Biocontrol of Litchi Downy Blight Dependent on Streptomyces abikoensis TJGA-19 Fermentation Filtrate Antagonism Competition with Peronophythora litchii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples, Pathogen, and Inoculation Materials
2.2. Actinomycete Isolation and Antagonistic Isolate Screen
2.3. Identification of Antagonistic Actinomycete TJGA-19
2.4. Preparation of the TJGA-19 Fermentation Filtrate
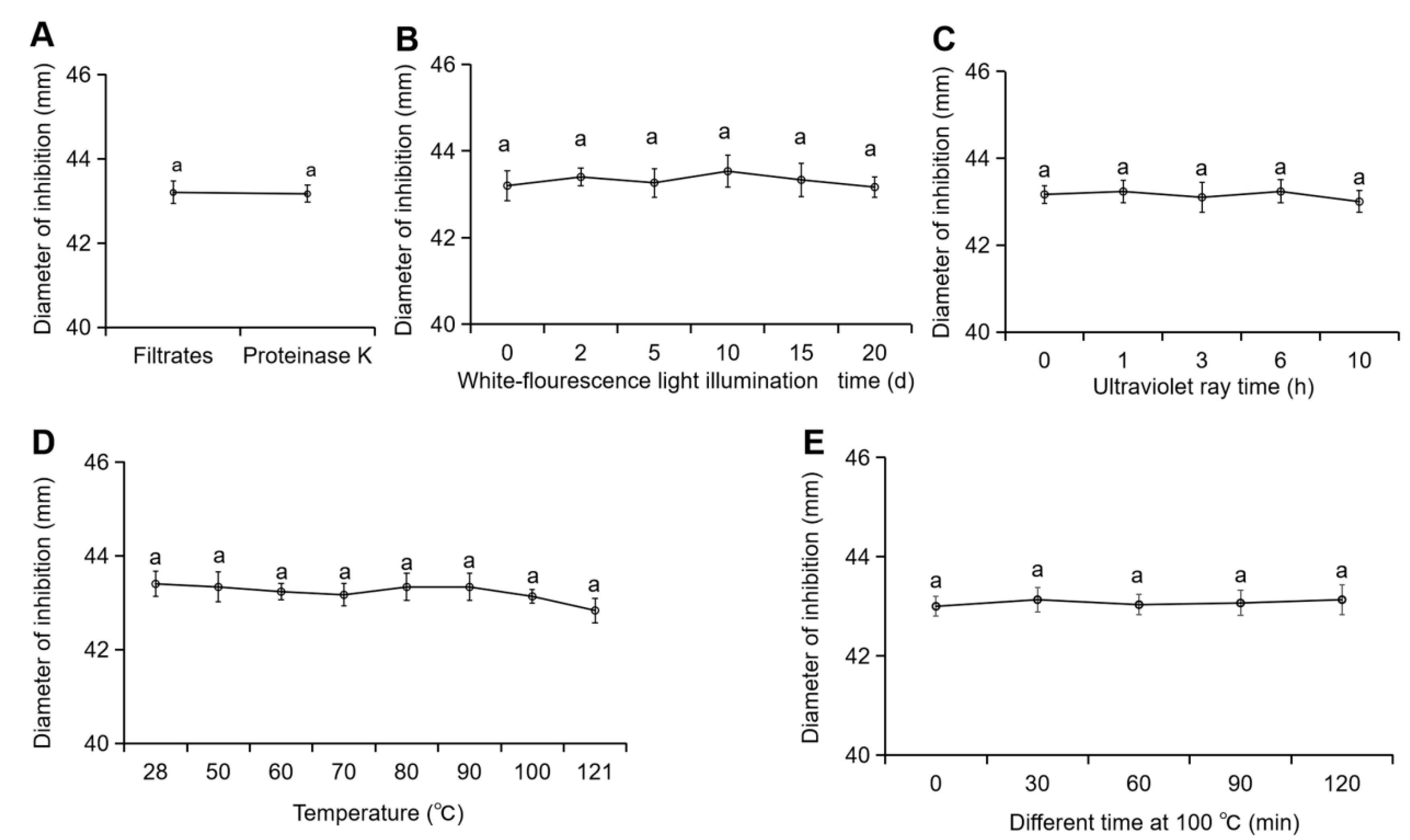
2.5. Measurement of the Stability of the TJGA-19 Fermentation Filtrate
2.6. Extraction of Bioactive Metabolites and Determination of Antifungal Activity
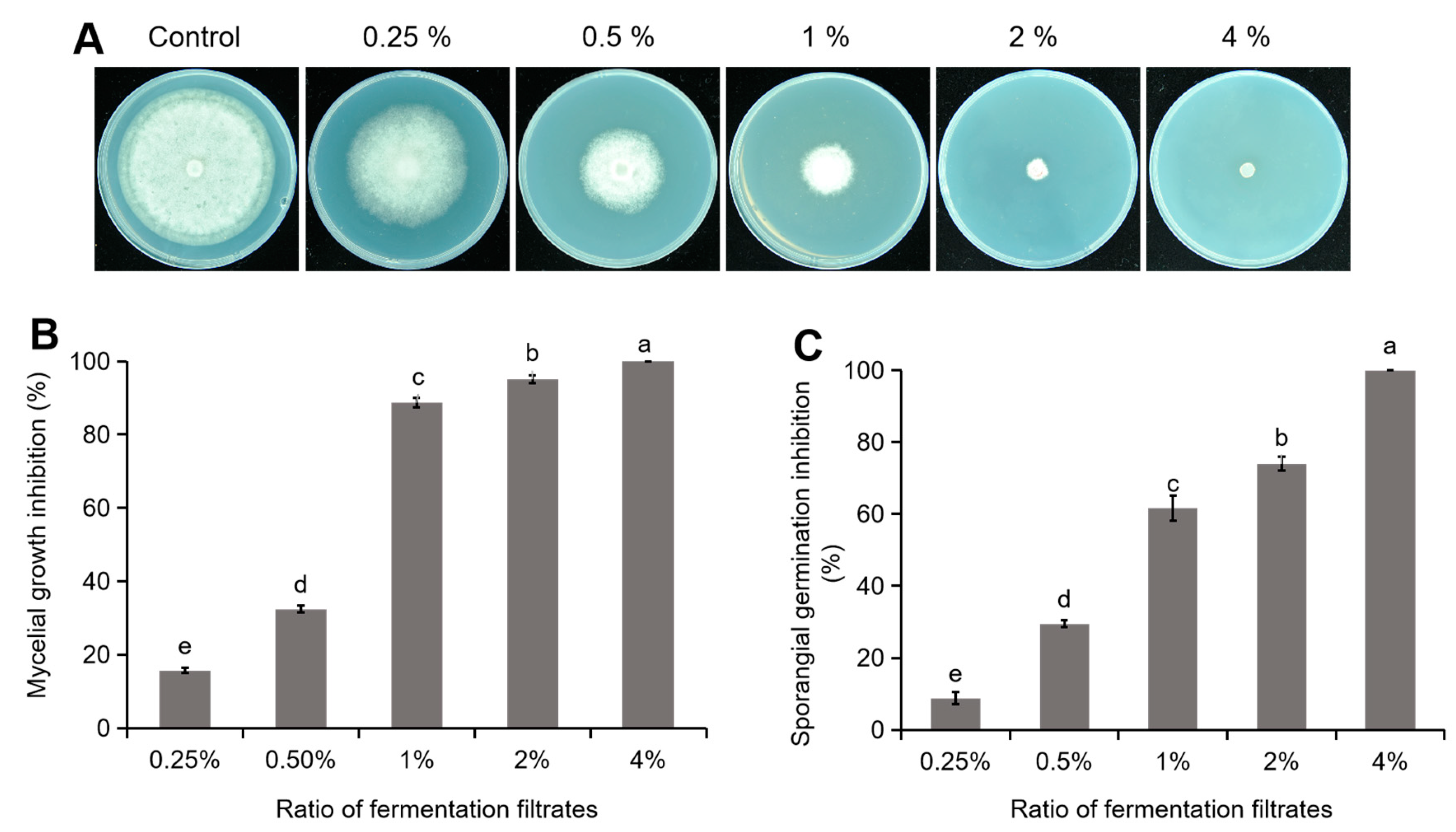
2.7. Bioactivity Assay on the TJGA-19 Fermentation Filtrate
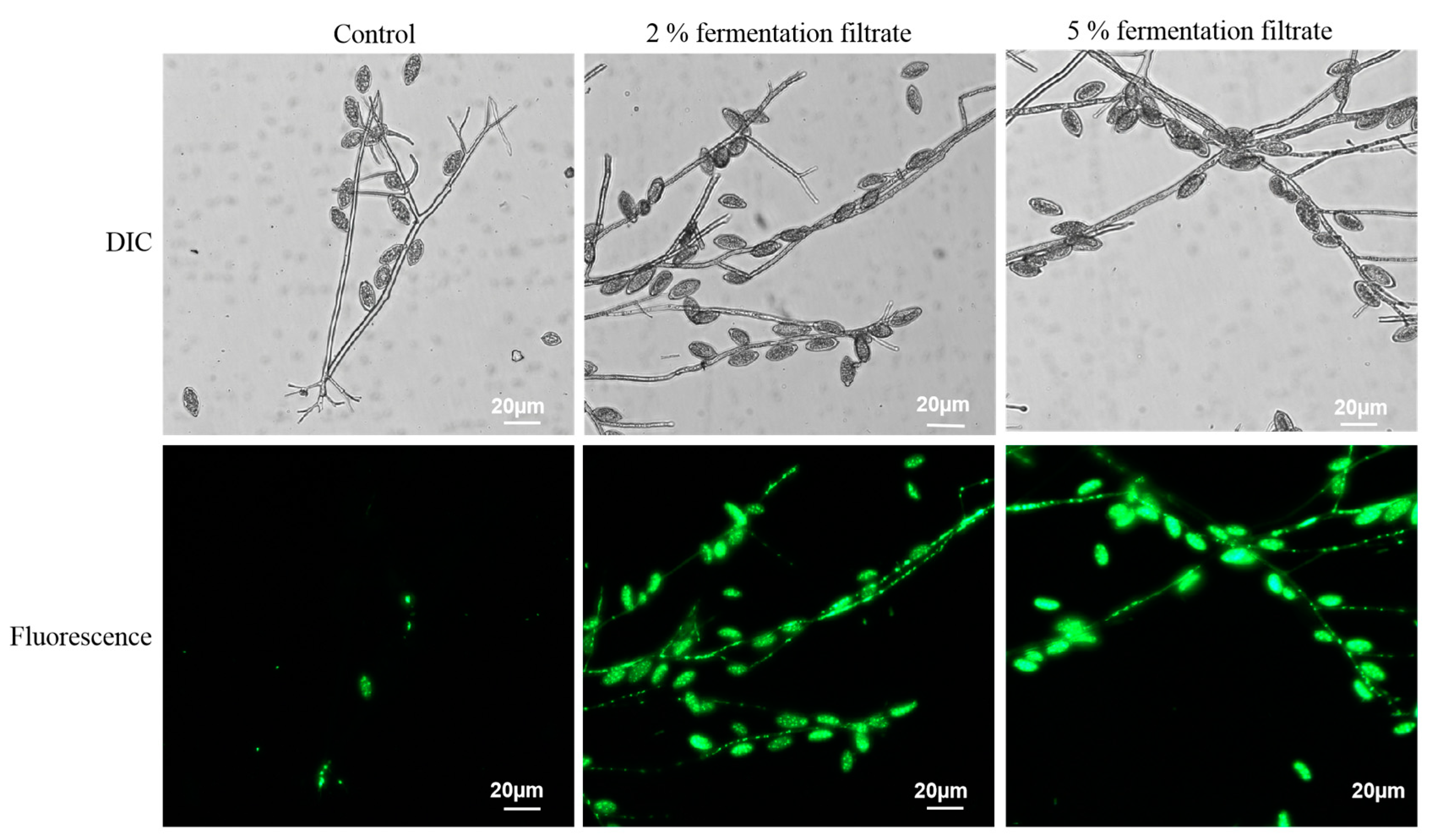
2.8. Plasma Membrane Permeabilization Assay
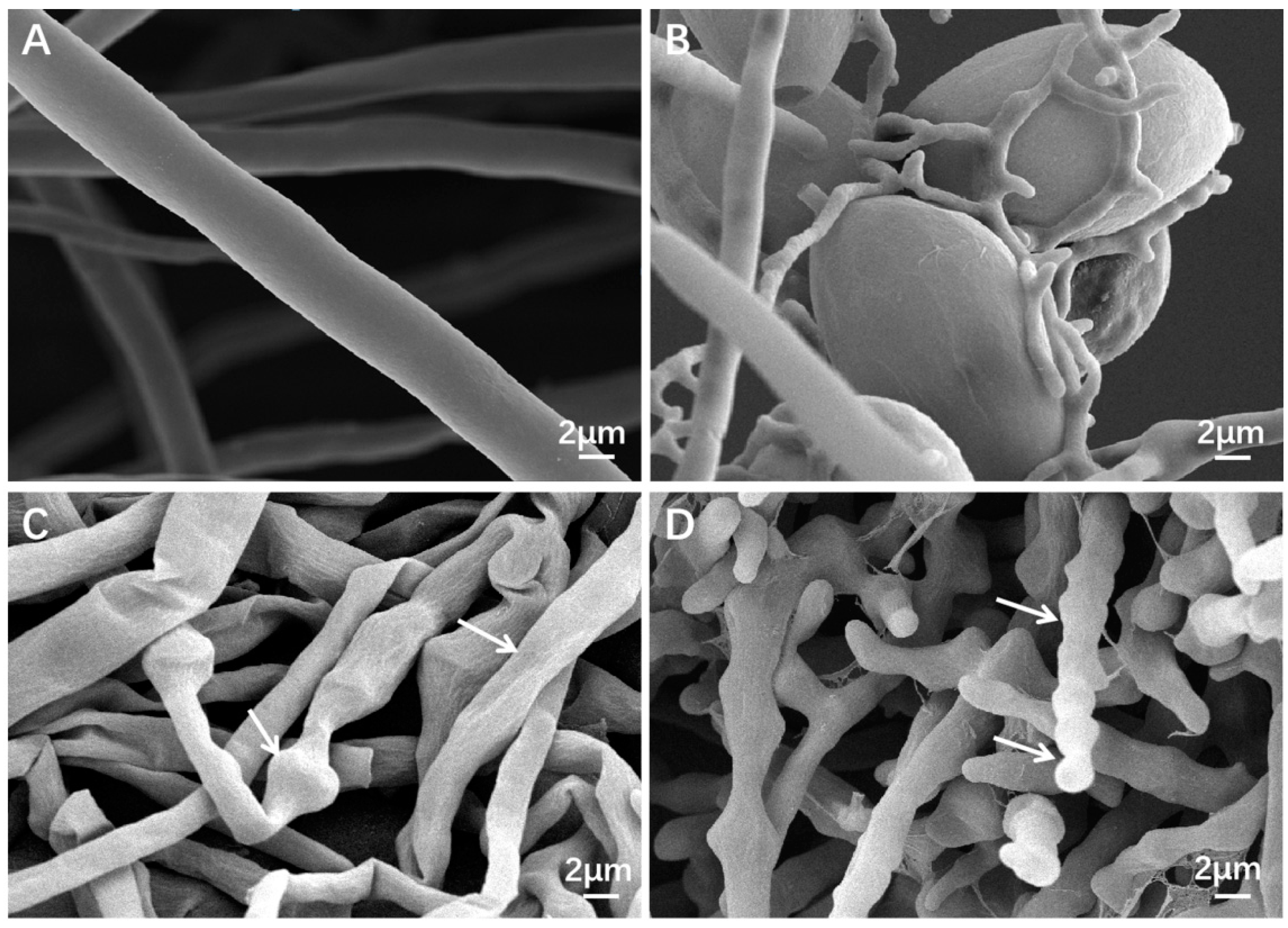
2.9. The Effect of Fermentation Filtrate on the Morphology and Ultrastructure of P. litchii
2.10. In Vivo Bioassay on Detached Litchi Leaf and Litchi Fruit
2.11. Statistical Analysis
3. Results
3.1. Isolation, Screening Antagonistic Actinomycetes, and Identification of TJGA-19
3.2. Determination of the Stability of TJGA-19 Fermentation Filtrate
3.3. Antifungal Activity of the TJGA-19 Fermentation Filtrate Extraction
3.4. Inhibitory Effect of TJGA-19 Fermentation Filtrate
3.5. Disruption of Plasma Membrane Permeabilization of P. litchii
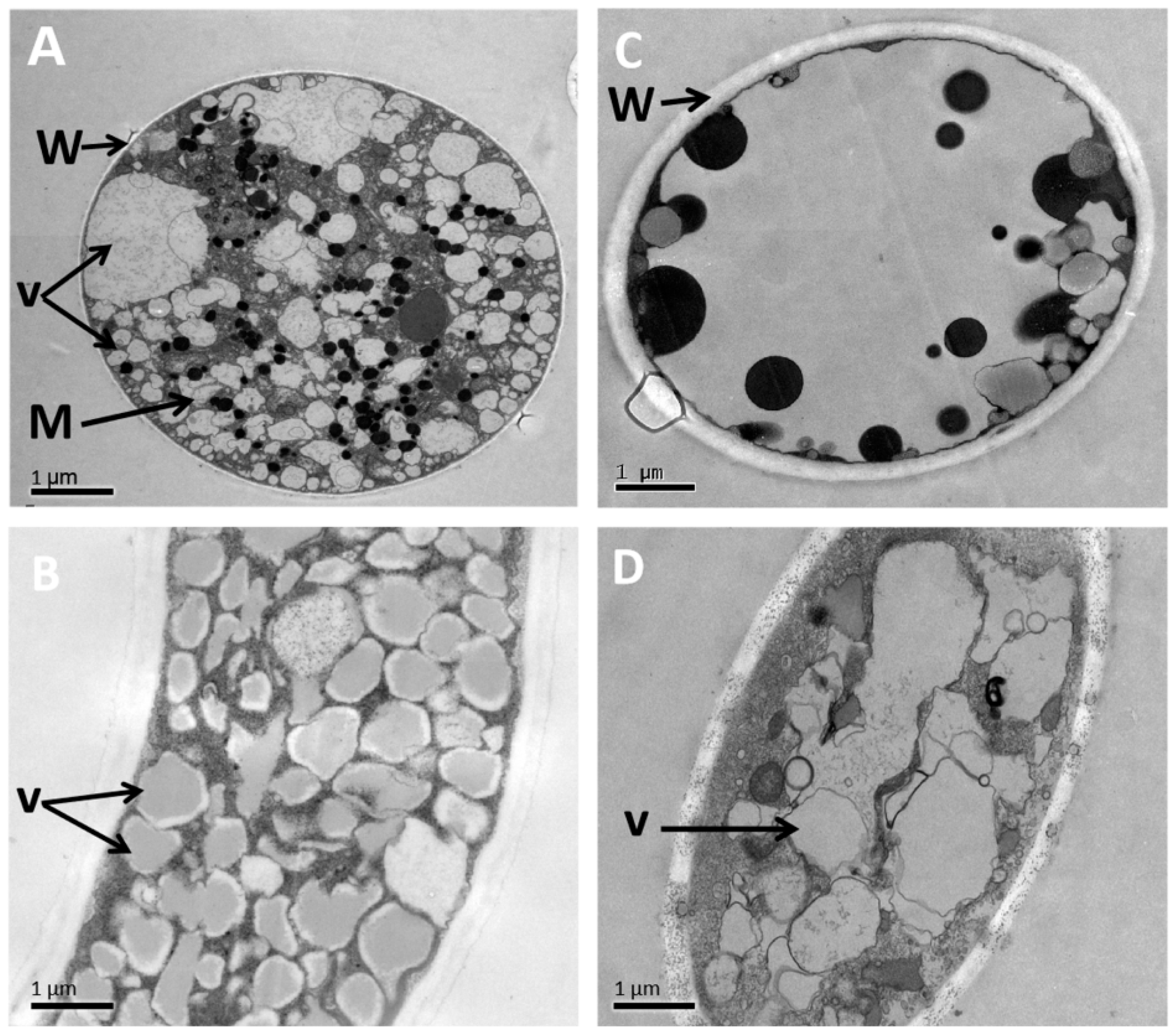
3.6. Alteration of the Morphology and Ultrastructure of P. litchii
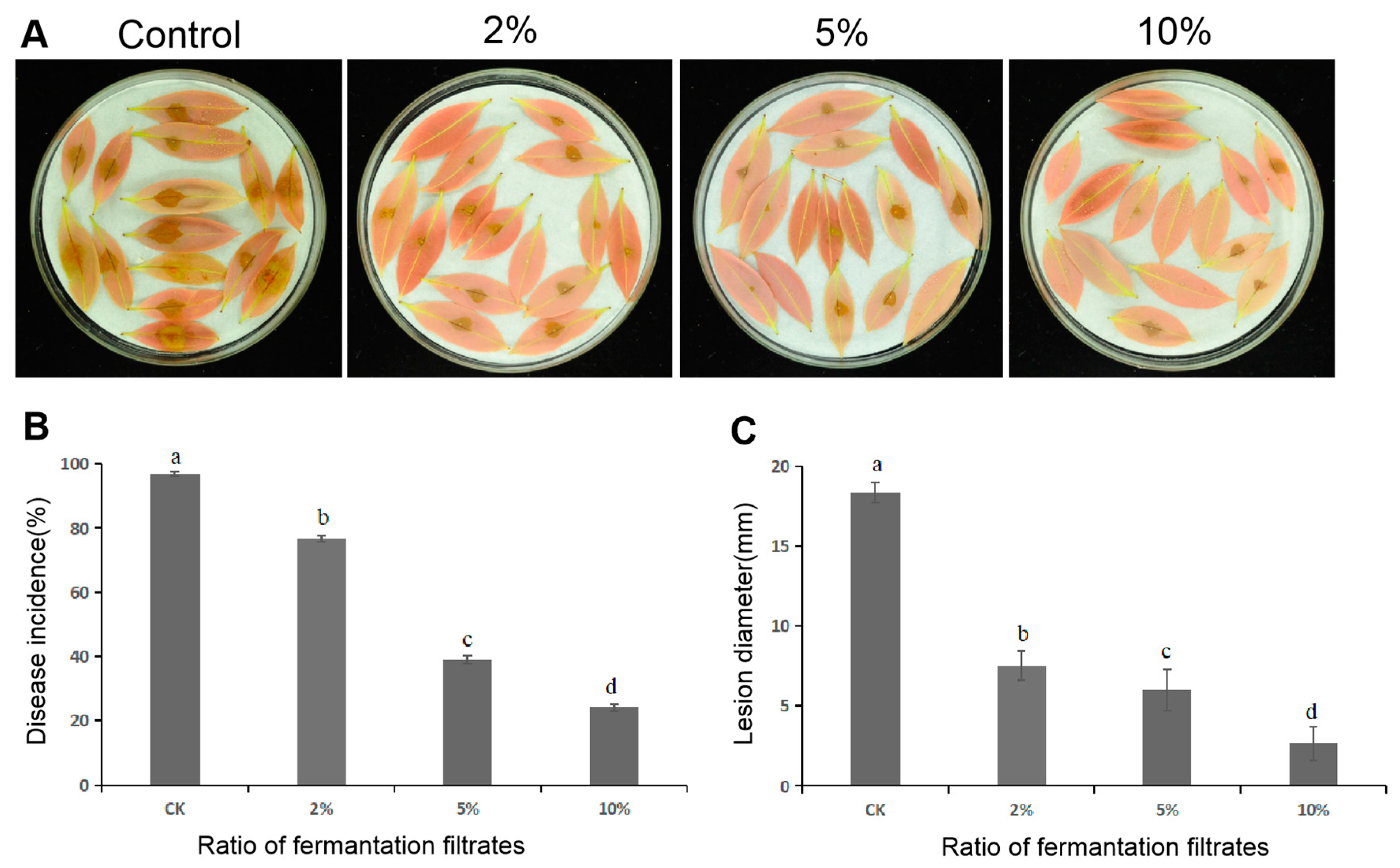
3.7. Delayed Disease Development of Litchi Downy Blight
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food. Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef] [PubMed]
- Situ, J.; Jiang, L.; Fan, X.; Yang, W.; Li, W.; Xi, P.; Deng, Y.; Kong, G.; Jiang, Z. An RXLR effector PlAvh142 from Peronophythora litchii triggers plant cell death and contributes to virulence. Mol. Plant Pathol. 2020, 21, 415–428. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, L.; Hu, J.; Liu, P.; Zhang, Y.; Meng, Q.; Li, B.; Si, N.; Liu, C.; Liu, X. Baseline sensitivity of natural population and resistance risk of Peronophythora litchii to four novel QoI fungicides. Eur. J. Plant Pathol. 2016, 146, 71–83. [Google Scholar] [CrossRef]
- Tomas-Grau, R.H.; Hael-Conrad, V.; Requena-Serra, F.J.; Perato, S.M.; Caro, M.D.P.; Salazar, S.M.; Díaz-Ricci, J.C. Biological control of strawberry grey mold disease caused by Botrytis cinerea mediated by Colletotrichum acutatum extracts. BioControl 2020, 65, 461–473. [Google Scholar] [CrossRef]
- Jin, P.; Tan, Z.; Wang, H.; Liu, W.; Miao, W. Antimicrobial effect of Bacillus licheniformis HN-5 bacitracin A on rice pathogen Pantoea ananatis. BioControl 2021, 66, 249–257. [Google Scholar] [CrossRef]
- Wang, Y.; Cobb, R.E.; Zhao, H. High-efficiency genome editing of Streptomyces species by an engineered CRISPR/Cas system. In Methods in Enzymology; O’Connor, S.E., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 575. [Google Scholar]
- Tian, Y.; Jiang, Y.; Wen, Z.; Guan, L.; Ouyang, X.; Ding, W.; Ma, Z. Identification of novel sphydrofuran-derived derivatives with lipid-lowering activity from the active crude extracts of Nocardiopsis sp. ZHD001. Int. J. Mol. Sci. 2023, 24, 2822. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef]
- Khadayat, K.; Sherpa, D.D.; Malla, K.P.; Shrestha, S.; Rana, N.; Marasini, B.P.; Khanal, S.; Rayamajhee, B.; Bhattarai, B.R.; Parajuli, N.; et al. Molecular identification and antimicrobial potential of Streptomyces species from Nepalese soil. Int. J. Microbiol. 2020, 2020, 8817467. [Google Scholar] [CrossRef]
- Singh, T.A.; Passari, A.K.; Jajoo, A.; Bhasin, S.; Gupta, V.K.; Hashem, A.; Alqarawi, A.A.; Abd Allah, E.F. Tapping into actinobacterial genomes for natural product discovery. Front. Microbiol. 2021, 12, 655620. [Google Scholar] [CrossRef]
- Song, L.; Jiang, N.; Wei, S.; Lan, Z.; Pan, L. Isolation, screening, and identification of actinomycetes with antifungal and enzyme activity assays against Colletotrichum dematium of Sarcandra glabra. Mycobiology 2020, 48, 37–43. [Google Scholar] [CrossRef]
- Li, N.; Chen, S.; Yan, Z.; Han, J.; Ta, Y.; Pu, T.; Wang, Y. Antimicrobial activity and identification of the biosynthetic gene cluster of X-14952B from Streptomyces sp. 135. Front. Microbiol. 2021, 12, 703093. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Karthic, C.; Mostafa, A.A.; Mohammed Al-Enazi, N.; Abdel-Raouf, N.; Nageh Sholkamy, E. Antifungal activity of Streptomyces sp. SLR03 against tea fungal plant pathogen Pestalotiopsis theae. J. King Saud Univ. Sci. 2020, 32, 3258–3264. [Google Scholar] [CrossRef]
- Njenga, W.P.; Mwaura, F.B.; Wagacha, J.M.; Gathuru, E.M. Methods of isolating actinomycetes from the soils of menengai crater in Kenya. Arch. Microbiol. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Mirsam, H.; Suriani; Aqil, M.; Azrai, M.; Efendi, R.; Muliadi, A.; Sembiring, H.; Azis, A.I. Molecular characterization of indigenous microbes and its potential as a biological control agent of Fusarium stem rot disease (Fusarium verticillioides) on maize. Heliyon 2022, 8, e11960. [Google Scholar] [CrossRef] [PubMed]
- Shirliong, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–314. [Google Scholar] [CrossRef]
- Shimizu, M.; Nakagawa, Y.; Sato, Y.; Furumai, T.; Igarashi, Y.; Onaka, H.; Yoshida, R.; Kunoh, H. Studies on endophytic actinomycetes (I) Streptomyces sp. isolated from rhododendron and its antifungal activity. J. Gen. Plant Pathol. 2000, 66, 360–366. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pokhrel, A.R.; Nguyen, C.T.; Pham, V.T.T.; Dhakal, D.; Lim, H.N.; Jung, H.J.; Kim, T.; Yamaguchi, T.; Sohng, J.K. Streptomyces sp. VN1, a producer of diverse metabolites including non-natural furan-type anticancer compound. Sci. Rep. 2020, 10, 1756. [Google Scholar] [CrossRef]
- Weisburg, W.G.S.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Wang, J.; Wang, R.; Gao, J.; Liu, H.; Tang, W.; Liu, Z.; Li, X. Identification of three Streptomyces strains and their antifungal activity against the rubber anthracnose fungus Colletotrichum siamense. J. Gen. Plant Pathol. 2023, 89, 67–76. [Google Scholar] [CrossRef]
- Wang, X.; Liang, L.; Shao, H.; Ye, X.; Yang, X.; Chen, X.; Shi, Y.; Zhang, L.; Xu, L.; Wang, J. Isolation of the novel strain Bacillus amyloliquefaciens F9 and identification of lipopeptide extract components responsible for activity against Xanthomonas citri subsp. citri. Plants 2022, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Carvalho, A.O.; Rodrigues, R.; Trindade, F.G.; Da Cunha, M.; Gomes, V.M. Thionin-like peptide from Capsicum annuum fruits: Mechanism of action and synergism with fluconazole against Candida species. BMC Microbiol. 2016, 16, 12. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, W.; Hu, S.; Kang, Y.; Zhang, Y.; Ng, T.B. Effects of Oudemansiella radicata polysaccharide on postharvest quality of oyster mushroom (Pleurotus ostreatus) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2020, 166, 111207. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.; Ser, H.; Khan, T.M.; Chuah, L.; Pusparajah, P.; Chan, K.; Goh, B.; Lee, L. The Potential of Streptomyces as biocontrol agents against the Rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 2017, 8, 3. [Google Scholar] [CrossRef]
- Cao, P.; Li, C.; Wang, H.; Yu, Z.; Xu, X.; Wang, X.; Zhao, J.; Xiang, W. Community structures and antifungal activity of root-associated endophytic actinobacteria in healthy and diseased cucumber plants and Streptomyces sp. HAAG3-15 as a promising biocontrol agent. Microorganisms 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Bai, L.; Zhong, J. Exogenous 1,4-butyrolactone stimulates A-factor-like cascade and validamycin biosynthesis in Streptomyces hygroscopicus 5008. Biotechnol. Bioeng. 2013, 110, 2984–2993. [Google Scholar] [CrossRef]
- Kim, J.D.; Kang, J.E.; Kim, B.S. Postharvest disease control efficacy of the polyene macrolide lucensomycin produced by Streptomyces plumbeus strain CA5 against gray mold on grapes. Postharvest Biol. Technol. 2020, 162, 111115. [Google Scholar] [CrossRef]
- Marian, M.; Ohno, T.; Suzuki, H.; Kitamura, H.; Kuroda, K.; Shimizu, M. A novel strain of endophytic Streptomyces for the biocontrol of strawberry anthracnose caused by Glomerella cingulata. Microbiol. Res. 2020, 234, 126428. [Google Scholar] [CrossRef]
- Li, X.; Jing, T.; Zhou, D.; Zhang, M.; Qi, D.; Zang, X.; Zhao, Y.; Li, K.; Tang, W.; Chen, Y.; et al. Biocontrol efficacy and possible mechanism of Streptomyces sp. H4 against postharvest anthracnose caused by Colletotrichum fragariae on strawberry fruit. Postharvest Biol. Technol. 2021, 175, 111401. [Google Scholar] [CrossRef]
- Zhou, D.; Jing, T.; Chen, Y.; Yun, T.; Qi, D.; Zang, X.; Zhang, M.; Wei, Y.; Li, K.; Zhao, Y.; et al. Biocontrol potential of a newly isolated Streptomyces sp. HSL-9B from mangrove forest on postharvest anthracnose of mango fruit caused by Colletotrichum gloeosporioides. Food Control 2022, 135, 108836. [Google Scholar] [CrossRef]
- Choudhary, B.; Nagpure, A.; Gupta, R.K. Biological control of toxigenic citrus and papaya-rotting fungi by Streptomyces violascens MT7 and its extracellular metabolites. J. Basic Microbiol. 2015, 55, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zhang, Y.; Huang, F.; Liu, S.; Gao, T.; Zhang, Y. Diversity and antimicrobial activities of culturable actinomycetes from Odontotermes formosanus (Blattaria: Termitidae). BMC Microbiol. 2022, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Park, A.R.; Hwang, I.M.; Kim, J. Identification and delineation of action mechanism of antifungal agents: Reveromycin E and its new derivative isolated from Streptomyces sp. JCK-6141. Postharvest Biol. Technol. 2021, 182, 111700. [Google Scholar] [CrossRef]
- Evangelista-Martínez, Z.; Contreras-Leal, E.A.; Corona-Pedraza, L.F.; Gastélum-Martínez, L. Biocontrol potential of Streptomyces sp. CACIS-1.5CA against phytopathogenic fungi causing postharvest fruit diseases. Egypt. J. Biol. Pest Control 2020, 30, 117. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, M.; Xu, D.; Wu, Y.; Liu, T.; Xi, P.; Wang, R.; Zhao, J.; Jiang, Z. Biocontrol of Litchi Downy Blight Dependent on Streptomyces abikoensis TJGA-19 Fermentation Filtrate Antagonism Competition with Peronophythora litchii. Fermentation 2023, 9, 1011. https://doi.org/10.3390/fermentation9121011
Xing M, Xu D, Wu Y, Liu T, Xi P, Wang R, Zhao J, Jiang Z. Biocontrol of Litchi Downy Blight Dependent on Streptomyces abikoensis TJGA-19 Fermentation Filtrate Antagonism Competition with Peronophythora litchii. Fermentation. 2023; 9(12):1011. https://doi.org/10.3390/fermentation9121011
Chicago/Turabian StyleXing, Mengyu, Dandan Xu, Yinggu Wu, Tong Liu, Pinggen Xi, Rui Wang, Jing Zhao, and Zide Jiang. 2023. "Biocontrol of Litchi Downy Blight Dependent on Streptomyces abikoensis TJGA-19 Fermentation Filtrate Antagonism Competition with Peronophythora litchii" Fermentation 9, no. 12: 1011. https://doi.org/10.3390/fermentation9121011
APA StyleXing, M., Xu, D., Wu, Y., Liu, T., Xi, P., Wang, R., Zhao, J., & Jiang, Z. (2023). Biocontrol of Litchi Downy Blight Dependent on Streptomyces abikoensis TJGA-19 Fermentation Filtrate Antagonism Competition with Peronophythora litchii. Fermentation, 9(12), 1011. https://doi.org/10.3390/fermentation9121011




