis from Rice Straw Hemicellulosic Hydrolysate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Biomass
2.2. Microorganisms Used
2.3. Optimization of Dilute Acid Pre-Treatment of RS
2.4. Large-Scale Pre-Treatment of RS
2.5. Preparation of RS-Derived Fermentation Medium
2.6. Inoculum Preparation
2.7. Effect of Toxic Inhibitors on Fermentation Efficiency of C. tropicalis and M. caribbica
2.8. Adaptive Evolution of Yeasts in RS Hemicellulosic Hydrolysate
2.9. Evaluation of Fermentation Potential of Un-Adapted and Adapted Yeasts
2.10. Repeated Batch Fermentation
2.11. Analytical Methods
2.12. Statistical Analysis
3. Results and Discussion
3.1. Effect of Inhibitors on Xylitol Production
3.1.1. Effect of Acetic Acid on Xylitol Production
3.1.2. Effect of Furfural and Hydroxymethylfurfural on Xylitol Production
3.2. Adaptation of Yeasts in RS Hydrolysate
3.3. Comparative Assessment of Fermentation in RS Hydrolysate with Un-Adapted and Adapted Yeasts
3.4. Repeated Batch Fermentation in Non-Detoxified and Detoxified Hydrolysate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bianchini, I.D.A.; Sene, L.; da Cunha, M.A.A.; Felipe, M.D.G.D.A. Short-term adaptation strategy improved xylitol production by Candida guilliermondii on sugarcane bagasse hemicellulosic hydrolysate. Bioenergy Res. 2022, 15, 1182–1194. [Google Scholar] [CrossRef]
- Logeswaran, J.; Shamsuddin, A.H.; Silitonga, A.S.; Mahlia, T.M.I. Prospect of using rice straw for power generation: A review. Environ. Sci. Pollut. Res. 2020, 27, 25956–25969. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Mathew, A.K.; Sindhu, R.; Pandey, A.; Binod, P. Potential of rice straw for bio-refining: An overview. Bioresour. Technol. 2016, 215, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, M.M.; Zhou, Z.W.; Huang, T.; Chen, X.L.; Wang, Y. Isolation of cellulose with ionic liquid from steam exploded rice straw. Ind. Crops Prod. 2011, 33, 734–738. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.F.; de Arruda, P.V.; Sene, L.; da Silva, S.S.; Chandel, A.K.; de Almeida Felipe, M.D.G. Xylitol bioproduction: State-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Crit. Rev. Biotechnol. 2019, 39, 924–943. [Google Scholar] [CrossRef] [PubMed]
- Global Xylitol Market Size/Share Worth 1475.87 Million by 2030 at a 6% CAGR: Custom Market Insights. Available online: https://www.globenewswire.com/ (accessed on 20 November 2022).
- Zhang, B.; Ren, L.; Zhao, Z.; Zhang, S.; Xu, D.; Zeng, X.; Li, F. High-temperature xylitol production through simultaneous co-utilization of glucose and xylose by engineered Kluyveromyces marxianus. Biochem. Eng. J. 2021, 165, 107820. [Google Scholar] [CrossRef]
- Kumar, V.; Krishania, M.; Sandhu, P.; Ahluwalia, P.; Gnansounou, E.; Sangwan, R.S. Efficient detoxification of corn cob hydrolysate with ion-exchange resin for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour. Technol. 2017, 251, 416–419. [Google Scholar] [CrossRef]
- Prakash, G.; Varma, A.J.; Prabhune, A.; Shouche, Y.; Rao, M. Microbial production of xylitol from d-xylose and sugarcane bagasse hemicellulose using newly isolated thermotolerant yeast Debaryomyces hansenii. Bioresour. Technol. 2011, 102, 3304–3308. [Google Scholar] [CrossRef]
- Mohamad, N.L.; Mustapa Kamal, S.M.; Mokhtar, M.N. Xylitol biological production: A review of recent studies. Food Rev. Int. 2015, 31, 74–89. [Google Scholar] [CrossRef]
- Jia, H.; Shao, T.; Zhong, C.; Li, H.; Jiang, M.; Zhou, H.; Wei, P. Evaluation of xylitol production using corncob hemicellulosic hydrolysate by combining tetrabutylammonium hydroxide extraction with dilute acid hydrolysis. Carbohydr. Polym. 2016, 151, 676–683. [Google Scholar] [CrossRef]
- Tadioto, V.; Milani, L.M.; Barrilli, É.T.; Baptista, C.W.; Bohn, L.; Dresch, A.; Harakava, R.; Fogolari, O.; Mibielli, G.M.; Bender, J.P.; et al. Analysis of glucose and xylose metabolism in new indigenous Meyerozyma caribbica strains isolated from corn residues. World J. Microbiol. Biotechnol. 2022, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Dhanarajan, G.; Sarkarb, D.; Sen, R. Multi-fold enhancement in sustainable production of biomass, lipids and biodiesel from oleaginous yeast: An artificial neural network-genetic algorithm approach. Sustain. Energy Fuels 2020, 4, 6075–6084. [Google Scholar] [CrossRef]
- Moremi, M.E.; Van Rensburg, E.L.J.; La Grange, D.C. The improvement of bioethanol production by pentose-fermenting yeasts isolated from herbal preparations, the gut of dung beetles, and marula wine. Int. J. Microbiol. 2020, 2020, 5670936. [Google Scholar] [CrossRef] [PubMed]
- Sukpipat, W.; Komeda, H.; Prasertsan, P.; Asano, Y. Purification and characterization of xylitol dehydrogenase with L-arabitol dehydrogenase activity from the newly isolated pentose-fermenting yeast Meyerozyma caribbica 5XY2. J. Biosci. Bioeng. 2016, 123, 20–27. [Google Scholar] [CrossRef]
- Arcaño, Y.D.; Valmaña García, O.D.; Mandelli, D.; Carvalho, W.A.; Pontes, L.A.M. Xylitol: A review on the progress and challenges of its production by chemical route. Catal. Today 2020, 344, 2–14. [Google Scholar] [CrossRef]
- Silva-Fernandes, T.; Santos, J.C.; Hasmann, F.; Rodrigues, R.; Filho, H.I.; Felipe, M. Biodegradable alternative for removing toxic compounds from sugarcane bagasse hemicellulosic hydrolysates for valorization in biorefineries. Bioresour. Technol. 2017, 243, 384–392. [Google Scholar] [CrossRef]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef]
- Yewale, T.; Panchwagh, S.; Rajagopalan, S.; Dhamole, P.B.; Jain, R. Enhanced xylitol production using immobilized Candida tropicalis with non-detoxified corn cob hemicellulosic hydrolysate. 3 Biotech 2016, 6, 75. [Google Scholar] [CrossRef]
- Zahed, O.; Jouzani, G.S.; Abbasalizadeh, S.; Khodaiyan, F.; Tabatabaei, M. Continuous co-production of ethanol and xylitol from rice straw hydrolysate in a membrane bioreactor. Folia Microbiol. 2015, 61, 179–189. [Google Scholar] [CrossRef]
- Rao, R.S.; Jyothi, C.P.; Prakasham, R.S.; Sarma, P.N.; Rao, L.V. Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour. Technol. 2006, 97, 1974–1978. [Google Scholar] [CrossRef]
- Costa Nogueira, C.D.; Araújo Padilha, C.E.D.; Medeiros Dantas, J.M.D.; Macedo de Medeiros, F.G.; Araújo Guilherme, A.D.; Santana Souza, D.F.D.; dos Santos, E.S. In-situ detoxification strategies to boost bio-alcohol production from lignocellulosic biomass. Renew. Energ. 2021, 180, 914–936. [Google Scholar] [CrossRef]
- Zhang, P.; Wells, Y.; Liang, J.; Love, D.; Parker, A.; Botella, C. Effect of diluted hydrolysate as yeast propagation medium on ethanol production. Bioresour. Technol. 2019, 271, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Landaeta, R.; Aroca, G.; Acevedo, F.; Teixeira, J.A.; Mussatto, S.I. Adaptation of a flocculent Saccharomyces cerevisiae strain to lignocellulosic inhibitors by cell recycle batch fermentation. Appl. Energy 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Kaur, S.; Guleria, P.; Sidana, A.; Yadav, S.K. Efficient process for xylitol production from nitric acid pretreated rice straw derived pentosans by Candida tropicalis GS18. Biomass Bioenergy 2022, 166, 106618. [Google Scholar] [CrossRef]
- Pooja; Purohit, A.; Kaur, S.; Yadav, S.K. Identification of a yeast Meyerozyma caribbica M72 from mahua flower for efficient transformation of rice straw into ethanol. Biomass Conv. Bioref. 2021, 1–13. [Google Scholar] [CrossRef]
- Sharma, B.; Larroche, C.; Dussap, C.G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 31, 123630. [Google Scholar] [CrossRef]
- Bhavana, B.K.; Mudliar, S.N.; Bokade, V.V.; Debnath, S. Effect of furfural, acetic acid and 5-hydroxymethylfurfural on yeast growth and xylitol fermentation using Pichia stipitis NCIM 3497. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Ping, Y.; Ling, H.Z.; Song, G.; Ge, J.P. Xylitol production from non-detoxified corncob hemicellulose acid hydrolysate by Candida tropicalis. Biochem. Eng. J. 2013, 75, 86–91. [Google Scholar] [CrossRef]
- Pant, S.; Ritika; Prakash, A.; Kuila, A. Integrated production of ethanol and xylitol from Brassica juncea using Candida sojae JCM 1644. Bioresour. Technol. 2022, 351, 126903. [Google Scholar] [CrossRef]
- Cheng, K.K.; Zhang, J.A.; Ling, H.Z.; Ping, W.X.; Huang, W.; Ge, J.P.; Xu, J.M. Optimization of pH and acetic acid concentration for bioconversion of hemicellulose from corncobs to xylitol by Candida tropicalis. Biochem. Eng. J. 2009, 43, 203–207. [Google Scholar] [CrossRef]
- Lima, L.H.A.; Felipe, M.G.A.; Vitolo, M.; Torres, F.A.G. Effect of acetic acid present in bagasse hydrolysate on the activities of xylose reductase and xylitol dehydrogenase in C. guilliermondii. Appl. Microbiol. Biotechnol. 2004, 65, 734–738. [Google Scholar] [CrossRef]
- Modig, T.; Lidén, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ma, M.; Song, M. Evolutionarily engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways. Mol. Genet. Genom. 2009, 282, 233–244. [Google Scholar]
- Horvath, I.S.; Franzen, C.J.; Taherzadeh, M.J.; Niklasson, C.; Liden, G. Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl. Environ. Microbiol. 2003, 69, 4076–4086. [Google Scholar] [CrossRef]
- Matos, Í.T.S.R.; do Carmo, E.J.; de Assunc, E.N.; de Almeida, R.A.; Soares, V.M.; Filho, S.A. Xylitol production and furfural consumption by a wild type Geotrichum sp. Electron. J. Biotechnol. 2016, 24, 21–25. [Google Scholar] [CrossRef]
- Bura, R.; Vajzovic, A.; Doty, S.L. Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 I: Production of xylitol and ethanol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1003–1011. [Google Scholar] [CrossRef]
- Perna, M.D.; Bastos, R.G.; Ceccato-Antonini, S.R. Single and combined effects of acetic acid, furfural, and sugars on the growth of the pentose-fermenting yeast Meyerozyma guilliermondii. 3 Biotech 2018, 8, 119. [Google Scholar] [CrossRef]
- Trichez, D.; Steindorff, A.S.; Soares, C.E.; Formighieri, E.F.; Almeida, J.R. Physiological and comparative genomic analysis of new isolated yeasts Spathaspora sp. JA1 and Meyerozyma caribbica JA9 reveal insights into xylitol production. FEMS Yeast Res. 2019, 19, 34. [Google Scholar] [CrossRef]
- Nagarajan, A.; Thulasinathan, B.; Arivalagan, P.; Alagarsamy, A.; Muthuramalingam, J.B.; Thangarasu, S.D.; Thangavel, K. Particle size influence on the composition of sugars in corncob hemicellulose hydrolysate for xylose fermentation by Meyerozyma caribbica. Bioresour. Technol. 2021, 340, 125677. [Google Scholar] [CrossRef]
- Ko, J.K.; Enkh-Amgalan, T.; Gong, G.; Um, Y.; Lee, S.M. Improved bioconversion of lignocellulosic biomass by Saccharomyces cerevisiae engineered for tolerance to acetic acid. GCB Bioenergy 2020, 12, 90–100. [Google Scholar] [CrossRef]
- Pramasari, D.A.; Oktaviani, M.; Thontowi, A.; Purnawan, A.; Ermawar, R.A.; Sondari, D.; Ningrum, R.S.; Laksana, R.P.B.; Lianawati, A.; Fahrezi, M.Z.M.; et al. The use of hemicellulose acid hydrolysate for hydrolysis of sugarcane trash and its fermentation for producing xylitol. Ind. Crops Prod. 2023, 193, 116163. [Google Scholar] [CrossRef]
- Tiwari, S.; Jadhav, R.; Avchar, R.; Lanjekar, V.; Datar, M.; Baghela, A. Nectar yeast community of tropical flowering plants and assessment of their osmotolerance and xylitol-producing potential. Curr. Microbiol. 2021, 79, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, L.; Li, F.; Hua, D.; Ma, C.; Ma, Y.; Xu, P. Kinetics of d-lactic acid production by Sporolactobacillus sp. strain CASD using repeated batch fermentation. Bioresour. Technol. 2010, 101, 6499–6505. [Google Scholar] [CrossRef] [PubMed]
- Sirisansaneeyakul, S.; Wannawilai, S.; Chisti, Y. Repeated fed-batch production of xylitol by Candida magnoliae TISTR 5663. J. Chem. Technol. Biotechnol. 2012, 88, 1121–1129. [Google Scholar] [CrossRef]
- Raposo, R.S.; de Almeida, M.C.M.D.; de Oliveira, M.C.M.A.; da Fonseca, M.M.; Cesario, M.T. A Burkholderia sacchari cell factory: Production of poly-3-hydroxybutyrate, xylitol and xylonic acid from xylose-rich sugar mixtures. New Biotechnol. 2017, 34, 12–22. [Google Scholar] [CrossRef] [PubMed]
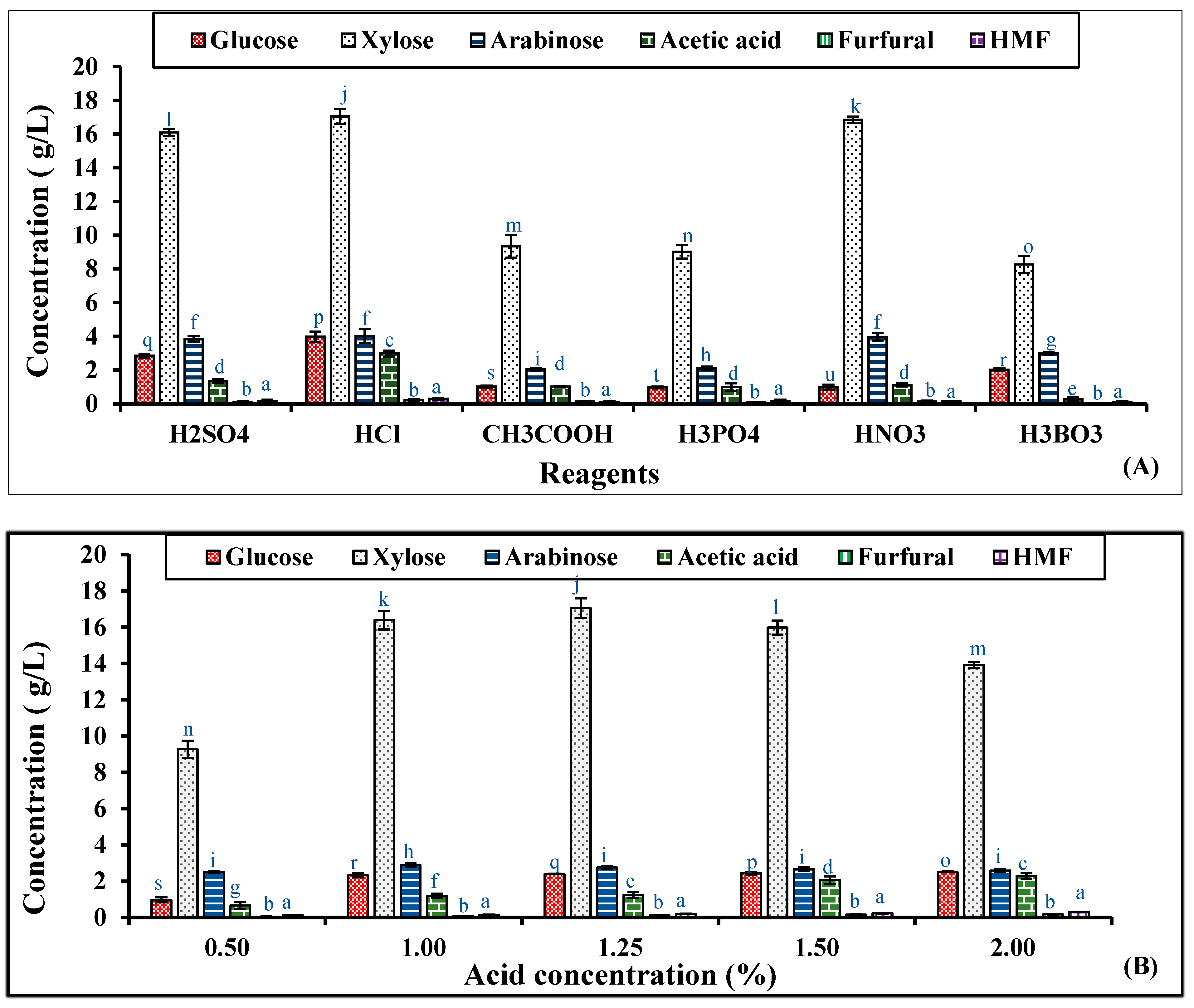

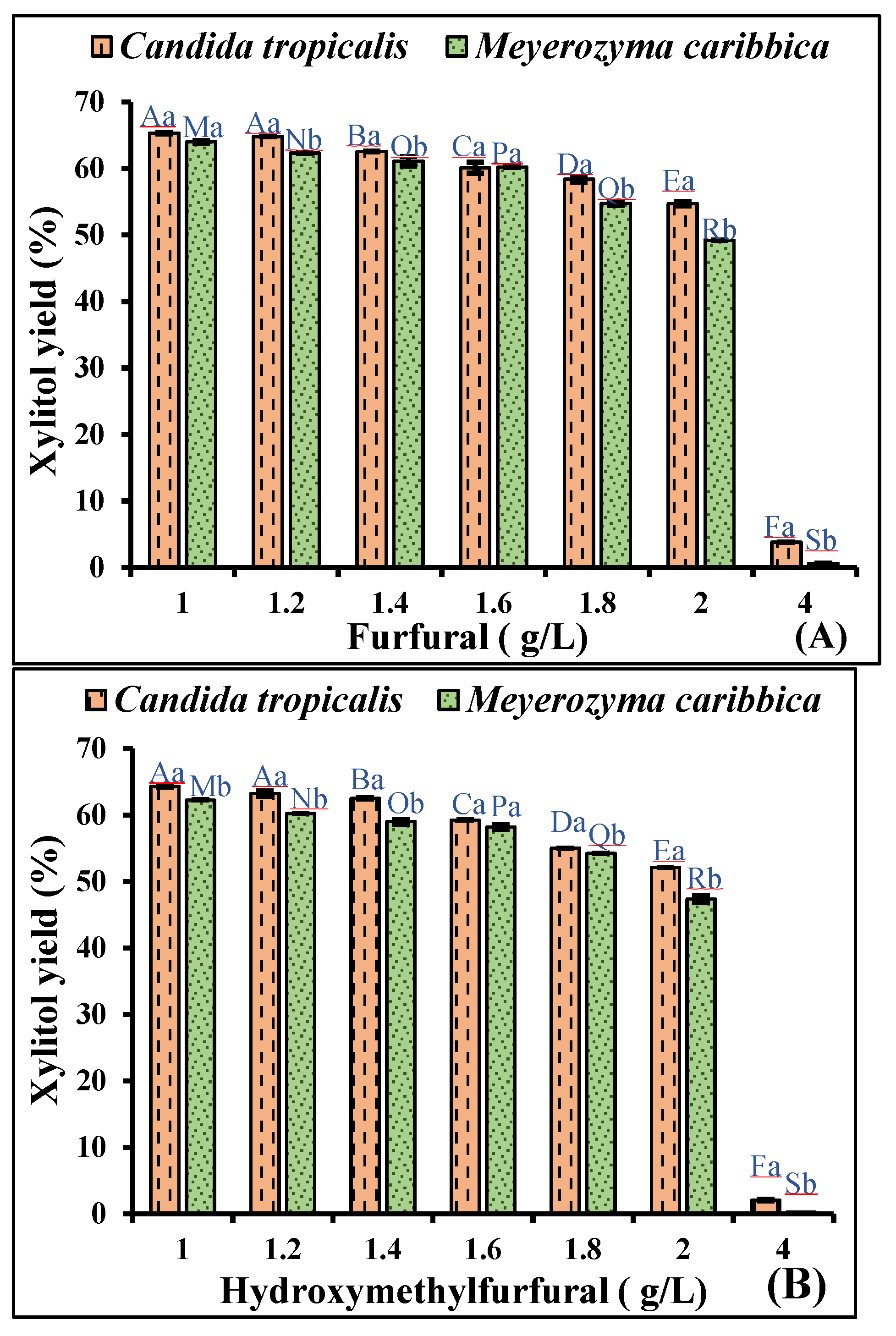

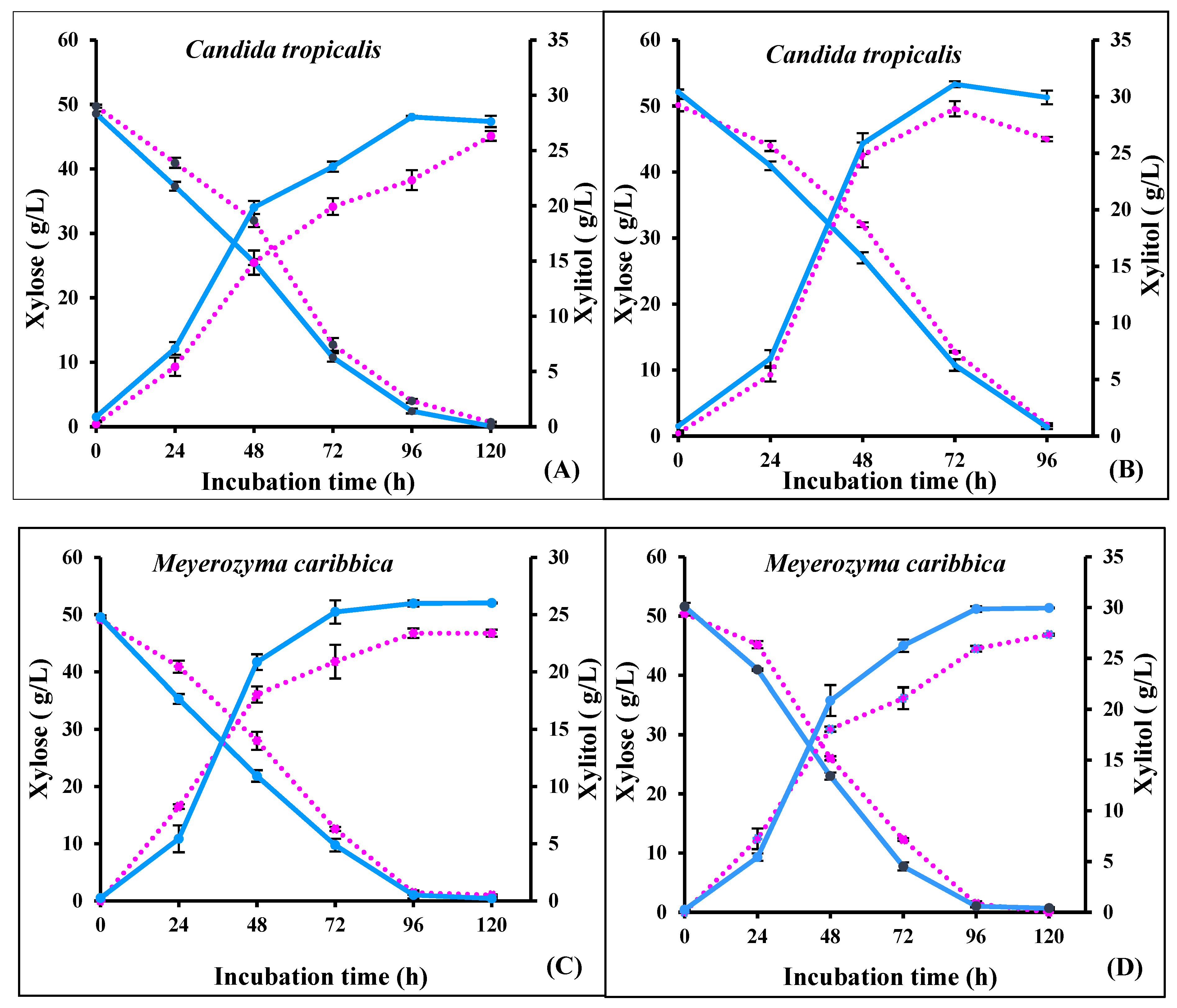
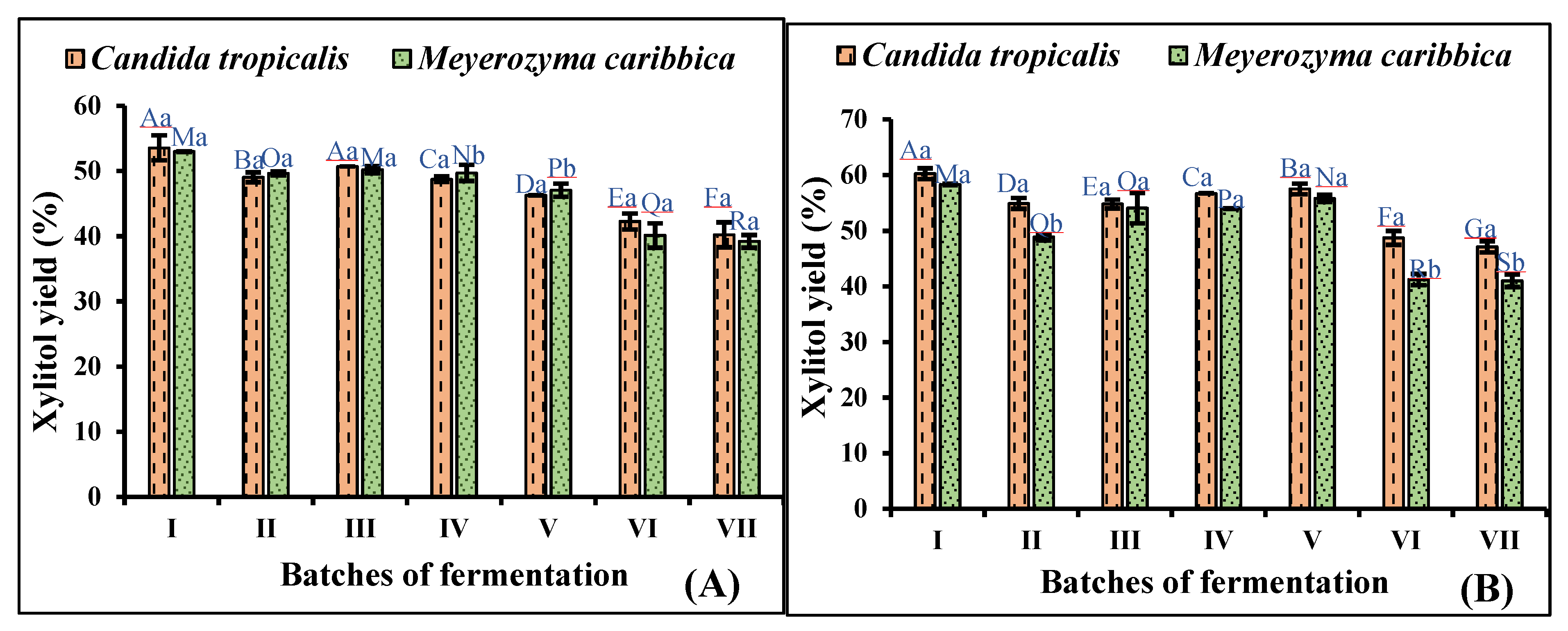
| Concentration (g/L) | Raw Hydrolysate | Detoxified Hydrolysate | Concentrated Non-detoxified Hydrolysate | Concentrated Detoxified Hydrolysate |
|---|---|---|---|---|
| Glucose | 2.4 ± 0.24 | 1.97 ± 0.05 | 6.98 ± 0.03 | 6.30 ± 0.14 |
| Xylose | 16.72 ± 1.04 | 15.97 ± 0.98 | 49.16 ± 0.16 | 52.15 ± 1.12 |
| Arabinose | 2.75 ± 0.45 | 2.70 ± 0.02 | 8.08 ± 0.12 | 8.14 ± 0.04 |
| Acetic acid | 1.03 ± 0.17 | 0.44 ± 0.03 | 3.04 ± 0.06 | 1.35 ± 0.02 |
| Furfural | 0.08 ± 0.03 | - | - | - |
| HMF | 0.16 ± 0.02 | - | 0.44 ± 0.04 | - |
| Fermentation Parameters | Xylitol Concentration (g/L) | Xylitol Yield (g/g) | ||||
|---|---|---|---|---|---|---|
| Acetic Acid Concentration (g/L) | pH | Time (h) | Candida tropicalis | Meyerozyme caribbica | Candida tropicalis | Meyerozyma caribbica |
| Control | 4.5 | 48 | 33.26 ± 0.11 Da | 33.92 ± 0.23 Na | 0.67 | 0.65 |
| 5.5 | 48 | 35.12 ± 0.79 Aa | 34.88 ± 0.12 Ma | 0.70 | 0.69 | |
| 6.5 | 48 | 34.23 ± 0.13 Ca | 34.02 ± 0.28 Na | 0.68 | 0.66 | |
| 2.17 ± 0.27 | 4.5 | 48 | 31.51 ± 0.13 Ga | 31.24 ± 0.016 Sa | 0.63 | 0.62 |
| 5.5 | 48 | 33.65 ± 0.01 Da | 32.13 ± 0.02 Qb | 0.67 | 0.64 | |
| 6.5 | 48 | 32.07 ± 0.04 Da | 31.89 ± 0.03 Ra | 0.64 | 0.63 | |
| 3.95 ± 0.06 | 4.5 | 72 | 26.49 ± 0.017 Ka | 24.45 ± 0.015 Vb | 0.53 | 0.49 |
| 5.5 | 48 | 34.02 ± 0.48 Da | 33.07 ± 0.87 Oa | 0.68 | 0.66 | |
| 6.5 | 48 | 34.50 ±0.20 Ba | 32.23 ± 0.007 Pb | 0.69 | 0.65 | |
| 6.02 ± 0.02 | 4.5 | 72 | 29.00 ± 0.16 Ja | 22.05 ± 0.021 Wb | 0.58 | 0.47 |
| 5.5 | 72 | 31.58 ± 0.70 Fa | 27.04 ± 0.013 Ub | 0.63 | 0.60 | |
| 6.5 | 72 | 32.48 ± 0.36 Ea | 28.12 ± 0.047 Tb | 0.65 | 0.60 | |
| 10 ± 0.11 | 4.5 | - | 6.57 ± 0.28 La | 2.36 ± 0.016 Zb | 0.13 | 0.13 |
| 5.5 | 72 | 29.76 ± 0.028 Ha | 12.04 ± 0.043 Yb | 0.59 | 0.501 | |
| 6.5 | 72 | 29.24 ± 0.16 Ia | 13.97 ± 0.007 Xb | 0.59 | 0.55 | |
| Microorganism | Raw Material/Method of Detoxification | Initial Xylose (g/L) | Xylitol Titre (g/L) | Xylitol Yield (g/g) | Xylitol Productivity (g/L·h) | References |
|---|---|---|---|---|---|---|
| C. tropicalis | Rice straw/Activated charcoal and neutralization | 52.13 | 31.1 | 0.61 | 0.54 | Present study |
| M. caribbica | Rice straw/Activated charcoal and neutralization | 51.57 | 29.95 | 0.58 | 0.42 | Present study |
| C. tropicalis | Rice straw/non-detoxified | 49.74 | 28.03 | 0.57 | 0.29 | Present study |
| M. caribbica | Rice straw/non-detoxified | 49.12 | 26.02 | 0.52 | 0.21 | Present study |
| C. tropicalis MTCC 6192 | Rice straw | 45 | 25.83 | 0.60 | 0.26 | [35] |
| C. tropicalis | Rice straw/Charcoal and ion exchange resins | 46.2 | 26.5 | 0.58 | 0.53 | [20] |
| C. tropicalis BCRC 20520 | Wood chip/Ca (OH)2 neutralized and activated charcoal | 60 | 32.5 | 0.54 | 0.73 | [41] |
| M. caribbica | Sugarcane bagasse/non-detoxified | 40 | 11.37 | 0.54 | 0.10 | [39] |
| M. caribbica | Sugarcane trash/non detoxified | 35.2 | 6.49 | - | - | [42] |
| M. caribbica | D-xylose | 40 | 21.56 | 0.539 | 0.29 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, S.; Guleria, P.; Yadav, S.K. Evaluation of Fermentative Xylitol Production Potential of Adapted Strains of Meyerozyma caribbica and Candida tropicalis from Rice Straw Hemicellulosic Hydrolysate. Fermentation 2023, 9, 181. https://doi.org/10.3390/fermentation9020181
Kaur S, Guleria P, Yadav SK. Evaluation of Fermentative Xylitol Production Potential of Adapted Strains of Meyerozyma caribbica and Candida tropicalis from Rice Straw Hemicellulosic Hydrolysate. Fermentation. 2023; 9(2):181. https://doi.org/10.3390/fermentation9020181
Chicago/Turabian StyleKaur, Sundeep, Payal Guleria, and Sudesh Kumar Yadav. 2023. "Evaluation of Fermentative Xylitol Production Potential of Adapted Strains of Meyerozyma caribbica and Candida tropicalis from Rice Straw Hemicellulosic Hydrolysate" Fermentation 9, no. 2: 181. https://doi.org/10.3390/fermentation9020181
APA StyleKaur, S., Guleria, P., & Yadav, S. K. (2023). Evaluation of Fermentative Xylitol Production Potential of Adapted Strains of Meyerozyma caribbica and Candida tropicalis from Rice Straw Hemicellulosic Hydrolysate. Fermentation, 9(2), 181. https://doi.org/10.3390/fermentation9020181




