Key Aromatic Volatile Compounds from Roasted Cocoa Beans, Cocoa Liquor, and Chocolate
Abstract
1. Introduction
2. Extraction and Quantification of Volatile Cocoa Compounds
3. Flavor Precursors in Cocoa Beans
3.1. Volatile Compounds Generated from Precursors
3.2. Specific Volatile Compounds
3.2.1. Fermented and Dried Beans
3.2.2. Roasted Beans
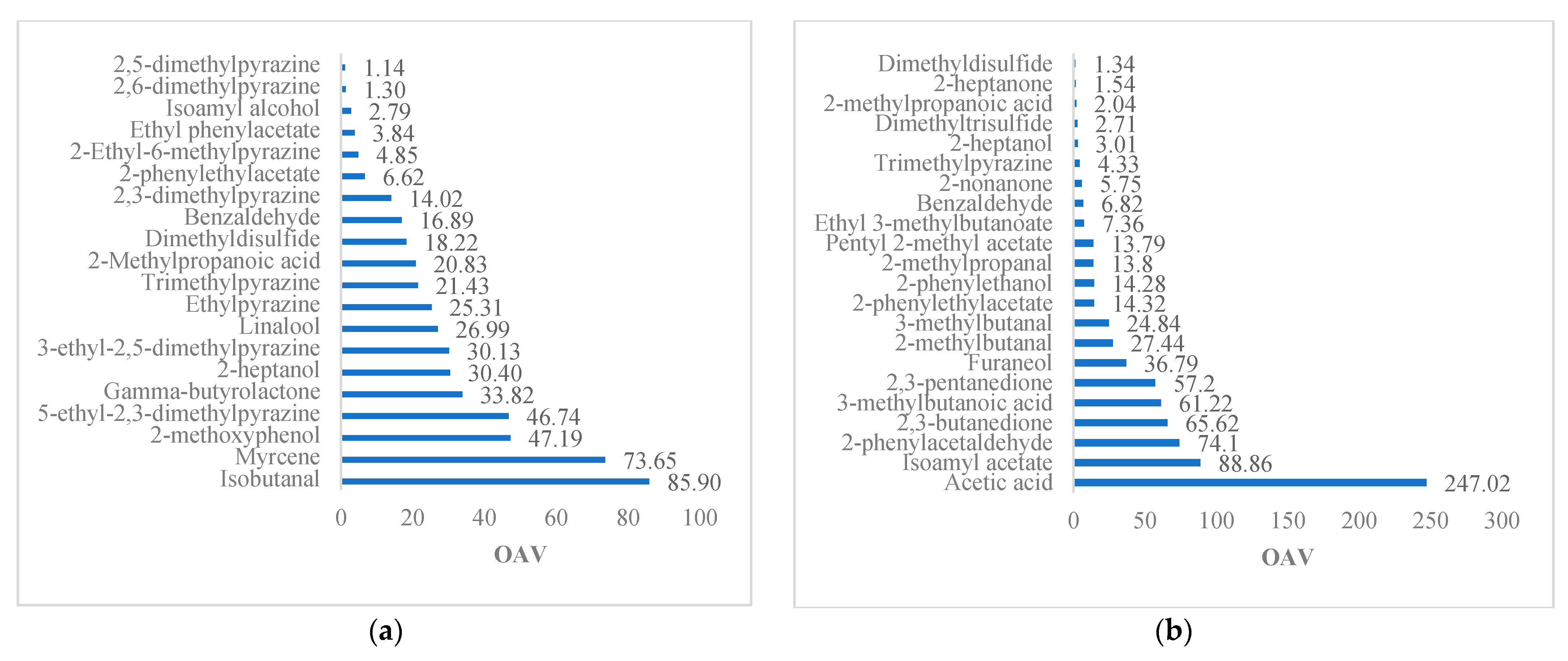
3.2.3. Cocoa Liquor
3.2.4. Chocolate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the raw seed to chocolate: Volatile profile of Blanco de Criollo in dif-ferent phases of the processing chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of aromatic compounds precursors during fermentation of Criollo and Forastero cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef]
- Rottiers, H.; Sosa, D.A.T.; Lemarcq, V.; De Winne, A.; De Wever, J.; Everaert, H.; Jaime, J.A.B.; Dewettinck, K.; Messens, K. A multipronged flavor comparison of Ecuadorian CCN51 and Nacional cocoa cultivars. Eur. Food Res. Technol. 2019, 245, 2459–2478. [Google Scholar] [CrossRef]
- Rottiers, H.; Sosa, D.A.T.; De Winne, A.; Ruales, J.; De Clippeleer, J.; De Leersnyder, I.; De Wever, J.; Everaert, H.; Messens, K.; Dewettinck, K. Dynamics of volatile compounds and flavor precursors during spontaneous fermentation of fine flavor Tri-nitario cocoa beans. Eur. Food Res. Technol. 2019, 245, 1917–1937. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Key aroma compounds in fermented Forastero cocoa beans and changes induced by roasting. Eur. Food Res. Technol. 2019, 245, 1907–1915. [Google Scholar] [CrossRef]
- Qin, X.-W.; Lai, J.-X.; Tan, L.-H.; Hao, C.-Y.; Li, F.-P.; He, S.-Z.; Song, Y.-H. Characterization of volatile compounds in Criollo, Forastero, and Trinitario cocoa seeds (Theobroma cacao L.) in China. Int. J. Food Prop. 2017, 20, 2261–2275. [Google Scholar] [CrossRef]
- Utrilla-Vázquez, M.; Rodríguez-Campos, J.; Avendaño-Arazate, C.H.; Gschaedler, A.; Lugo-Cervantes, E. Analysis of volatile compounds of five varieties of Maya cocoa during fermentation and drying processes by Venn diagram and PCA. Food Res. Int. 2020, 129, 108834. [Google Scholar] [CrossRef]
- Hinneh, M.; Van de Walle, D.; Tzompa-Sosa, D.A.; De Winne, A.; Termote, S.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; De Cooman, L.; Dewettinck, K. Tuning the aroma profiles of FORASTERO cocoa liquors by varying pod storage and bean roasting temperature. Food Res. Int. 2019, 125, 108550. [Google Scholar] [CrossRef]
- Seyfried, C.; Granvogl, M. Characterization of the Key Aroma Compounds in Two Commercial Dark Chocolates with High Cocoa Contents by Means of the Sensomics Approach. J. Agric. Food Chem. 2019, 67, 5827–5837. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.D.; Van Durme, J.; Van De Walle, D.; De Winne, A.; Delbaere, C.; De Clercq, N.; Phan, T.T.Q.; Nguyen, C.P.; Dewettinck, K. Quality Attributes of Dark Chocolate Produced from Vietnamese Cocoa Liquors. J. Food Qual. 2016, 39, 311–322. [Google Scholar] [CrossRef]
- Tuenter, E.; Delbaere, C.; De Winne, A.; Bijttebier, S.; Custers, D.; Foubert, K.; Van Durme, J.; Messens, K.; Dewettinck, K.; Pieters, L. Non-volatile and volatile composition of West African bulk and Ecuadorian fine-flavor cocoa liquor and chocolate. Food Res. Int. 2019, 130, 108943. [Google Scholar] [CrossRef]
- Füllemann, D.; Steinhaus, M. Characterization of Odorants Causing Smoky Off-Flavors in Cocoa. J. Agric. Food Chem. 2020, 68, 10833–10841. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal com-ponents analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Koua, B.K.; Koffi, P.M.E.; Gbaha, P. Evolution of shrinkage, real density, porosity, heat and mass transfer coefficients during indirect solar drying of cocoa beans. J. Saudi Soc. Agric. Sci. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Kongor, J.E.; Takrama, J.; Budu, A.S. Changes in nib acidification and biochemical composition during fer-mentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int. Food Res. J. 2013, 20, 1843–1853. [Google Scholar]
- Souza, C.D.S.; Block, J.M. Impact of the addition of cocoa butter equivalent on the volatile compounds profile of dark chocolate. J. Food Sci. Technol. 2018, 55, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Marty-Terrade, S.; Marangoni, A.G. Impact of Cocoa Butter Origin on Crystal Behavior. Cocoa Butter Relat. Compd. 2012, 245–274. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Matrix effects on flavour volatiles release in dark chocolates varying in particle size distribution and fat content using GC–mass spectrometry and GC–olfactometry. Food Chem. 2009, 113, 208–215. [Google Scholar] [CrossRef]
- Valle-Epquín, M.G.; Balcázar-Zumaeta, C.R.; Auquiñivín-Silva, E.A.; Fernández-Jeri, A.B.; Idrogo-Vásquez, G.; Castro-Alayo, E.M. The roasting process and place of cultivation influence the volatile fingerprint of Criollo cocoa from Amazonas, Peru. Sci. Agropecu. 2020, 11, 599–610. [Google Scholar] [CrossRef]
- Hinneh, M.; Semanhyia, E.; Van de Walle, D.; De Winne, A.; Tzompa-Sosa, D.A.; Scalone, G.L.L.; De Meulenaer, B.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; et al. Assessing the influence of pod storage on sugar and free amino acid profiles and the implications on some Maillard reaction related flavor volatiles in Forastero cocoa beans. Food Res. Int. 2018, 111, 607–620. [Google Scholar] [CrossRef]
- Marseglia, A.; Musci, M.; Rinaldi, M.; Palla, G.; Caligiani, A. Volatile fingerprint of unroasted and roasted cocoa beans (Theobroma cacao L.) from different geographical origins. Food Res. Int. 2020, 132, 109101. [Google Scholar] [CrossRef]
- Hinneh, M.; Van de Walle, D.; Tzompa-Sosa, D.A.; Haeck, J.; Abotsi, E.E.; De Winne, A.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; De Cooman, L.; et al. Comparing flavor profiles of dark chocolates refined with melanger and conched with Stephan mixer in various alternative chocolate production techniques. Eur. Food Res. Technol. 2019, 245, 837–852. [Google Scholar] [CrossRef]
- Engeseth, N.J.; Pangan, M.F.A. Current context on chocolate flavor development—A review. Curr. Opin. Food Sci. 2018, 21, 84–91. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Effect of Fermentation Method, Roasting and Conching Conditions on the Aroma Volatiles of Dark Chocolate. J. Food Process. Preserv. 2012, 36, 446–456. [Google Scholar] [CrossRef]
- Acierno, V.; Yener, S.; Alewijn, M.; Biasioli, F.; Van Ruth, S. Factors contributing to the variation in the volatile composition of chocolate: Botanical and geographical origins of the cocoa beans, and brand-related formulation and processing. Food Res. Int. 2016, 84, 86–95. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Palla, G. Cocoa: Production, Chemistry, and Use. Encycl. Food Health 2015, 185–190. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Gysel, L.; Maridueña-Zavala, M.G.; Molina-Miranda, M.J. Time-Related Changes in Volatile Com-pounds during Fermentation of Bulk and Fine-Flavor Cocoa (Theobroma cacao) Beans. J. Food Qual. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Samaniego, I.; Espín, S.; Quiroz, J.; Ortiz, B.; Carrillo, W.; García-Viguera, C.; Mena, P. Effect of the growing area on the methylxanthines and flavan-3-ols content in cocoa beans from Ecuador. J. Food Compos. Anal. 2020, 88, 103448. [Google Scholar] [CrossRef]
- Anecacao, 2019. Cacao Nacional|Anecacao Ecuador. Cacao Nac. Available online: http://www.anecacao.com/index.php/es/quienes-somos/cacao-nacional.html (accessed on 8 December 2020).
- Dang, Y.K.T.; Nguyen, H.V.H. Effects of Maturity at Harvest and Fermentation Conditions on Bioactive Compounds of Cocoa Beans. Plant Foods Hum. Nutr. 2019, 74, 54–60. [Google Scholar] [CrossRef]
- Pedan, V.; Weber, C.; Do, T.; Fischer, N.; Reich, E.; Rohn, S. HPTLC fingerprint profile analysis of cocoa proanthocyanidins depending on origin and genotype. Food Chem. 2018, 267, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Scollo, E.; Neville, D.C.; Oruna-Concha, M.J.; Trotin, M.; Cramer, R. UHPLC–MS/MS analysis of cocoa bean proteomes from four different genotypes. Food Chem. 2020, 303, 125244. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O. Cocoa Cultivation and Practices. In Chocolate Science and Technology; Wiley Blackwell: Singapore, 2010; pp. 16–18. [Google Scholar]
- Rottiers, H.; Sosa, D.A.T.; Van de Vyver, L.; Hinneh, M.; Everaert, H.; De Wever, J.; Messens, K.; Dewettinck, K. Discrimination of Cocoa Liquors Based on Their Odor Fingerprint: A Fast GC Electronic Nose Suitability Study. Food Anal. Methods 2019, 12, 475–488. [Google Scholar] [CrossRef]
- Scalone, G.L.L.; Textoris-Taube, K.; De Meulenaer, B.; De Kimpe, N.; Wöstemeyer, J.; Voigt, J. Cocoa-specific flavor components and their peptide precursors. Food Res. Int. 2019, 123, 503–515. [Google Scholar] [CrossRef]
- Calva-Estrada, S.; Utrilla-Vázquez, M.; Vallejo-Cardona, A.; Roblero-Pérez, D.; Lugo-Cervantes, E. Thermal properties and volatile compounds profile of commercial dark-chocolates from different genotypes of cocoa beans (Theobroma cacao L.) from Latin America. Food Res. Int. 2020, 136, 109594. [Google Scholar] [CrossRef]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of turning, pod storage and fermentation time on microbial ecology and volatile composition of cocoa beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.; Contreras-Ramos, S.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Torres-Moreno, M.; Tarrega, A.; Blanch, C. Effect of cocoa roasting time on volatile composition of dark chocolates from different origins determined by HS-SPME/GC-MS. CyTA—J. Food 2021, 19, 81–95. [Google Scholar] [CrossRef]
- Lemarcq, V.; Van de Walle, D.; Monterde, V.; Sioriki, E.; Dewettinck, K. Assessing the flavor of cocoa liquor and chocolate through instrumental and sensory analysis: A critical review. Crit. Rev. Food Sci. Nutr. 2021, 62, 5523–5539. [Google Scholar] [CrossRef]
- Schlüter, A.; Hühn, T.; Kneubühl, M.; Chatelain, K.; Rohn, S.; Chetschik, I. Novel Time- and Location-Independent Postharvest Treatment of Cocoa Beans: Investigations on the Aroma Formation during “Moist Incubation” of Unfermented and Dried Cocoa Nibs and Comparison to Traditional Fermentation. J. Agric. Food Chem. 2020, 68, 10336–10344. [Google Scholar] [CrossRef]
- van Ruth, S.M. Methods for gas chromatography-olfactometry: A review. Biomol. Eng. 2001, 17, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hinneh, M.; Abotsi, E.E.; Van de Walle, D.; Tzompa-Sosa, D.A.; De Winne, A.; Simonis, J.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; De Cooman, L.; et al. Pod storage with roasting: A tool to diversifying the flavor profiles of dark chocolates produced from ‘bulk’ cocoa beans? (Part I: Aroma profiling of chocolates). Food Res. Int. 2019, 119, 84–98. [Google Scholar] [CrossRef]
- Parker, J. Introduction to aroma compounds in foods. In Flavour Development, Analysis and Perception In Food and Beverages; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Servent, A.; Boulanger, R.; Davrieux, F.; Pinot, M.-N.; Tardan, E.; Forestier-Chiron, N.; Hue, C. Assessment of cocoa ( Theobroma cacao L.) butter content and composition throughout fermentations. Food Res. Int. 2018, 107, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Budu, A.S.; Saalia, F.K. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 2013, 50, 1097–1105. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Budu, A.S.; Takrama, J.; Saalia, F.K. Effect of pulp preconditioning on acidification, proteolysis, sugars and free fatty acids concentration during fermentation of cocoa (Theobroma cacao) beans. Int. J. Food Sci. Nutr. 2011, 62, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Quao, J.; Takrama, F.S.; Budu, A.S.; Saalia, F.K. Changes in total polyphenols, o-diphenols and anthocyanin concentrations during fermentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int. Food Res. J. 2012, 19, 1071–1077. [Google Scholar]
- D’Souza, R.N.; Grimbs, A.; Grimbs, S.; Behrends, B.; Corno, M.; Ullrich, M.S.; Kuhnert, N. Degradation of cocoa proteins into oligopeptides during spontaneous fermentation of cocoa beans. Food Res. Int. 2018, 109, 506–516. [Google Scholar] [CrossRef]
- Kumari, N.; Kofi, K.J.; Grimbs, S.; D’Souza, R.N.; Kuhnert, N.; Vrancken, G.; Ullrich, M.S. Biochemical fate of vicilin storage protein during fermentation and drying of cocoa beans. Food Res. Int. 2016, 90, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Textoris-Taube, K.; Wöstemeyer, J. pH-Dependency of the proteolytic formation of cocoa- and nutty-specific aroma precursors. Food Chem. 2018, 255, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Sunoj, S.; Igathinathane, C.; Visvanathan, R. Nondestructive determination of cocoa bean quality using FT-NIR spectroscopy. Comput. Electron. Agric. 2016, 124, 234–242. [Google Scholar] [CrossRef]
- Afoakwa, E.; Jennifer, Q.; Agnes, S.B.; Jemmy, S.T.; Firibu, K.S. Influence of pulp-preconditioning and fermentation on fer-mentative quality and appearance of ghanaian cocoa (Theobroma cacao) beans. Int. Food Res. J. 2012, 19, 127–133. [Google Scholar]
- Ilangantileke, S.G.; Wahyudi, T.; Bailon, M.G. Assessment methodology to predict quality of cocoa beans for export. J. Food Qual. 1991, 14, 481–496. [Google Scholar] [CrossRef]
- Brunetto, M.D.R.; Gallignani, M.; Orozco, W.; Clavijo, S.; Delgado, Y.; Ayala, C.; Zambrano, A. The effect of fermentation and roasting on free amino acids profile in Criollo cocoa (Theobroma cacao L.) grown in Venezuela. Braz. J. Food Technol. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Crafack, M.; Keul, H.; Eskildsen, C.E.; Petersen, M.A.; Saerens, S.; Blennow, A.; Skovmand-Larsen, M.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Impact of starter cultures and fermentation techniques on the volatile aroma and sensory profile of chocolate. Food Res. Int. 2014, 63, 306–316. [Google Scholar] [CrossRef]
- Reineccius, G.A.; Andersen, D.A.; Kavanagh, T.E.; Keeney, P.G. Identification and Quantification of the Free Sugars in Cocoa Beans. J. Agric. Food Chem. 1972, 20, 199–202. [Google Scholar] [CrossRef]
- Domínguez-Pérez, L.A.; Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Artisanal cocoa bean fermentation: From cocoa bean proteins to bioactive peptides with potential health benefits. J. Funct. Foods 2020, 73, 104134. [Google Scholar] [CrossRef]
- Kumari, N.; Grimbs, A.; D’Souza, R.N.; Verma, S.K.; Corno, M.; Kuhnert, N.; Ullrich, M.S. Origin and varietal based proteomic and peptidomic fingerprinting of Theobroma cacao in non-fermented and fermented cocoa beans. Food Res. Int. 2018, 111, 137–147. [Google Scholar] [CrossRef]
- Tchouatcheu, G.A.N.; Noah, A.M.; Lieberei, R.; Niemenak, N. Effect of cacao bean quality grade on cacao quality evaluation by cut test and correlations with free amino acids and polyphenols profiles. J. Food Sci. Technol. 2019, 56, 2621–2627. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Coll, F.V. Factors Affecting the Formation of Alkylpyrazines during Roasting Treatment in Natural and Alkalinized Cocoa Powder. J. Agric. Food Chem. 2002, 50, 3743–3750. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Prandi, B.; Palla, G.; Sforza, S. Influence of fermentation level and geographical origin on cocoa bean oligopeptide pattern. Food Chem. 2016, 211, 431–439. [Google Scholar] [CrossRef]
- Marseglia, A.; Palla, G.; Caligiani, A. Presence and variation of γ-aminobutyric acid and other free amino acids in cocoa beans from different geographical origins. Food Res. Int. 2014, 63, 360–366. [Google Scholar] [CrossRef]
- ICCO. (n.d.). Cultivo de cacao - Organización Internacional del Cacao. Available online: https://www.icco.org/growing-cocoa/ (accessed on 27 May 2021).
- Niether, W.; Smit, I.; Armengot, L.; Schneider, M.; Gerold, G.; Pawelzik, E. Environmental Growing Conditions in Five Production Systems Induce Stress Response and Affect Chemical Composition of Cocoa (Theobroma cacao L.) Beans. J. Agric. Food Chem. 2017, 65, 10165–10173. [Google Scholar] [CrossRef] [PubMed]
- Selamat, J.; Harun, S.M.; Ghazali, N.M. Formation of Methyl Pyrazine during Cocoa Bean Fermentation. Pertanika 1994, 17, 27–32. [Google Scholar]
- Counet, C.; Ouwerx, C.; Rosoux, D.; Collin, S. Relationship between procyanidin and flavor contents of cocoa liquors from different origins. J. Agric. Food Chem. 2004, 52, 6243–6249. [Google Scholar] [CrossRef]
- Rentería, O.; Pliego-Arreaga, R.; Regalado, C.; Amaro-Reyes, A.; García-Almendárez, B.E. Enhancing isoamyl acetate biosynthesis by Pichia fermentans. Rev. Mex. Ing. Química 2021, 12, 505–511. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Ziegleder, G. Linalool contents as characteristic of some flavor grade cocoas. Z. Lebensm. Unters. Forsch. 1990, 191, 306–309. [Google Scholar] [CrossRef]
- Maga, J.A. Pyrazine update. Food Rev. Int. 1992, 8, 479–558. [Google Scholar] [CrossRef]
- Ziegleder, G. Composition of flavor extracts of raw and roasted cocoas. Z. Lebensm. Unters. Forsch. 1991, 192, 521–525. [Google Scholar] [CrossRef]
- Perotti, P.; Cordero, C.; Bortolini, C.; Rubiolo, P.; Bicchi, C.; Liberto, E. Cocoa smoky off-flavor: Chemical characterization and objective evaluation for quality control. Food Chem. 2020, 309, 125561. [Google Scholar] [CrossRef] [PubMed]
- Lemarcq, V.; Tuenter, E.; Bondarenko, A.; Van de Walle, D.; De Vuyst, L.; Pieters, L.; Sioriki, E.; Dewettinck, K. Roasting-induced changes in cocoa beans with respect to the mood pyramid. Food Chem. 2020, 332, 127467. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Ieri, F.; Campo, M.; Paolino, D.; Restuccia, D.; Romani, A. Biogenic Amines, Phenolic, and Aroma-Related Compounds of Unroasted and Roasted Cocoa Beans with Different Origin. Foods 2019, 8, 306. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; He, C.; Song, H.; Liu, Y.; Zhang, Y.; Wang, Y.; Guo, J.; Yang, H.; Su, X. Characterization and comparison of key aroma-active compounds of cocoa liquors from five different areas. Int. J. Food Prop. 2017, 20, 2396–2408. [Google Scholar] [CrossRef]
- Misnawi, J.; Ariza, B.T.S. Use of gas Chromatography-Olfactometry in combination with solid phase micro extraction for cocoa liquor aroma analysis. Int. Food Res. J. 2011, 18, 829–835. [Google Scholar]
- Dand, R. Cocoa bean processing and the manufacture of chocolate. In The International Cocoa Trade, 3rd ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 268–289. [Google Scholar] [CrossRef]
- Beckett, S.T.; Paggios, K.; Roberts, I. Conching. In Beckett’s Industrial Chocolate Manufacture and Use; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef] [PubMed]
- Toker, O.S.; Palabiyik, I.; Konar, N. Chocolate quality and conching. Trends Food Sci. Technol. 2019, 91, 446–453. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Relationship of sensory and instrumental aroma measurements of dark chocolate as influenced by fermentation method, roasting and conching conditions. J. Food Sci. Technol. 2013, 50, 909–917. [Google Scholar] [CrossRef]
- Toker, O.S.; Palabiyik, I.; Pirouzian, H.R.; Aktar, T.; Konar, N. Chocolate aroma: Factors, importance and analysis. Trends Food Sci. Technol. 2020, 99, 580–592. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; He, C.; Song, H.; Guo, J.; Wang, Y.; Yang, H.; Su, X. A comparative study of aroma-active compounds between dark and milk chocolate: Relationship to sensory perception. J. Sci. Food Agric. 2015, 95, 1362–1372. [Google Scholar] [CrossRef]
- Kitani, Y.; Putri, S.P.; Fukusaki, E. Investigation of the effect of processing on the component changes of single-origin chocolate during the bean-to-bar process. J. Biosci. Bioeng. 2022, 134, 138–143. [Google Scholar] [CrossRef]
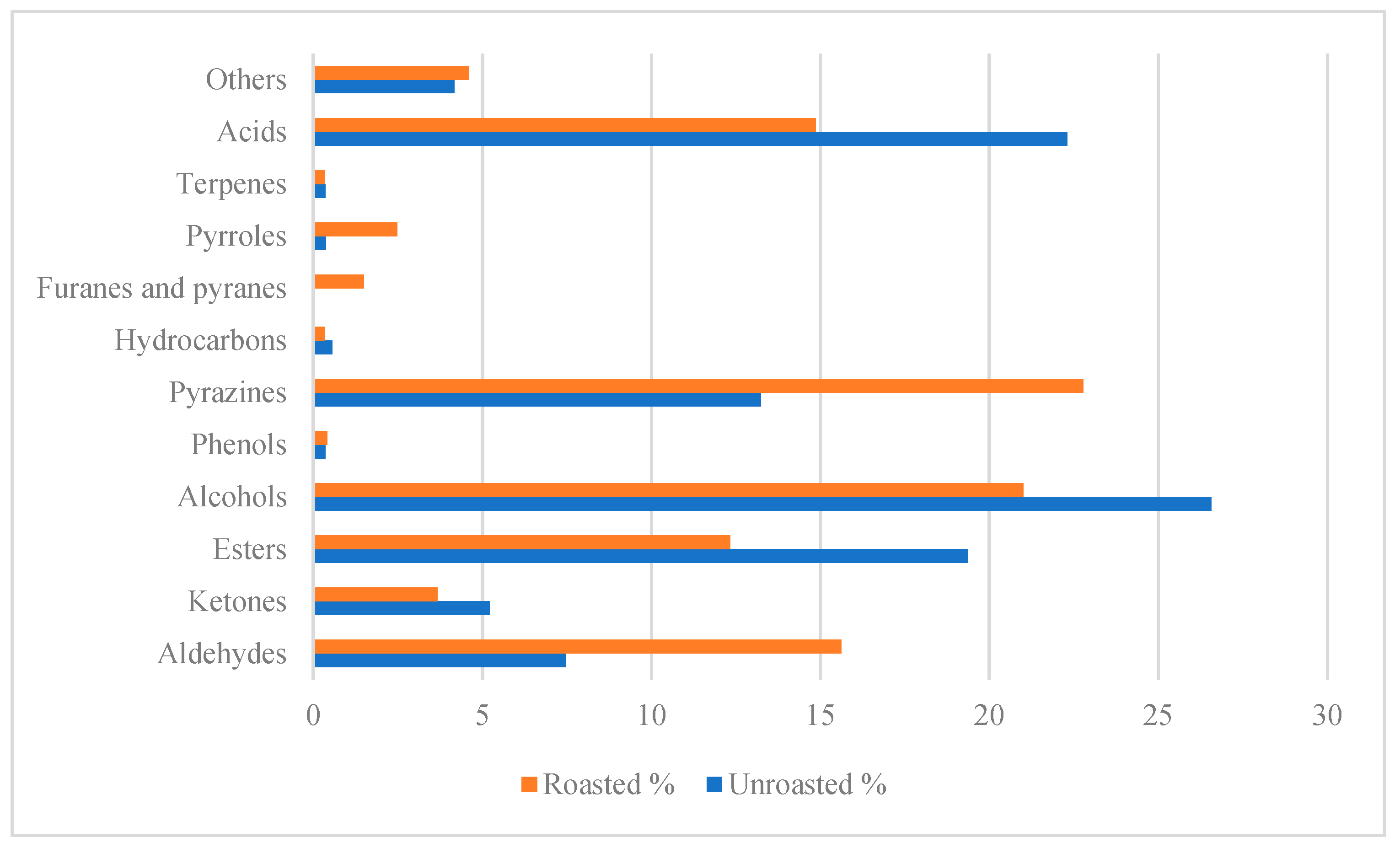
| No | Volatile | Odor | Dilution Factors (FD) | |
|---|---|---|---|---|
| Unroasted | Roasted | |||
| 1 | 4-hydroxy-2,5-dimethyl-3 (2H)-furanone | Like caramel | 128 | 8192 |
| 1 | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | Caramel-like | 128 | 8192 |
| 2 | 2- and 3-Methylbutanale | Malty | 64 | 4096 |
| 3 | Phenylacetaldehyde | Honey-like | 64 | 4096 |
| 4 | 2–and 3-Methylbutanoic acid | Rancid | 8192 | 4096 |
| 5 | 2-Acetyl-1-pyrroline | Popcorn-like | 32 | 1024 |
| 6 | Acetic acid | Acetic | 2048 | 1024 |
| 7 | 2-Methoxyphenol | Smoky | 256 | 512 |
| 8 | 2-Phenylethanol | Flowery | 256 | 512 |
| 9 | 2-Ethyl-3,5-dimethylpyrazine | Earthy | 64 | 256 |
| 10 | Linalool | Flowery | 64 | 256 |
| 11 | Methyl propanoic acid | Rancid | 256 | 256 |
| 12 | 2-Methyl-3-(methyldithio)furan | Cooked meat-like | 128 | 256 |
| 13 | Ethyl methyl propanoate | Fruity | 64 | 128 |
| 14 | Ethyl 2-methylbutanoate | Fruity | 256 | 128 |
| 15 | Dimethyl trisulfide | Sulfur-like | 64 | 128 |
| 16 | 2,3,5-Trimethylpyrazine | Earthy | 32 | 128 |
| 17 | Phenylethyl acetate | Flowery | 64 | 128 |
| 18 | δ-Decenolactone | Coconut-like | 256 | 128 |
| 19 | Phenylethyl acetic acid | Sweet | 256 | 128 |
| 20 | Ethyl 3-methylbutanoate | Fruity | 32 | 64 |
| 21 | 2,3-Diethyl-5-methylpyrazine | Earthy | 2 | 64 |
| 22 | Butanoic acid | Rancid | 128 | 64 |
| 23 | δ-Octenolactone | Coconut-like | 32 | 64 |
| 24 | cis-Isoeugenol | Smoky | 64 | 64 |
| 25 | 3-Hydroxy-4,5-dimethyl-2(5H)-furanone | Spiced | 1024 | - |
| * Variety | ** Matrix | Volatile Compounds | Total Volatiles | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acids | Alcohols | Aldehydes | Ketones | Esters | Ethers | Pyrazines | Lactones | Furans | Furanones | Pyrans, Pyrodons and Pyrroles | Terpenes and Terpenoids | Other | ||||
| CR | CL | 2 | 4 | 4 | 2 | 7 | 7 | 6 | 2 | 34 | [2] | |||||
| TR | DB | 3 | 10 | 5 | 6 | 7 | 4 | 1 | 5 | 41 | [4] | |||||
| NC, CCN51 | RB | 4 | 10 | 7 | 10 | 11 | 9 | 4 | 4 | 3 | 6 | 2 | 70 | [5] | ||
| FR | DB | 6 | 3 | 4 | 1 | 3 | 5 | 2 | 1 | 2 | 1 | 3 | 31 | [6] | ||
| CR, FR, TR | UDB | 4 | 12 | 4 | 4 | 7 | 2 | 2 | 5 | 9 | 3 | 52 | [7] | |||
| CR | DB | 6 | 7 | 10 | 5 | 11 | 5 | 1 | 4 | 1 | 50 | [8] | ||||
| CH | 6 | 1 | 9 | 7 | 11 | 8 | 1 | 1 | 2 | 1 | 2 | 49 | [10] | |||
| CH | 4 | 4 | 7 | 6 | 3 | 6 | 2 | 1 | 2 | 35 | [11] | |||||
| NC, FR | CL | 3 | 11 | 8 | 7 | 9 | 12 | 1 | 1 | 2 | 4 | 8 | 1 | 67 | [12] | |
| FR | DB | 10 | 9 | 3 | 5 | 11 | 1 | 39 | [14] | |||||||
| RB | 9 | 8 | 10 | 1 | 3 | 2 | 1 | 5 | 39 | [21] | ||||||
| FR | CL | 3 | 7 | 7 | 10 | 8 | 15 | 4 | 3 | 10 | 6 | 7 | 80 | [23] | ||
| CR, FR, NC | DB | 9 | 12 | 14 | 5 | 2 | 12 | 6 | 60 | [28] | ||||||
| CH | 11 | 14 | 13 | 11 | 8 | 16 | 2 | 5 | 3 | 2 | 85 | [37] | ||||
| FR | DB | 11 | 12 | 3 | 5 | 20 | 4 | 3 | 58 | [39] | ||||||
| FR | CH | 4 | 4 | 4 | 3 | 3 | 5 | 1 | 1 | 1 | 6 | 1 | 33 | [44] | ||
| FR | CL | 2 | 9 | 7 | 5 | 9 | 9 | 2 | 6 | 3 | 52 | [57] | ||||
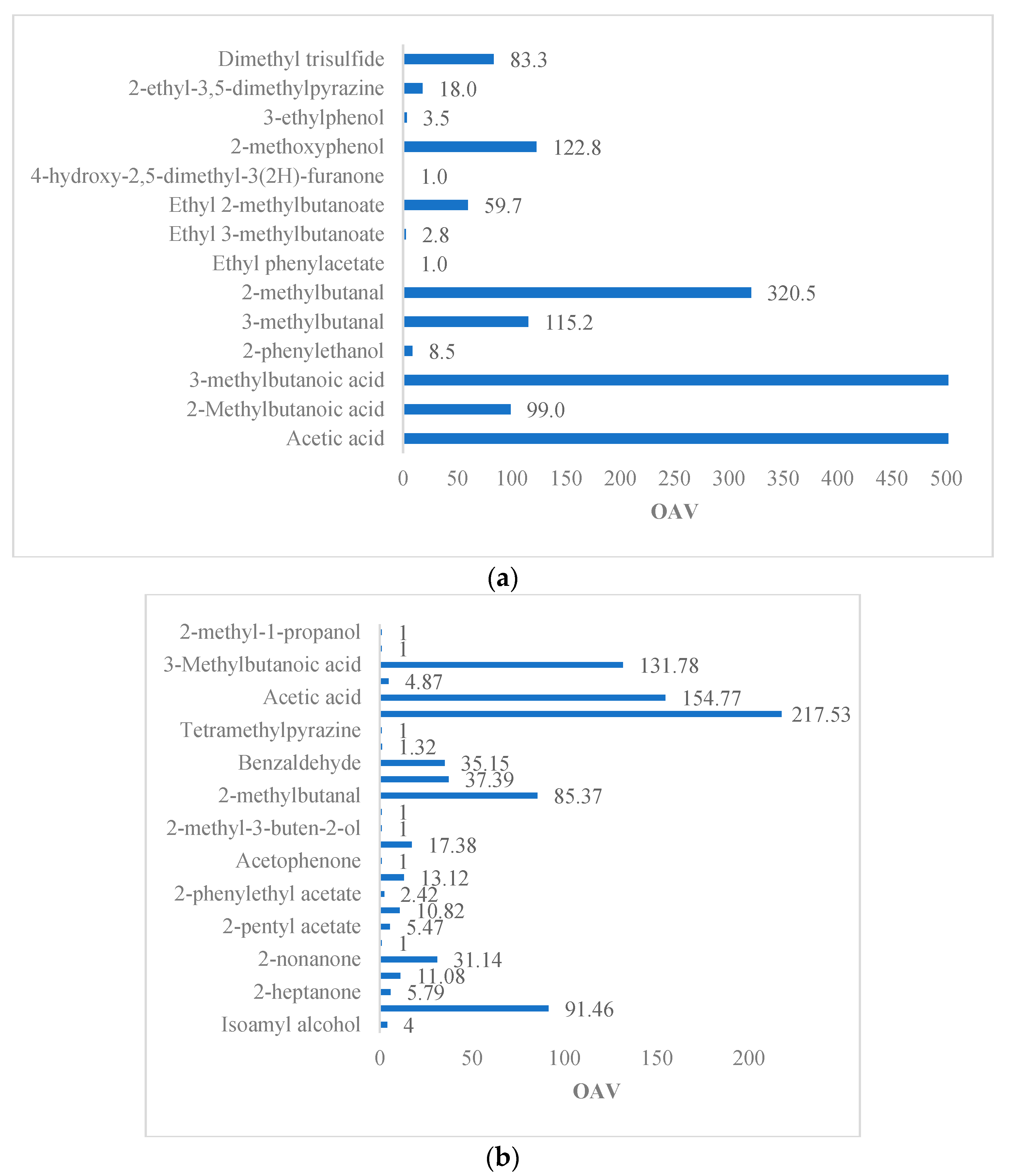
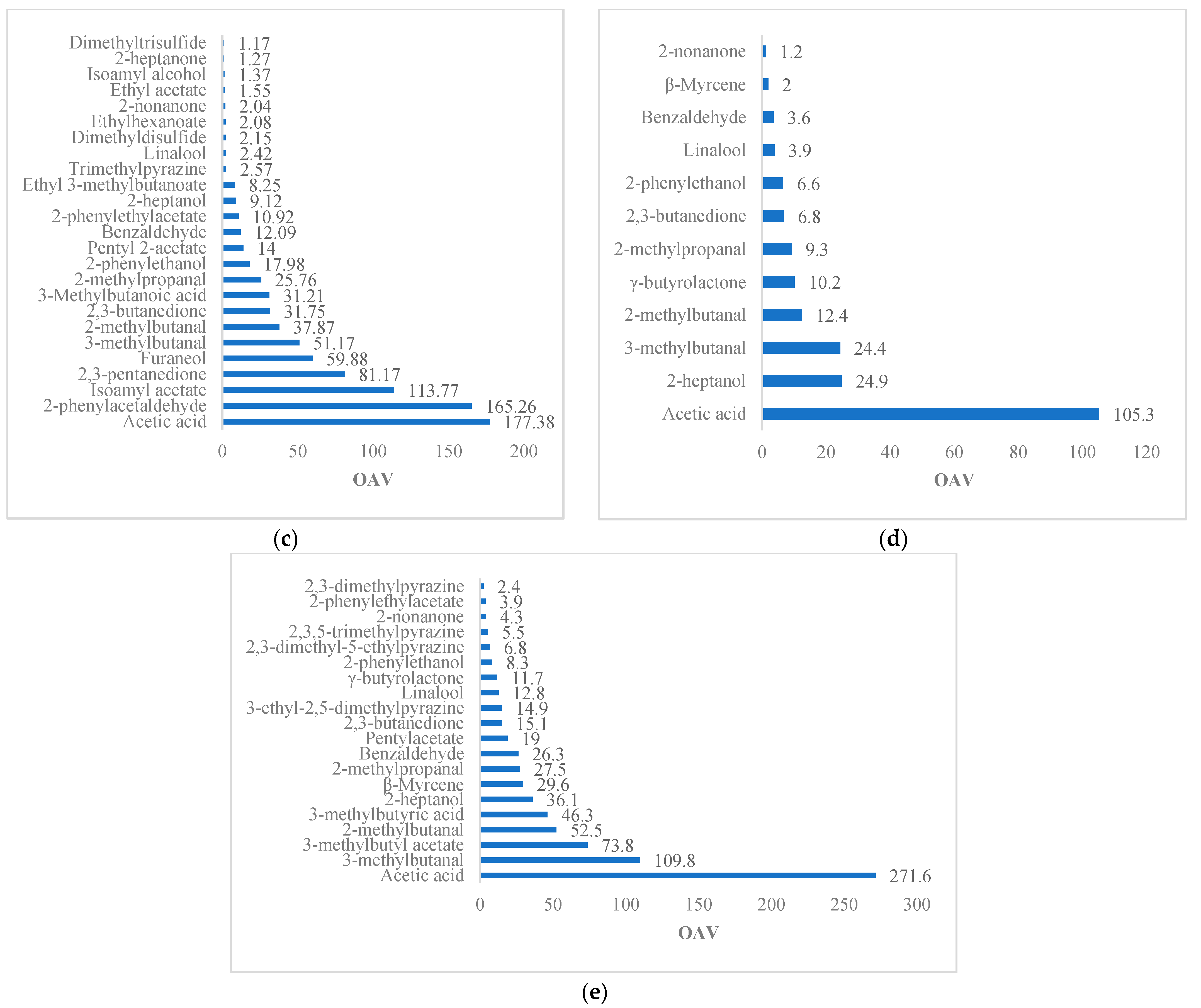
| Reference | Variety | Method | Matrix | Volatile | OTV (μg/kg) | OAV | Odor Description |
|---|---|---|---|---|---|---|---|
| [5] | Trinitario (Sacha Gold) | HS-SPME GC–MS | Dried beans | Isoamyl alcohol | 100 | 4 | Banana, fruity, fermented, cognac |
| Isoamyl acetate | 9.6 | 91.46 | Banana, fruity | ||||
| 2-heptanone | 300–98,000 | 5.79 | Fruity, coconut, floral, cheesy | ||||
| 2-heptanol | 263 | 11.08 | Citrus, fruity | ||||
| 2-nonanone | 100 | 31.14 | Fruity, fresh, sweet | ||||
| Ethyl acetate | 940–22,000 | 1 | Pineapple, fruity, sweet, grape | ||||
| 2-pentyl acetate | 13–27,000 | 5.47 | Fruity, orange, tropical | ||||
| 2-Phenylacetaldehyde | 22–154 | 10.82 | Floral, honey | ||||
| 2-phenylethyl acetate | 137–233 | 2.42 | Floral, honey | ||||
| 2-phenylethanol | 211 | 13.12 | Floral, honey | ||||
| Acetophenone | 5629 | 1 | Floral | ||||
| Linalool | 37 | 17.38 | Floral, pink, sweet, green, citrus | ||||
| 2-methyl-3-buten-2-ol | 480 | 1 | Herbal, earthy | ||||
| 2-octanol | 100 | 1 | Spicy, green, woody, earthy | ||||
| 2-methylbutanal | 2.2–152 | 85.37 | Chocolate | ||||
| 3-methylbutanal | 5.4–80 | 37.39 | Chocolate | ||||
| Benzaldehyde | 60 | 35.15 | Sweet, bitter almond, cherry, woody | ||||
| Trimethylpyrazine | 290 | 1.32 | Cocoa, roasted nuts | ||||
| Tetramethylpyrazine | 38,000 | 1 | Chocolate, cocoa, coffee | ||||
| 2,3-butanedione | 3–10 | 217.53 | Buttery | ||||
| Acetic acid | 124 | 154.77 | Sour vinegar | ||||
| 2-methylpropanoic acid | 190–755 | 4.87 | Rancid butter | ||||
| 3-methylbutanoic acid | 22 | 131.78 | Rancid sweat | ||||
| Ethanol | 3 × 104–6 × 105 | 1 | Alcoholic | ||||
| 2-methyl-1-propanol | 1000 | 1 | Wine, ethereal | ||||
| Acetic acid | 124 | 8467.7 | bitter, vinegar | ||||
| 2-methylbutanoic acid | 203 | 99.0 | spicy, sweaty | ||||
| 3-methylbutanoic acid | 11 | 6590.9 | spicy, sweaty | ||||
| 2-phenylethanol | 211 | 8.5 | flowery | ||||
| 3-methylbutanal | 5.4 | 115.2 | of malt | ||||
| 2-methylbutanal | 2.2 | 320.5 | of malt | ||||
| Fethyl enilacetate | 300 | 1.0 | flowery, fruity | ||||
| [42] | Trinitario | GC−OAEDA-AIDS | Dried beans | Ethyl 3-methylbutanoate | 0.98 | 2,8 | tasty |
| 2-phenylethyl acetate | 0.137 | 9489.05 | Nuts | ||||
| Ethyl 2-methylbutanoate | 0.37 | 59.7 | tasty | ||||
| 4-hydroxy-2,5-dimethyl-3 (2H)-furanone | 27 | 1.0 | like caramel | ||||
| 2-Methoxyphenol | 1.8 | 122.8 | Smoked | ||||
| 3-Ethylphenol | 2.2 | 3.5 | phenolic, animalic | ||||
| 2-ethyl-3,5-dimethylpyrazine | 2.2 | 18.0 | earthy | ||||
| 2-ethyl-3,6-dimethylpyrazine | 57 | 0.01 | earthy | ||||
| dimethyl trisulfide | 0.03 | 83.3 | Cabbage |
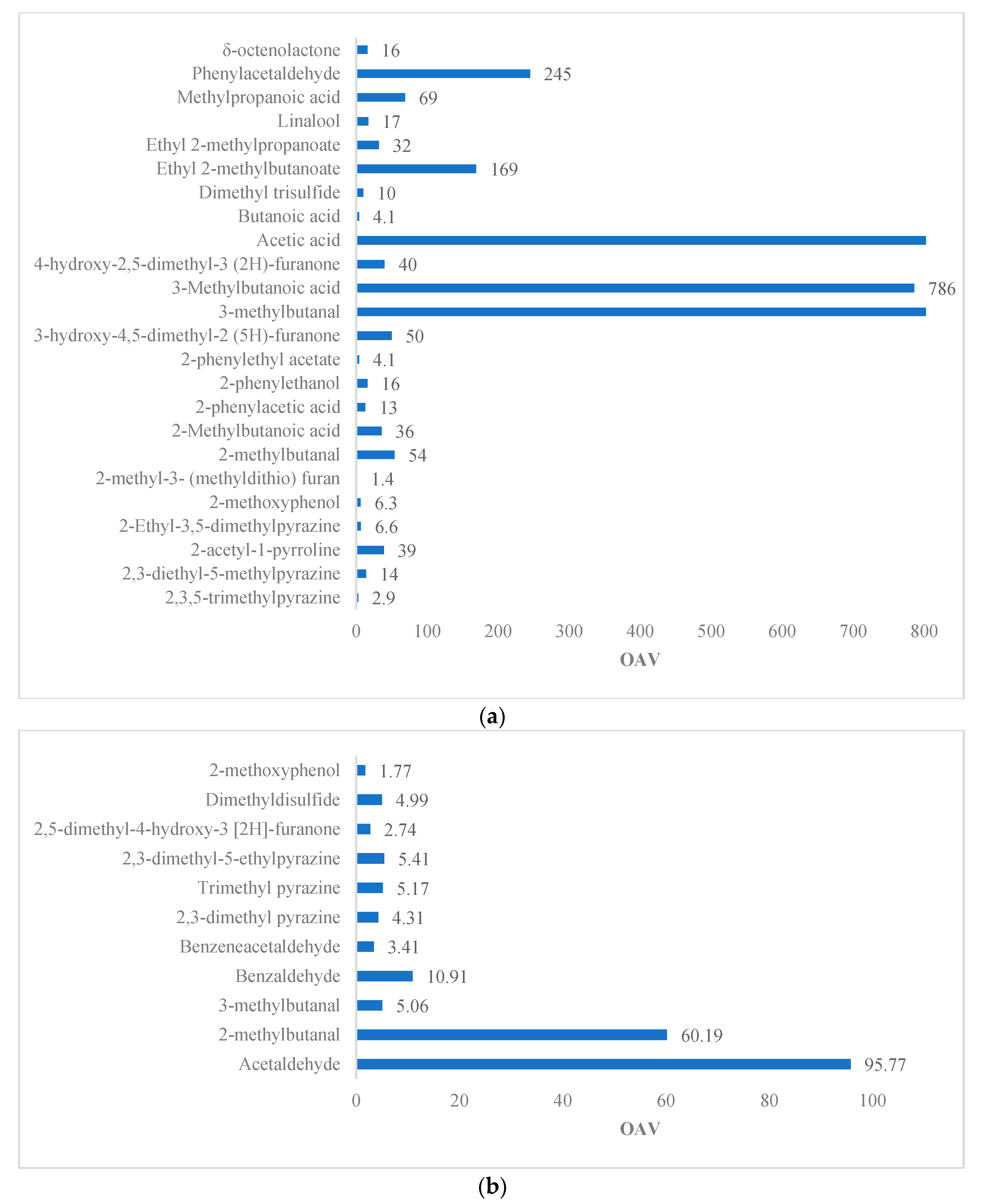
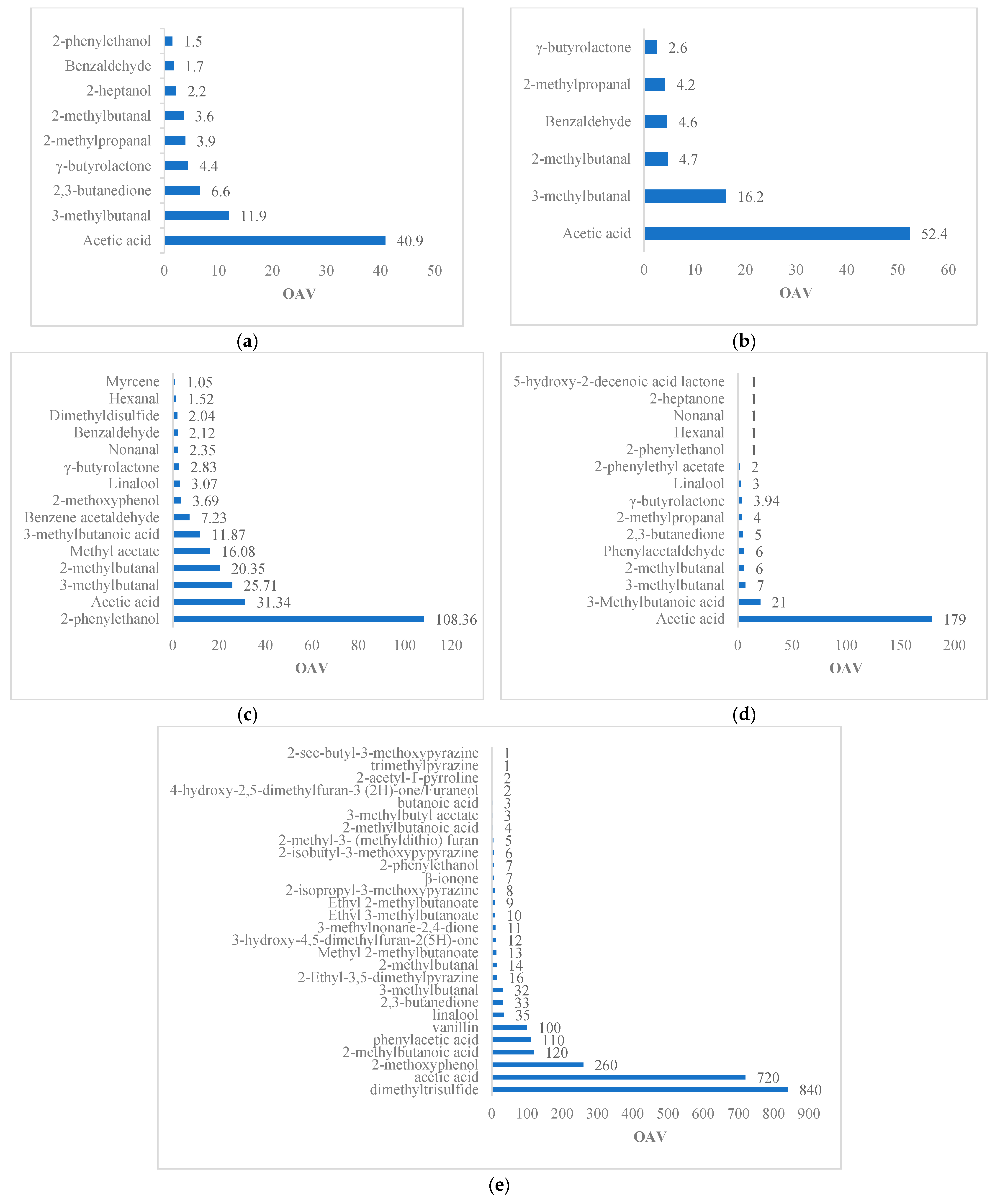
| Reference | Variety | Method | Matrix | Volatile | OTV (ng/g) | OAV | Odor Description |
|---|---|---|---|---|---|---|---|
| [6] | Forastero | SAFE-AEDA | Roasted beans | 2. 3. 5-trimethylpyrazine | 290 | 2.9 | Earthy |
| 2. 3-diethyl-5-methylpyrazine | 0.5 | 14 | Roasted potato | ||||
| 2-acetyl-1-pyrroline | 0.1 | 39 | Like popcorn | ||||
| 2-ethyl-3. 5-Dimethylpyrazine | 2.2 | 6.6 | Chocolate, sweet | ||||
| 2-Methoxyphenol | 16 | 6.3 | Smoked | ||||
| 2-methyl-3- (methyldithio) furan | 0.4 | 1.4 | Similar to cooked meat | ||||
| 2-methylbutanal | 140 | 54 | Malt | ||||
| 2-methylbutanoic acid | 203 | 36 | Rancid | ||||
| 2-phenylacetic acid | 360 | 13 | Floral smell, nasty geranium | ||||
| 2-phenylethanol | 211 | 16 | Flowery | ||||
| 2-phenylethyl acetate | 233 | 4.1 | Floral, honey | ||||
| 3-hydroxy-4. 5-dimethyl-2 (5H)-furanone | 0.2 | 50 | Spicy | ||||
| 3-methylbutanal | 13 | 2030 | Malt | ||||
| 3-methylbutanoic acid | 22 | 786 | Rancid | ||||
| 4-hydroxy-2. 5-dimethyl-3 (2H)-furanone | 25 | 40 | Like to caramel | ||||
| Acetic acid | 124 | 4920 | Sour, vinegar | ||||
| Butanoic acid | 135 | 4.1 | Rancid | ||||
| Dimethyl trisulfide | 2.5 | 10 | Sulfuric | ||||
| Ethyl 2-methylbutanoate | 0.26 | 169 | Tasty | ||||
| Ethyl 2-methylpropanoate | 1.24 | 32 | Tasty | ||||
| Linalool | 37 | 17 | Flowery | ||||
| Methylpropanoic acid | 190 | 69 | Rancid | ||||
| Phenylacetaldehyde | 22 | 245 | Like honey | ||||
| δ-octenolactone | 4730 | 16 | Like coconut | ||||
| [21] | Forastero | HS–SPME–GC–MS | Roasted beans | Acetaldehyde | 0.22 | 96.78 | Sour, fruity |
| 2-methylbutanal | 2.2–152 | 53.36 | Chocolate | ||||
| 3-methylbutanal | 5.9–80 | 5.35–79.32 | Chocolate | ||||
| Benzaldehyde | 60 | 18.08 | Sweet almond, bitter, cherry | ||||
| Benzeneacetaldehyde | 22–154 | 2.41 | Honey, sweet, pink, floral | ||||
| 2. 3-Dimethyl pyrazine | 123 | 1.45 | Caramel, cocoa | ||||
| Trimethylpyrazine | 290 | 1.64 | Cocoa, toasted walnuts, peanuts | ||||
| 2. 3-dimethyl-5-ethylpyrazine | 60 | 2.95 | Popcorn, burnt cocoa, toasted | ||||
| 2. 5-dimethyl-4-hydroxy-3 [2H]-furanone | 1.6–50 | 1.15–36.06 | - | ||||
| Dimethyl disulfide | 12 | 3.68 | Sulfur-like, cabbage, onion | ||||
| 2-Methoxyphenol | 10–70 | 6.81 | Smoked, repulsive |
| Variety | OAV * | Odor Contribution % | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fruity | Floral | Chocolate | Buttery | Spices | Caramel | Undesirables | Earthy | |||
| CCN51 | >1 | 31.82 | 13.64 | 22.73 | 13.64 | - | - | 18.18 | - | [4] |
| National | 4- | 16.00 | 2- | 8.00 | - | - | 16.00 | - | ||
| Forastero | 17.24 | 13.79 | 27.59 | 3.45 | 3.45 | 6.90 | 24.14 | 3.45 | [9] | |
| National | 16.67 | 25.00 | 33.33 | 8.33 | - | 8.33 | 8.33 | - | [12] | |
| ** | 2- | 2- | 3- | 5.00 | - | 15.00 | 1- | - | ||
| CCN51 | <1 | 25.00 | 12.50 | 25.00 | - | - | - | 37.50 | - | [4] |
| National | 7.69 | 7.69 | 30.77 | - | - | - | 53.85 | - | ||
| Foastero | 35.29 | 17.65 | 11.76 | 11.76 | - | 5.88 | 17.65 | - | [9] | |
| National | 25.00 | 12.50 | 37.50 | - | - | 12.50 | 12.50 | - | [12] | |
| Description | OAV | Odor Contribution % | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fruit | Floral | Chocolate | Buttery | Spice | Caramel | Undesirable | Earthy | |||
| Chocolate 90% cocoa | 25.00 | 14.29 | 21.43 | 3.57 | 7.14 | 3.57 | 25.00 | - | [10] | |
| Chocolate 40% cocoa liquor | 13.33 | 26.67 | 2.00 | 13.33 | 6.67 | 6.67 | 13.33 | - | [11] | |
| * Chocolate 51.6% cocoa | >1 | 11.11 | 11.11 | 44.44 | 11.11 | - | 11.11 | 11.11 | - | [12] |
| ** Chocolate 51.6% cocoa | - | - | 66.67 | - | - | 16.67 | 16.67 | - | ||
| Chocolate 70% cocoa liquor | 6.67 | 26.67 | 2.00 | - | 6.67 | 6.67 | 33.33 | - | [44] | |
| Chocolate 90% cocoa | 23.81 | 4.76 | 9.52 | 23.81 | 9.52 | 14.29 | 14.29 | - | [10] | |
| Chocolate 40% cocoa liquor | 11.11 | - | 66.67 | 11.11 | - | - | 11.11 | - | [11] | |
| * Chocolate 51.6%cocoa | <1 | 36.36 | 27.27 | 18.18 | - | - | 18.18 | - | - | [12] |
| ** Chocolate 51.6% cocoa | 35.71 | 28.57 | 14.29 | 7.14 | - | 14.29 | - | - | ||
| Chocolate 70% cocoa liquor | 40.91 | 13.64 | 18.18 | - | - | 4.55 | 18.18 | 4.55 | [44] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quelal, O.M.; Hurtado, D.P.; Benavides, A.A.; Alanes, P.V.; Alanes, N.V. Key Aromatic Volatile Compounds from Roasted Cocoa Beans, Cocoa Liquor, and Chocolate. Fermentation 2023, 9, 166. https://doi.org/10.3390/fermentation9020166
Quelal OM, Hurtado DP, Benavides AA, Alanes PV, Alanes NV. Key Aromatic Volatile Compounds from Roasted Cocoa Beans, Cocoa Liquor, and Chocolate. Fermentation. 2023; 9(2):166. https://doi.org/10.3390/fermentation9020166
Chicago/Turabian StyleQuelal, Orlando Meneses, David Pilamunga Hurtado, Andrés Arroyo Benavides, Pamela Vidaurre Alanes, and Norka Vidaurre Alanes. 2023. "Key Aromatic Volatile Compounds from Roasted Cocoa Beans, Cocoa Liquor, and Chocolate" Fermentation 9, no. 2: 166. https://doi.org/10.3390/fermentation9020166
APA StyleQuelal, O. M., Hurtado, D. P., Benavides, A. A., Alanes, P. V., & Alanes, N. V. (2023). Key Aromatic Volatile Compounds from Roasted Cocoa Beans, Cocoa Liquor, and Chocolate. Fermentation, 9(2), 166. https://doi.org/10.3390/fermentation9020166





