Assessing the Impact of Simultaneous Co-Fermentation on Malolactic Bioconversion and the Quality of Cider Made with Low-Acidity Apples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Washing and Sanitization of Apples
2.2.2. Processing of Cider
2.2.3. The Growth Curves of Yeast and LAB
2.2.4. Monitoring of Fermentation and Characterization of Ciders
2.2.5. Analysis of Volatile Compounds
2.2.6. Sensory Analysis
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Wild LAB in the Apple Must
3.2. Kinetics of Lactic Acid Bacteria in Cider Fermentation
3.3. Evolution of Organic Acids
3.4. Synthesis of Volatile Compounds
3.5. Sensorial Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The European Cider & Fruit Wine Association. European Cider Trends 2022; AICV: Brussels, Belgium, 2022. [Google Scholar]
- Alberti, A.; Nogueira, A. Cider. In Fermented and Distilled Alcoholic Beverages: A Technological, Chemical and Sensory Overview; Castilhos, M.B.M., Del Bianchi, V.L., Manfroi, V., Eds.; Nova Science Publishers: New York, NY, USA, 2021; pp. 59–92. [Google Scholar]
- Way, M.L.; Jones, J.E.; Longo, R.; Dambergs, R.G.; Swarts, N.D. A Preliminary Study of Yeast Strain Influence on Chemical and Sensory Characteristics of Apple Cider. Fermentation 2022, 8, 455. [Google Scholar] [CrossRef]
- Ma, S.; Neilson, A.P.; Lahne, J.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free Amino Acid Composition of Apple Juices with Potential for Cider Making as Determined by UPLC-PDA. J. Inst. Brew. 2018, 124, 467–476. [Google Scholar] [CrossRef]
- Hu, L.; Chen, X.; Lin, R.; Xu, T.; Xiong, D.; Li, L.; Zhao, Z. Quality Improvement in Apple Ciders during Simultaneous Co-Fermentation through Triple Mixed-Cultures of Saccharomyces cerevisiae, Pichia kudriavzevii, and Lactiplantibacillus plantarum. Foods 2023, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Zhao, H.; Zuo, W.F.; Zhang, T.L.; Zhang, Y.; Chen, X. Sen the Effects of Simultaneous and Sequential Inoculation of Yeast and Autochthonous Oenococcus oeni on the Chemical Composition of Red-Fleshed Apple Cider. LWT Food Sci. Technol. 2020, 124, 109184. [Google Scholar] [CrossRef]
- Reuss, R.M.; Stratton, J.E.; Smith, D.A.; Read, P.E.; Cuppett, S.L.; Parkhurst, A.M. Malolactic Fermentation as a Technique for the Deacidification of Hard Apple Cider. J. Food Sci. 2010, 75, C74–C78. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Du, J. A Comparative Study of the Effect of Bacteria and Yeasts Communities on Inoculated and Spontaneously Fermented Apple Cider. Food Microbiol. 2023, 111, 104195. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Troianou, V.; Paramithiotis, S.; Proksenia, N.; Kotseridis, Y. Evaluation of Malolactic Starters in White and Rosé Winemaking of Moschofilero Wines. Appl. Sci. 2022, 12, 5722. [Google Scholar] [CrossRef]
- du Plessis, H.W.; du Toit, M.; Hoff, J.W.; Hart, R.S.; Ndimba, B.K.; Jolly, N.P. Characterisation of Non-Saccharomyces Yeasts Using Different Methodologies and Evaluation of Their Compatibility with Malolactic Fermentation. S. Afr. J. Enol. Vitic. 2017, 38, 46–63. [Google Scholar] [CrossRef]
- Saguir, F.M.; Sajur, S.A.; Pérez, M.B.; Savino, M.J.; Maturano, C. Enzymatic Activities and Fermentation Products of Lactic Acid Bacteria from Fruits and Fermented Beverages. Incidence on Food Quality. Qual. Control Beverage Ind. 2019, 17, 491–528. [Google Scholar]
- Zhang, S.; Xing, X.; Chu, Q.; Sun, S.; Wang, P. Impact of Co-Culture of Lactobacillus plantarum and Oenococcus oeni at Different Ratios on Malolactic Fermentation, Volatile and Sensory Characteristics of Mulberry Wine. LWT Food Sci. Technol. 2022, 169, 113995. [Google Scholar] [CrossRef]
- Dierings, L.R.; Braga, C.M.; da Silva, K.M.; Wosiacki, G.; Nogueira, A. Population Dynamics of Mixed Cultures of Yeast and Lactic Acid Bacteria in Cider Conditions. Braz. Arch. Biol. Technol. 2013, 56, 837–847. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, F.; Dziugan, P.; Yao, Y.; Zhang, J.; Lv, Z.; Zhang, B. Development of Organic Acids and Volatile Compounds in Cider during Malolactic Fermentation. Czech J. Food Sci. 2014, 32, 69–76. [Google Scholar] [CrossRef]
- Blanpied, G.D.; Kenneth, J.S. Predicting Harvest Date Ewindows for Apples; Cornell Cooperative Extension: Middletown, NY, USA, 1992. [Google Scholar]
- Zielinski, A.A.F.; Braga, C.M.; Demiate, I.M.; Beltrame, F.L.; Nogueira, A.; Wosiacki, G. Development and Optimization of a HPLC-RI Method for the Determination of Major Sugars in Apple Juice and Evaluation of the Effect of the Ripening Stage. Food Sci. Technol. 2014, 34, 38–43. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- De Arruda Moura Pietrowski, G.; Dos Santos, C.M.E.; Sauer, E.; Wosiacki, G.; Nogueira, A. Influence of Fermentation with Hanseniaspora Sp. Yeast on the Volatile Profile of Fermented Apple. J. Agric. Food Chem. 2012, 60, 9815–9821. [Google Scholar] [CrossRef] [PubMed]
- Dutcosky, S.D. Análise Sensorial de Alimentos, 5th ed.; PUCPRESS: Curitiba, Brazil, 2019. [Google Scholar]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.-M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- De La Luz Mohedano, M.; Russo, P.; De Los Ríos, V.; Capozzi, V.; De Palencia, P.F.; Spano, G.; López, P. A Partial Proteome Reference Map of the Wine Lactic Acid Bacterium Oenococcus oeni ATCC BAA-1163. Open Biol. 2014, 4, 130154. [Google Scholar]
- Wang, Z.; Xu, K.; Cai, R.; Yue, T.; Yuan, Y.; Gao, Z. Construction of Recombinant Fusant Yeasts for the Production of Cider with Low Alcohol and Enhanced Aroma. Eur. Food Res. Technol. 2020, 246, 745–757. [Google Scholar] [CrossRef]
- Sánchez, A.; De Revel, G.; Antalick, G.; Herrero, M.; García, L.A.; Díaz, M. Influence of Controlled Inoculation of Malolactic Fermentation on the Sensory Properties of Industrial Cider. J. Ind. Microbiol. Biotechnol. 2014, 41, 853–867. [Google Scholar] [CrossRef]
- Antón-Díaz, M.J.; Suárez Valles, B.; Mangas-Alonso, J.J.; Fernández-García, O.; Picinelli-Lobo, A. Impact of Different Techniques Involving Contact with Lees on the Volatile Composition of Cider. Food Chem. 2016, 190, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhu, Y.; Zhu, R.; Bai, J.; Qiu, J.; Wu, Y.; Zhong, K.; Gao, H. Insight into the Characteristics of Cider Fermented by Single and Co-Culture with Saccharomyces cerevisiae and Schizosaccharomyces pombe Based on Metabolomic and Transcriptomic Approaches. LWT Food Sci. Technol. 2022, 163, 113538. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of Chemical Composition and Sensorial Properties of Ciders Fermented with Different Non-Saccharomyces Yeasts in Pure and Mixed Fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.D.; Zakalik, D.L.; Cook, B.S.; Krishna Kumar, S.; Peck, G.M. The Biochemical and Physiological Basis for Hard Cider Apple Fruit Quality. Plants People Planet 2023, 5, 178–189. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile Composition of Merlot Red Wine and Its Contribution to the Aroma: Optimization and Validation of Analytical Method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Fiore, A.; la Gatta, B.; Gerardi, C.; Grieco, F.; Tufariello, M. A Chemometric Approach to the Evaluation of Sparkling Ciders Produced by Champenoise and Charmat Methods. Food Biosci. 2023, 55, 102917. [Google Scholar] [CrossRef]
- Rosend, J.; Kuldjärv, R.; Rosenvald, S.; Paalme, T. The Effects of Apple Variety, Ripening Stage, and Yeast Strain on the Volatile Composition of Apple Cider. Heliyon 2019, 5, e01953. [Google Scholar] [CrossRef]
- Villière, A.; Arvisenet, G.; Bauduin, R.; Le Quéré, J.M.; Sérot, T. Influence of Cider-Making Process Parameters on the Odourant Volatile Composition of Hard Ciders. J. Inst. Brew. 2015, 121, 95–105. [Google Scholar] [CrossRef]
 wild LAB; ■ O. oeni); (B) sugar evolution (●, ○) and ethanol production (■;
wild LAB; ■ O. oeni); (B) sugar evolution (●, ○) and ethanol production (■;  ). Note: Empty symbols “Cider I (10 days of fermentation)” and filled “Cider II (20 days of fermentation)”.
). Note: Empty symbols “Cider I (10 days of fermentation)” and filled “Cider II (20 days of fermentation)”.
 wild LAB; ■ O. oeni); (B) sugar evolution (●, ○) and ethanol production (■;
wild LAB; ■ O. oeni); (B) sugar evolution (●, ○) and ethanol production (■;  ). Note: Empty symbols “Cider I (10 days of fermentation)” and filled “Cider II (20 days of fermentation)”.
). Note: Empty symbols “Cider I (10 days of fermentation)” and filled “Cider II (20 days of fermentation)”.
 ), volatile acidity (△), and pH (○); and organic acids L-malic acid (◆); L-lactic acid (■), and D-lactic acid (▲), during cider fermentation. Note: (A,C) corresponds to alcoholic fermentation with yeast inoculum over 10 days, Cider I. (B,D) corresponds to alcoholic fermentation with yeast and lactic bacteria inoculum over 20 days, Cider II.
), volatile acidity (△), and pH (○); and organic acids L-malic acid (◆); L-lactic acid (■), and D-lactic acid (▲), during cider fermentation. Note: (A,C) corresponds to alcoholic fermentation with yeast inoculum over 10 days, Cider I. (B,D) corresponds to alcoholic fermentation with yeast and lactic bacteria inoculum over 20 days, Cider II.
 ), volatile acidity (△), and pH (○); and organic acids L-malic acid (◆); L-lactic acid (■), and D-lactic acid (▲), during cider fermentation. Note: (A,C) corresponds to alcoholic fermentation with yeast inoculum over 10 days, Cider I. (B,D) corresponds to alcoholic fermentation with yeast and lactic bacteria inoculum over 20 days, Cider II.
), volatile acidity (△), and pH (○); and organic acids L-malic acid (◆); L-lactic acid (■), and D-lactic acid (▲), during cider fermentation. Note: (A,C) corresponds to alcoholic fermentation with yeast inoculum over 10 days, Cider I. (B,D) corresponds to alcoholic fermentation with yeast and lactic bacteria inoculum over 20 days, Cider II.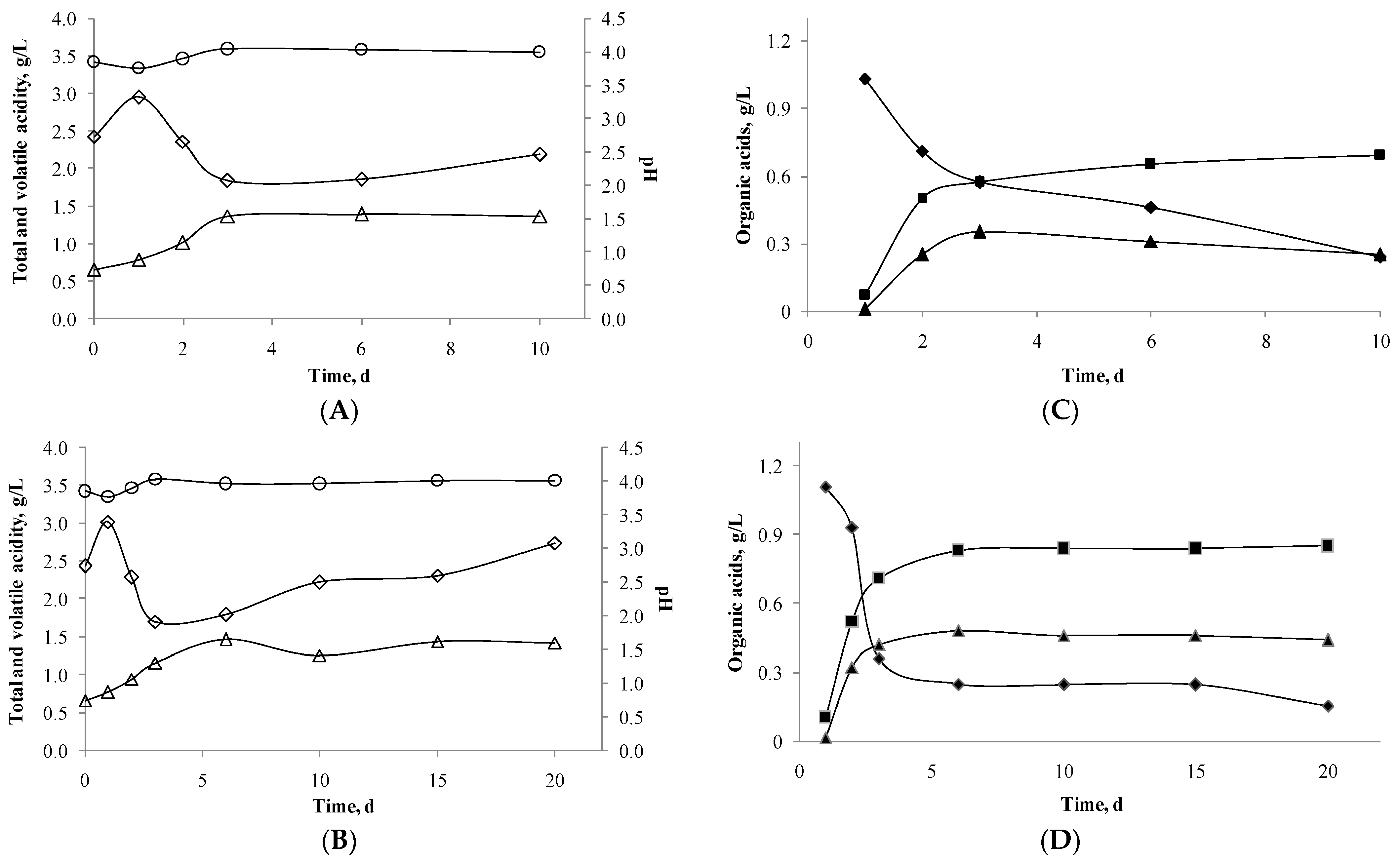
| Apples | Without Wash | After Wash | Sodium Hypochlorite Content * (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | |||||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| Commercial | 2.7 × 103 | 1.5 × 105 | 1.7 × 102 | 7.7 × 103 | 3.4 × 102 | 4.1 × 104 | <4.0 × 101 | 6.1 × 102 | 2.6 × 102 | 4.0 × 103 | 1.5 × 103 | 6.1 × 103 |
| Industrial | 6.2 × 105 | 3.7 × 106 | 5.0 × 102 | 1.1 × 104 | 2.6 × 102 | 6.8 × 102 | 6.6 × 102 | 3.7 × 103 | <4.0 × 101 | 1.0 × 104 | <4.0 × 101 | 1.4 × 103 |
| Analytical Parameters (g/L) | Apple Must | Ciders | |
|---|---|---|---|
| I * | II ** | ||
| Total sugars 1 | 118.95 ± 0.24 | 16.45 a ± 0.19 | 2.27 b ± 0.01 |
| Ethanol (%) | nd | 5.7 b ± 0.1 | 6.4 a ± 0.1 |
| Total acidity 2 | 2.43 ± 0.04 | 2.19 ± 0.01 | 2.74 ± 0.04 |
| Malic acid | 2.25 ± 0.15 | 0.24 a ± 0.08 | 0.15 b ± 0.05 |
| L-lactic acid | nd | 0.73 ± 0.04 | 0.85 ± 0.06 |
| D-lactic acid | nd | 0.69 ± 0.06 | 0.40 ± 0.04 |
| Volatile acidity 3 | 0.66 ± 0.10 | 1.37 ± 0.08 | 1.42 ± 0.12 |
| pH | 3.84 ± 0.02 | 3.99 ± 0.02 | 4.10 ± 0.01 |
| Yeast, UFC/mL | nd | 2.8 × 108 | 8.5 × 105 |
| LAB, UFC/mL | 2.8 × 104 | 1.6 × 102 | 3.8 × 107 |
| Volatile Compounds (mg/L) | Apple Must | Ciders | ||
|---|---|---|---|---|
| I * | II ** | Threshold | ||
| Ester | ||||
| Ethyl ethanoate | 3.79 ± 1.93 | 140.62 b ± 0.82 | 442.56 a ± 4.94 | 7.5 (1;2;3;6) |
| Ethyl butanoate | nd | 1.14 a ± 0.79 | 2.76 a ± 0.72 | 0.02 (1;2;3) |
| Isopentyl acetate | 0.39 ± 0.19 | 12.14 b ± 0.89 | 19.55 a ± 0.78 | 0.03 (1;2;3) |
| Hexyl ethanoate | 0.11 ± 0.09 | 1.15 a ± 0.93 | 1.71 a ± 0.84 | 0.67 (2;3;6) |
| 2-hydroxy ethyl propanoate | 44.39 ± 6.50 | 36.87 b ± 5.39 | 123.27 a ± 3.46 | 1.8 (3) |
| Ethyl octanoate | nd | 10.56 b ± 1.07 | 17.03 a ± 1.35 | 0.002 (1;6) |
| Ethyl decanoate | 0.04 ± 0.02 | 6.78 b ± 1.75 | 7.20 a ± 0.97 | 0.5 (1;6) |
| 1,4-ethyl butanoate | 6.46 ± 7.06 | 14.78 a ± 0.21 | nd | 0.02 (1;2;3) |
| Ethyl dodecanoate | 3.74 ± 1.08 | 4.77 b ± 0.01 | 11.43 a ± 0.25 | 0.5 (1) |
| Aldehyde | ||||
| Acetaldehyde (ethanal) | 1.54 ± 1.38 | 12.78 a ± 0.82 | nd | 0.5 (3) |
| Acid | ||||
| Butanoic acid | nd | 47.11 b ± 3.68 | 53.57 a ± 3.19 | 0.24 (5) |
| Octanoic acid | 2.22 ± 1.44 | 12.77 b ± 0.01 | 23.25 a ± 0.53 | 10 (1;6) |
| Higher alcohols | ||||
| 2-phenyl-ethanol | 11.22 ± 3.19 | 27.95 b ± 2.27 | 61.83 a ± 0.15 | 10 (1;2;3) |
| 3-methyl-1-butanol | 2.37 ± 1.19 | 187.54 b ± 6.51 | 351.36 a ± 1.46 | 30 (1;2;3) |
| 2-hexanol | nd | 16.72 b ± 1.40 | 25.86 a ± 6.33 | 15 (3) |
| Acetone | ||||
| 2-heptanone | 0.07 ± 0.04 | nd | 3.22 ± 0.67 | 0.0082 (4) |
| ∑ of compounds | 76.34 | 533.68 | 1144.6 | - |
| Hedonic Scale/Categories | Ciders | ||
|---|---|---|---|
| I * | II ** | ||
| Preference Test | I dislike extremely | 10 | 2 |
| I dislike very much | 7 | 5 | |
| I dislike moderately | 5 | 8 | |
| I dislike slightly | 8 | 9 | |
| I neither like nor dislike | 12 | 9 | |
| I like slightly | 27 | 27 | |
| I like moderately | 21 | 27 | |
| I like very much | 11 | 16 | |
| I like extremely | 3 | 1 | |
| Acceptance Index (p = 0.193) | 59.6 | 68.3 | |
| Purchase Intention Test | Definitely wouldn’t buy | 13 | 9 |
| Probably wouldn’t buy | 26 | 18 | |
| Might would buy/Might wouldn’t buy | 39 | 49 | |
| Probably would buy | 23 | 19 | |
| Definitely would buy | 3 | 9 | |
| Purchase intent % | 62.0 | 71.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerri, M.L.; Gomes, T.A.; Carraro, M.d.M.; Wojeicchowski, J.P.; Demiate, I.M.; Lacerda, L.G.; Alberti, A.; Nogueira, A. Assessing the Impact of Simultaneous Co-Fermentation on Malolactic Bioconversion and the Quality of Cider Made with Low-Acidity Apples. Fermentation 2023, 9, 1017. https://doi.org/10.3390/fermentation9121017
Cerri ML, Gomes TA, Carraro MdM, Wojeicchowski JP, Demiate IM, Lacerda LG, Alberti A, Nogueira A. Assessing the Impact of Simultaneous Co-Fermentation on Malolactic Bioconversion and the Quality of Cider Made with Low-Acidity Apples. Fermentation. 2023; 9(12):1017. https://doi.org/10.3390/fermentation9121017
Chicago/Turabian StyleCerri, Maria Luísa, Tatiane Aparecida Gomes, Matheus de Melo Carraro, José Pedro Wojeicchowski, Ivo Mottin Demiate, Luiz Gustavo Lacerda, Aline Alberti, and Alessandro Nogueira. 2023. "Assessing the Impact of Simultaneous Co-Fermentation on Malolactic Bioconversion and the Quality of Cider Made with Low-Acidity Apples" Fermentation 9, no. 12: 1017. https://doi.org/10.3390/fermentation9121017
APA StyleCerri, M. L., Gomes, T. A., Carraro, M. d. M., Wojeicchowski, J. P., Demiate, I. M., Lacerda, L. G., Alberti, A., & Nogueira, A. (2023). Assessing the Impact of Simultaneous Co-Fermentation on Malolactic Bioconversion and the Quality of Cider Made with Low-Acidity Apples. Fermentation, 9(12), 1017. https://doi.org/10.3390/fermentation9121017






