Enhanced β-Carotene Production in Mycolicibacterium neoaurum Ac-501/22 by Combining Mutagenesis, Strain Selection, and Subsequent Fermentation Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Nutrient Media Components
2.2. Microorganisms
2.3. Composition of Media for Cultivation and Maintenance of M. neoaurum Ac-501 and Ac-501/22
2.4. Cultivation of M. neoaurum Ac-501 and M. neoaurum Ac-501/22 on a Liquid Nutrient Medium
2.5. Mutagenesis
2.6. Carotenoid Content Determination
2.7. Carotenoid Determination by Thin-Layer Chromatography
2.8. Dry Biomass Obtaining
2.9. Fermentation of M. neoaurum Ac-501/22 in a 3 L Bioreactor
2.9.1. Obtaining of the Inoculate
2.9.2. Bioreactor Preparation
2.9.3. M. neoaurum Ac-501/22 Fermentation
2.10. M. neoaurum Ac-501/22 Identification
2.11. Data Treatment
3. Results
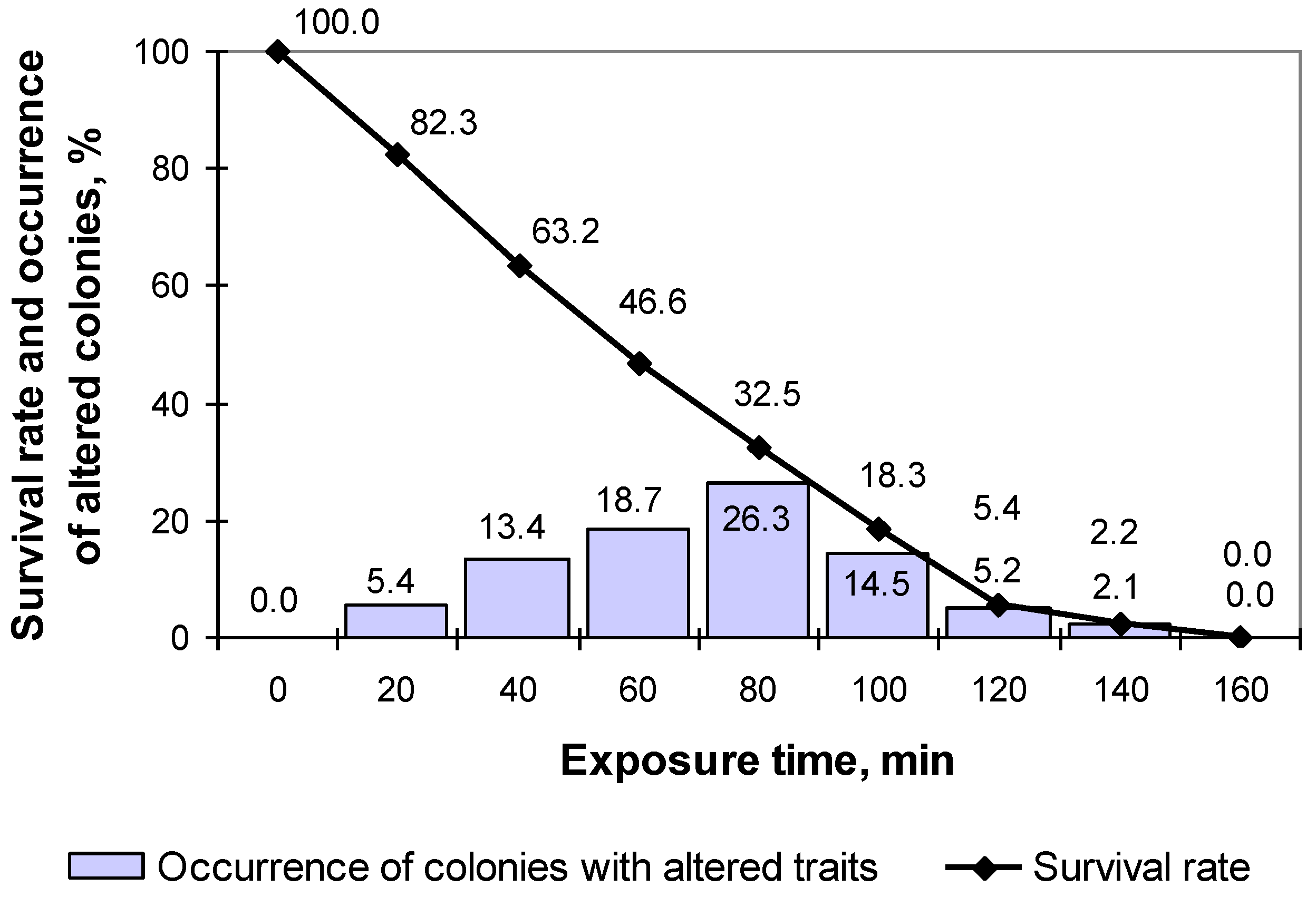
3.1. Breeding of M. neoaurum Ac-501/22 Characterized by an Enhanced Carotenoid Biosynthesis
3.2. Mutant Strain Identification
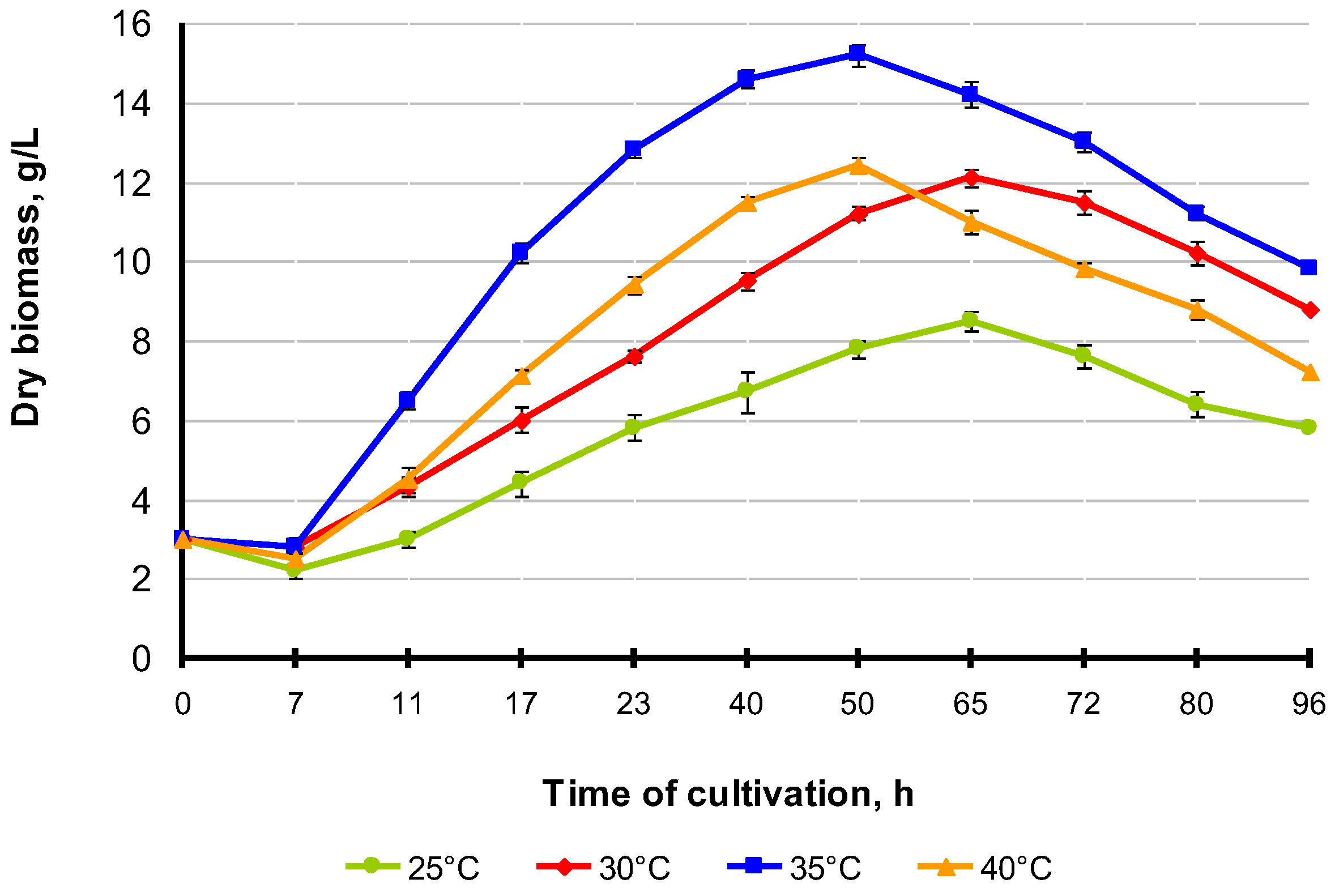
3.3. Effect of Temperature on the Growth of M. neoaurum Ac-501/22 and Carotenoid Accumulation
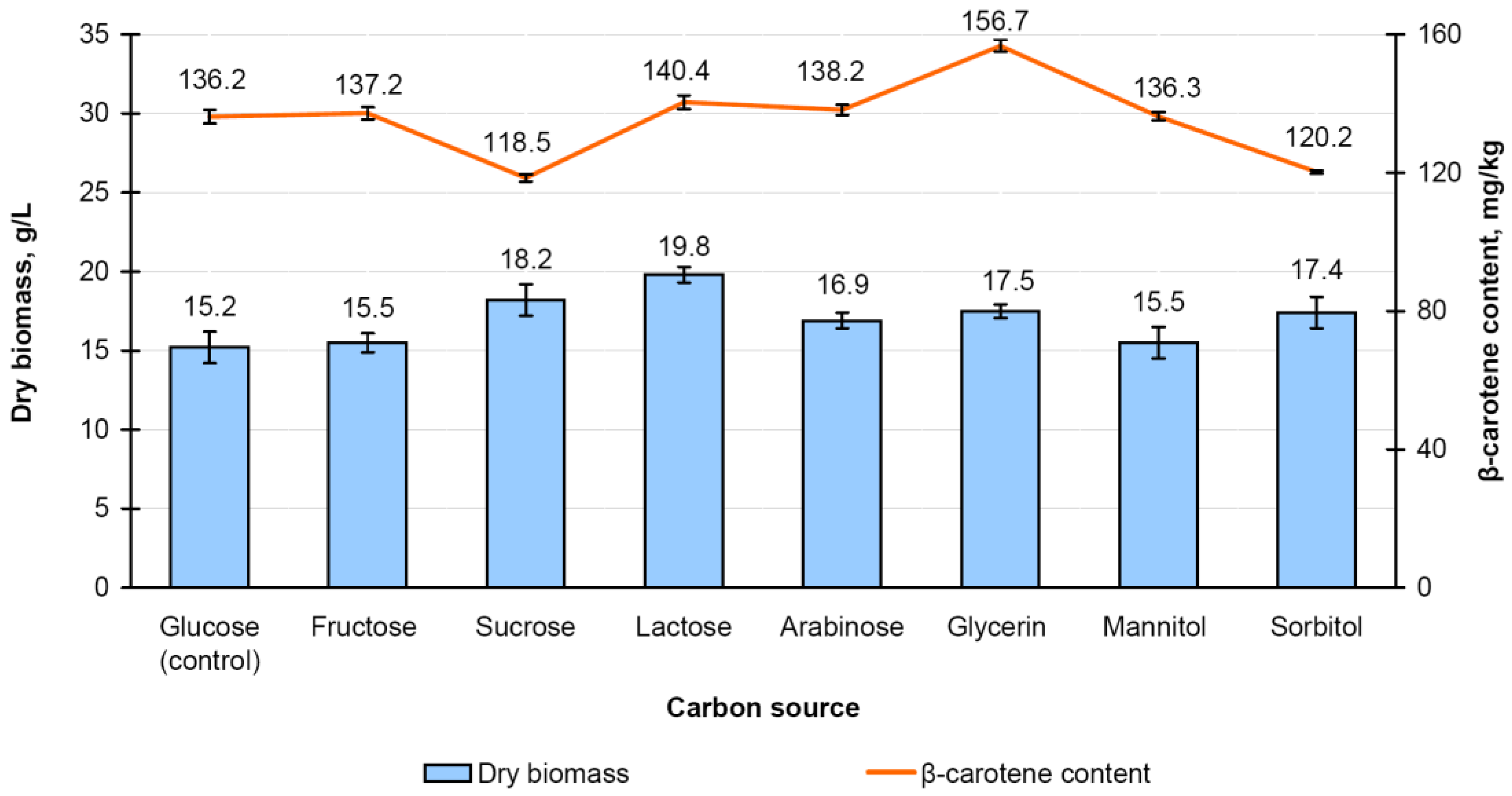
3.4. Effect of Carbon Source on Carotenoid Accumulation in the Mycolicibacterium neoaurum Ac-501/22 Biomass
3.5. Effect of Nitrogen Source on Carotenoid Accumulation in the M. neoaurum Ac-501/22 Biomass
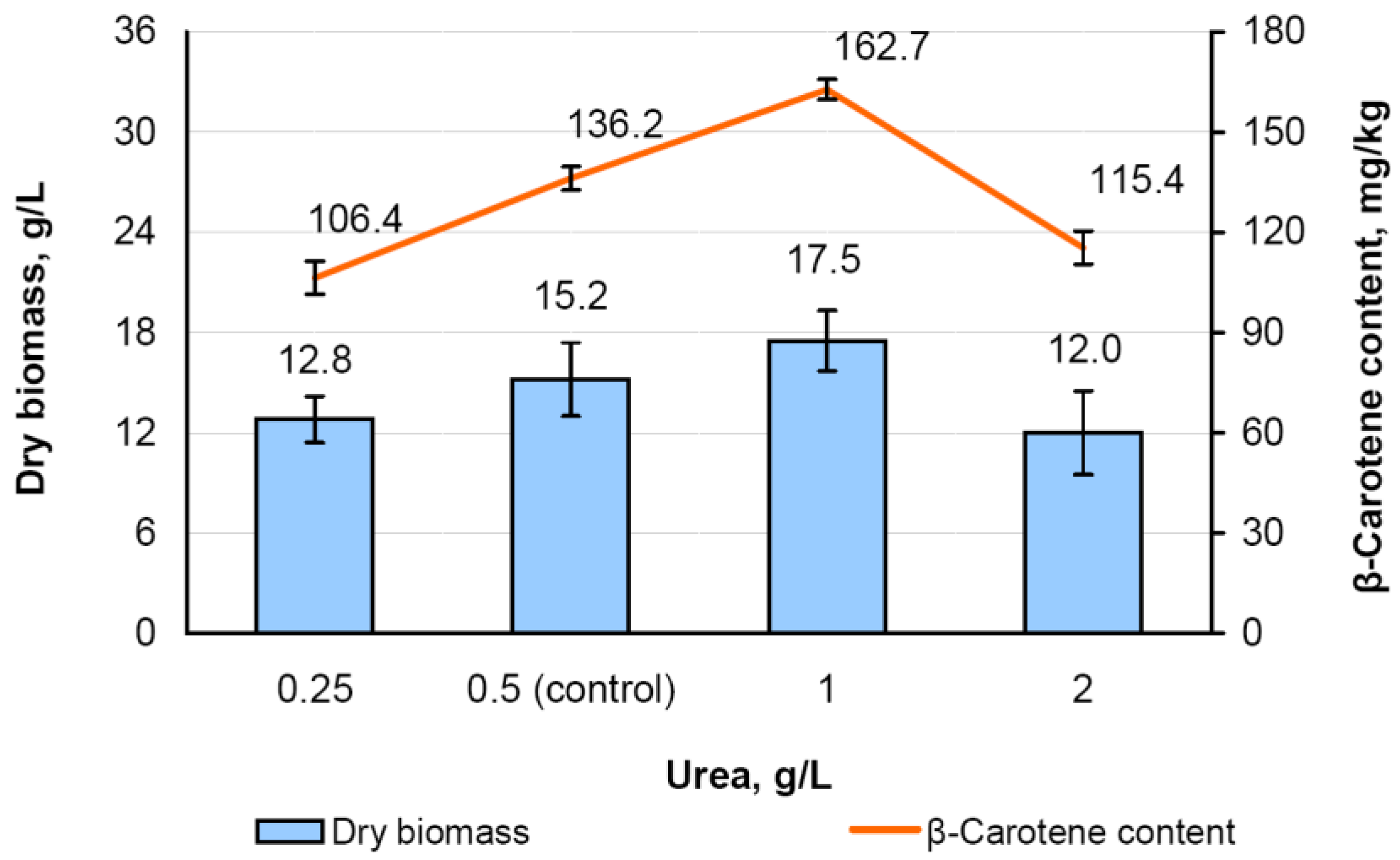
3.6. Effect of the Urea Content on Biomass Accumulation and Carotenoid Content in M. neoaurum Ac-501/22
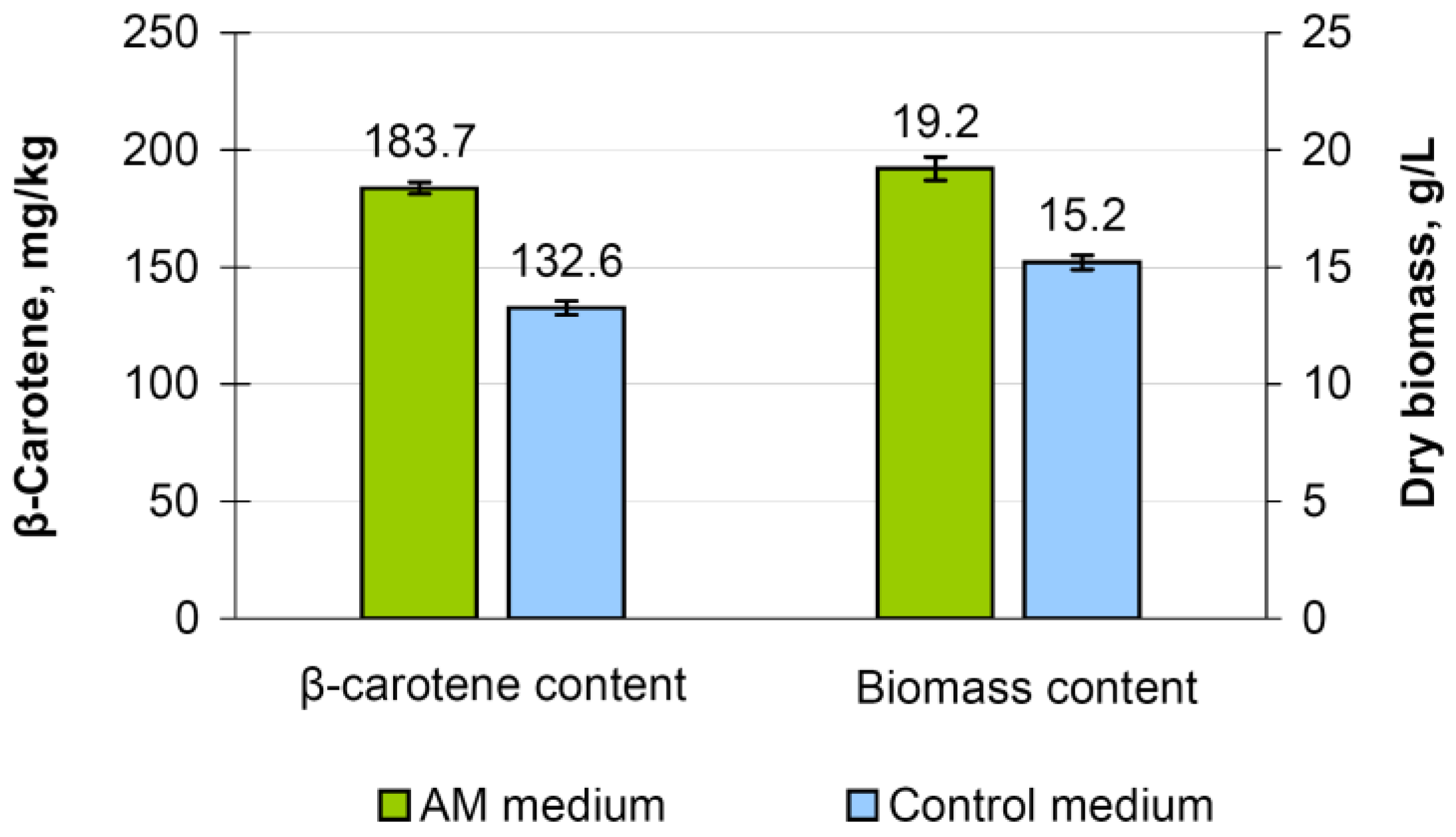
3.7. M. neoaurum Ac-501/22 Cultivation in a Modified Medium
3.8. M. neoaurum Ac-501/22 Fermentation in a 3 L Bioreactor under Laboratory Conditions
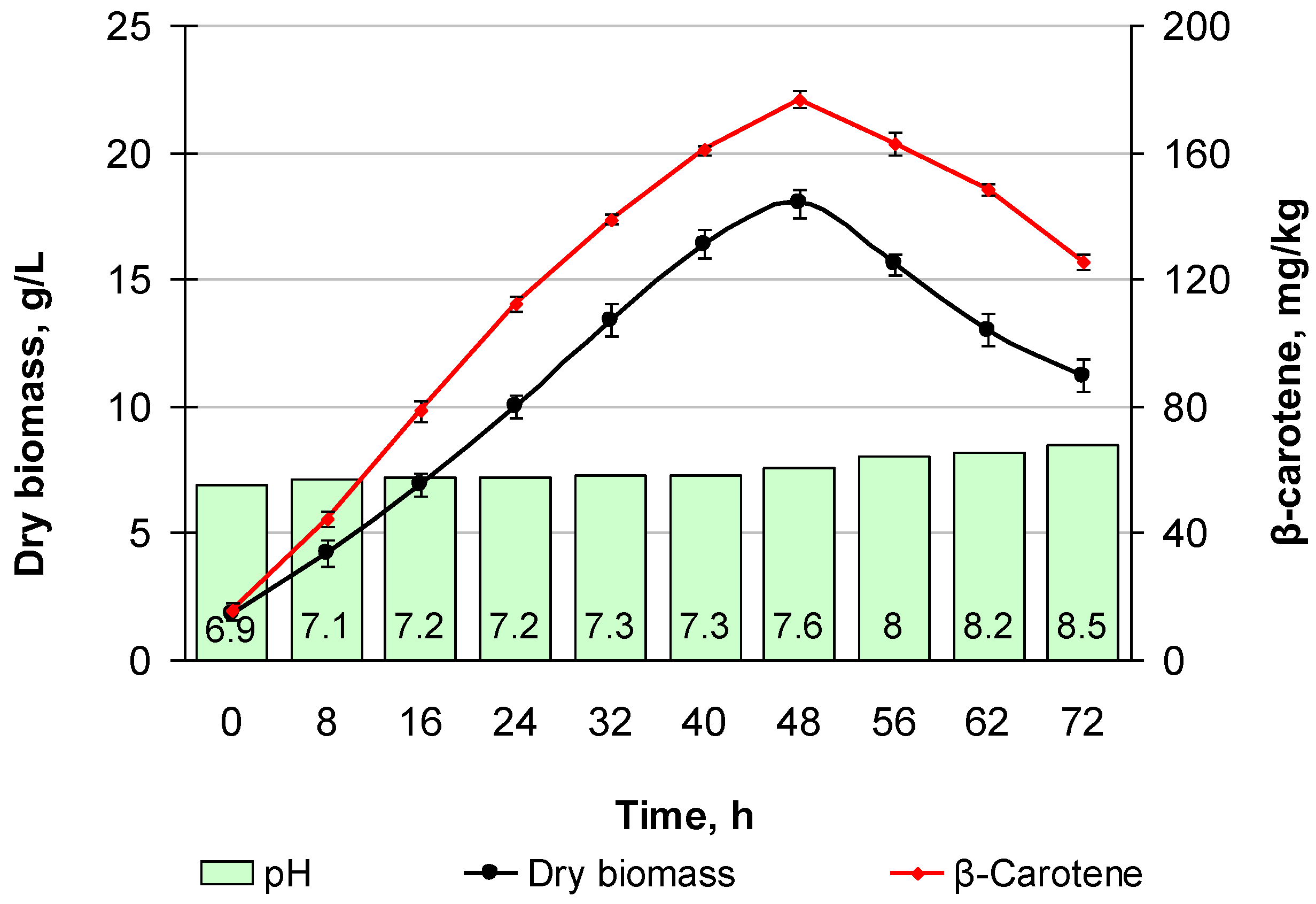
3.8.1. M. neoaurum Ac-501/22 Fermentation in the Registration Regime
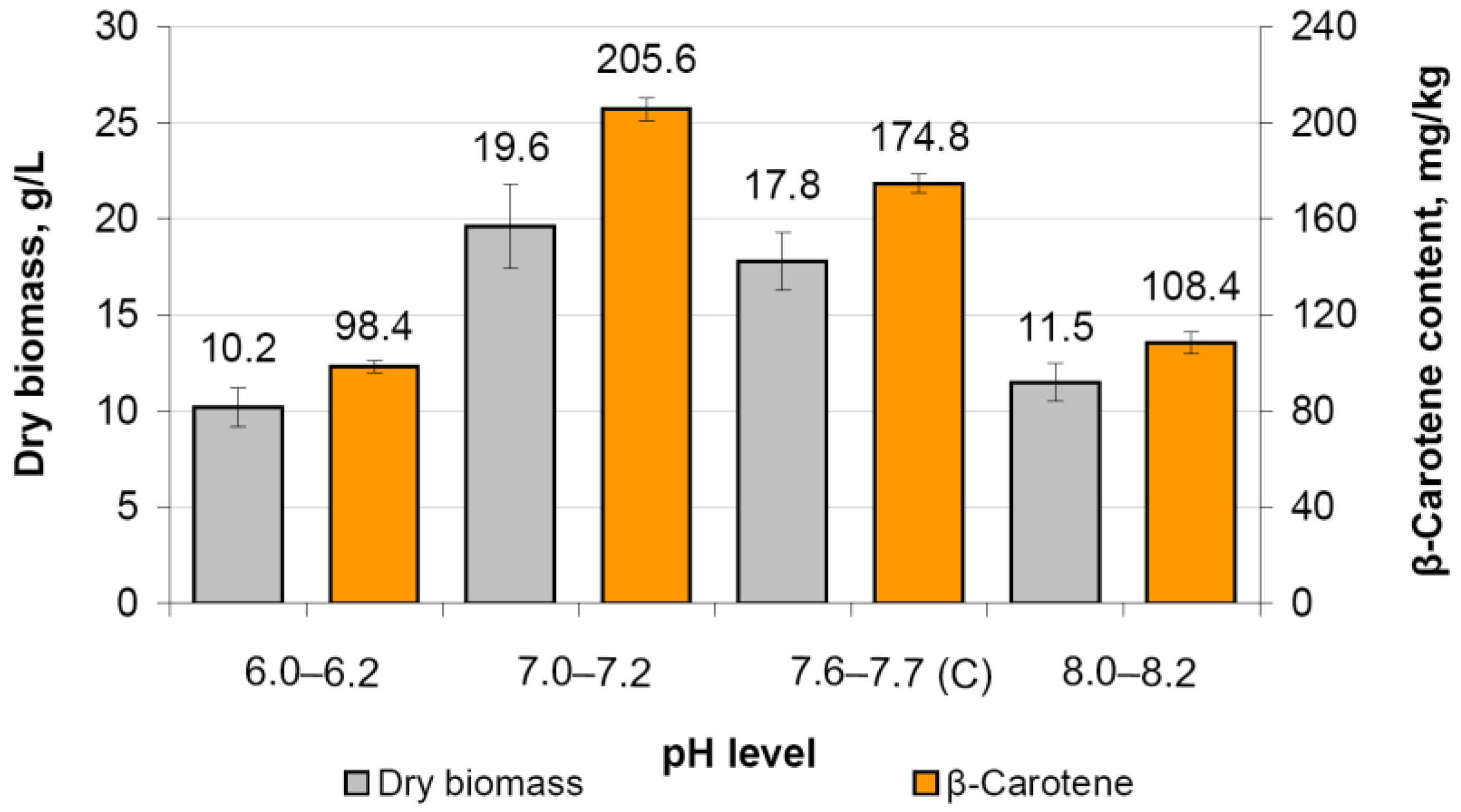
3.8.2. Fermentation of M. neoaurum Ac-501/22 under Controlled pH Regime
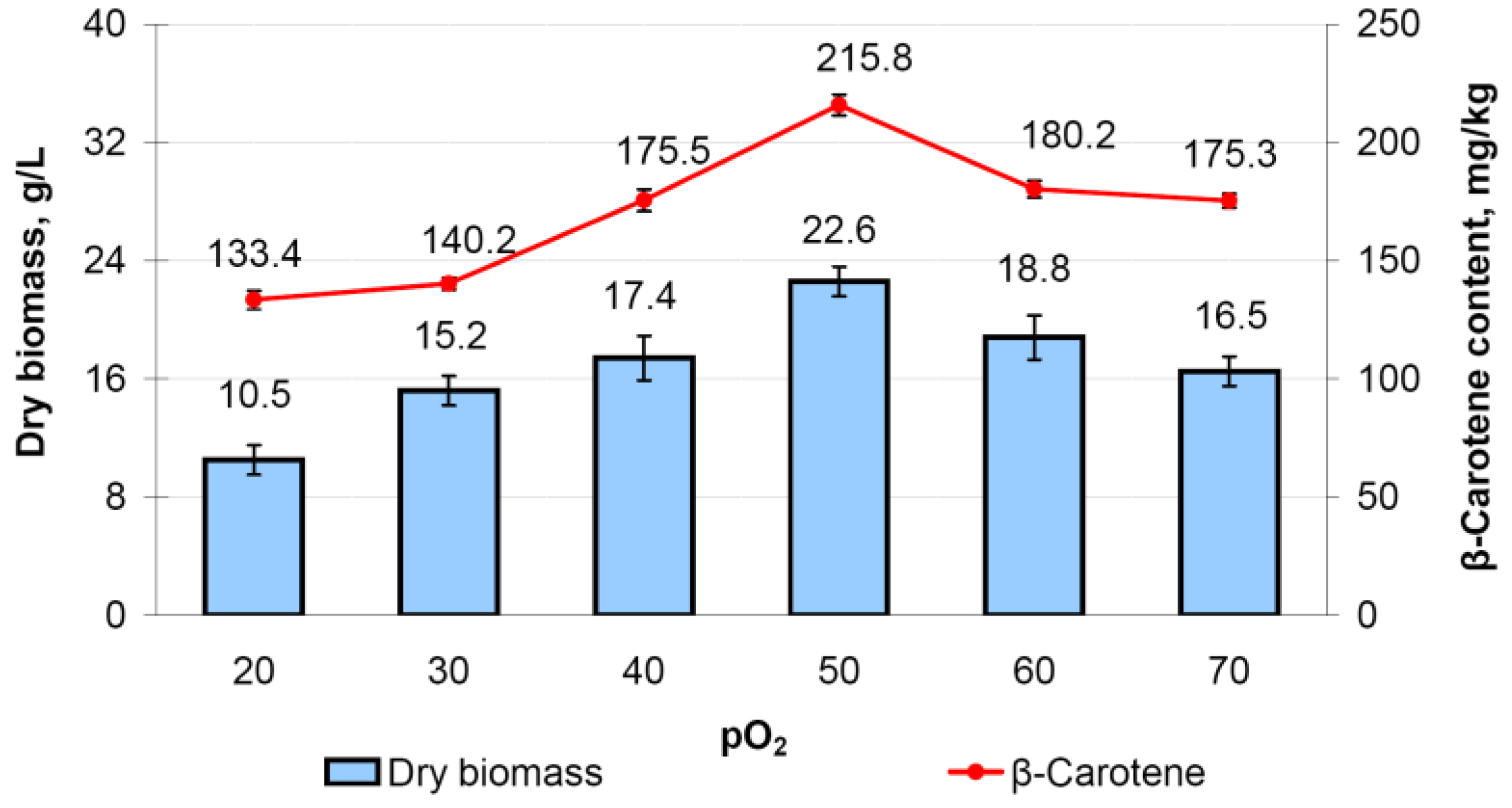
3.8.3. Determination of the Optimal pO2 Level for Fermentation of M. neoaurum Ac-501/22
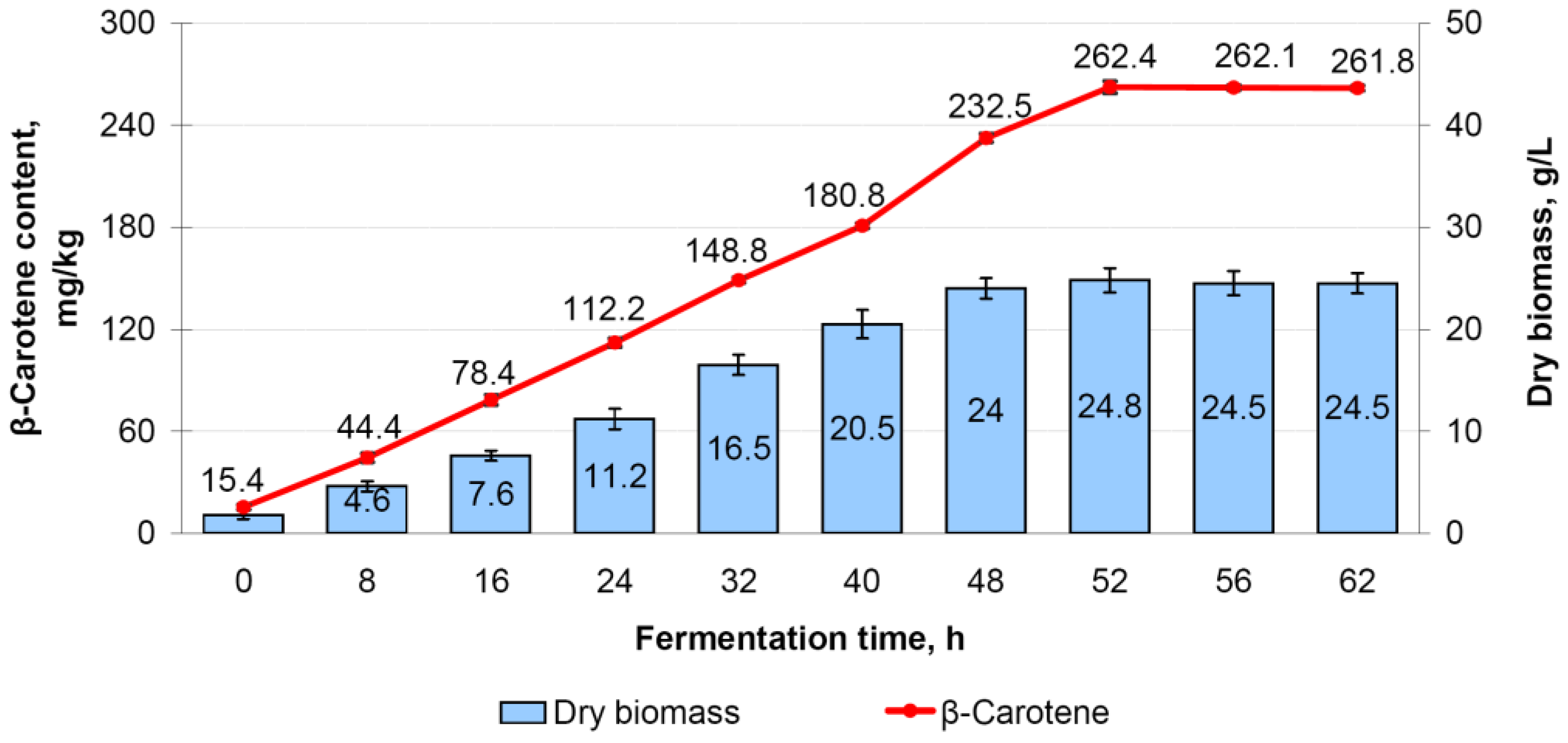
3.8.4. Productivity of M. neoaurum Ac-501/22 Cultivated in a 3 L Bioreactor with Controlled pH and pO2 Levels and Additional Carbon Source Supply
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from cyanobacteria: Biotechnological potential and optimization strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.H.; Rajesh Banu, J.; Kumar, G. Technological advances in the production of carotenoids and their applications—A critical review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef] [PubMed]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef] [PubMed]
- Ernst, H. Recent advances in industrial carotenoid synthesis. Pure Appl. Chem. 2002, 74, 2213–2226. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquacult. 2017, 10, 738–773. [Google Scholar] [CrossRef]
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol. Plant 2006, 126, 369–381. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, L.J.; Wu, C.Y.; Guo, K.N.; Li, J.H. Growth and antioxidant production of Spirulina in different NaCl concentrations. Biotechnol. Lett. 2016, 38, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Maddiboyina, B.; Vanamamalai, H.K.; Roy, H.; Ramaiah; Gandhi, S.; Kavisri, M.; Moovendhan, M. Food and drug industry applications of microalgae Spirulina platensis: A review. J. Basic Microbiol. 2023, 63, 573–583. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, G.D. Production of carotenoids by bacteria: Carotenoid productivity and availability. J. Life Sci. 2022, 32, 411–419. [Google Scholar]
- El Baky, H.H.A.; El Baroty, G.S.; Mostafa, E.M. Optimization growth of Spirulina (Arthrospira) platensis in photobioreactor under varied nitrogen concentration for maximized biomass, carotenoids and lipid contents. Recent. Pat. Food Nutr. Agric. 2018, 11, 40–48. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Alves, L. Ability of Gordonia alkanivorans strain 1B for high added value carotenoids production. RSC Adv. 2016, 5, 58055. [Google Scholar] [CrossRef]
- Tran, T.; Dawrs, S.N.; Norton, G.J.; Virdi, R.; Honda, J.R. Brought to you courtesy of the red, white, and blue pigments of nontuberculous mycobacteria. AIMS Microbiol. 2020, 6, 434–450. [Google Scholar] [CrossRef]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and identification of carotenoid-producing Rhodotorula sp. from Pinaceae forest ecosystems and optimization of in vitro carotenoid production. Biotechnol. Rep. 2021, 32, e00687. [Google Scholar] [CrossRef]
- Barreiro, C.; Barredo, J.L. Microbial Carotenoids: Methods and Protocols; Humana New York: New York, NY, USA, 2018; p. 344. [Google Scholar]
- Zeb, A.; Murkovic, M. Thin-layer chromatographic analysis of carotenoids in plant and animal samples. J. Planar Chromatogr. 2010, 23, 94–103. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Solovchenko, A.E. Express analysis of microalgal secondary carotenoids by TLC and UV-Vis spectroscopy. In Microbial Carotenoids; Methods in Molecular Biology; Springer: Amsterdam, The Netherlands, 2018; p. 7395. [Google Scholar]
- Bulygina, E.S.; Kuznetsov, B.B.; Marusina, A.I.; Turova, T.P.; Kravchenko, I.K.; Bykova, S.A.; Kolganova, T.V.; Gal’chenko, V.F. A study of nucleotide sequences of nifH genes of some methanotrophic bacteria. Mikrobiology 2002, 71, 425–432. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 84, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Checheta, O.V.; Safonova, E.F.; Slivkin, A.I. Structure of chemical compounds, methods and process control: Thin-layer chromatography characteristics of β-carotene. Pharm. Chem. J. 2012, 46, 321–323. [Google Scholar] [CrossRef]
- Tekucheva, D.N.; Nikolayeva, V.M.; Karpov, M.V.; Timakova, T.A.; Shutov, A.V.; Donova, M.V. Bioproduction of testosterone from phytosterol by Mycolicibacterium neoaurum strains: “one-pot”, two modes. Bioresour. Bioproces 2022, 9, 11. [Google Scholar] [CrossRef]
- Andriushina, V.A.; Rodina, N.V.; Stytsenko, T.C.; Hue, L.D.; Druzhinina, A.V.; Iaderets, V.V.; Voishvillo, N.E. Conversion of soybean sterols into 3,17-diketosteroids using actinobacteria Mycobacterium neoaurum, Pimelobacter simplex, and Rhodococcus erythropolis. Appl. Biochem. Microbiol. 2011, 47, 270–273. [Google Scholar] [CrossRef]
- Delegan, Y.; Yachkula, A.; Antipova, T.; Vainshtein, M. Evaluation of red-colored carotenoids in yeasts by the biomass color. Folia Microbiol. 2021, 66, 615–622. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “new” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef]
- Li, N.; Han, Z.; O’Donnell, T.J.; Kurasaki, R.; Kajihara, L.; Williams, P.G.; Tang, Y.; Su, W.W. Production and excretion of astaxanthin by engineered Yarrowia lipolytica using plant oil as both the carbon source and the biocompatible extractant. Appl. Microbiol. Biotechnol. 2020, 104, 6977–6989. [Google Scholar] [CrossRef]
- Verwaal, R.; Wang, J.; Meijnen, J.P.; Visser, H.; Sandmann, G.; van den Berg, J.A.; van Ooyen, A.J. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2007, 73, 4342–4350. [Google Scholar] [CrossRef]
- Sharpe, P.L.; Di Cosimo, D.J. Construction and utilization of carotenoid reporter systems: Identification of chromosomal integration sites that support suitable expression of biosynthetic genes and pathways. Methods Mol. Biol. 2012, 892, 219–243. [Google Scholar]
- Wang, C.; Zhao, S.; Shao, X.; Park, J.B.; Jeong, S.H.; Park, H.J.; Kwak, W.J.; Wei, G.; Kim, S.W. Challenges and tackles in metabolic engineering for microbial production of carotenoids. Microb. Cell Fact. 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, S.; Shimokata, K.; Tsukamura, M. Relationship between mycobacterial species and their carotenoid pigments. Microbiol. Immunol. 1988, 32, 473–479. [Google Scholar] [CrossRef]
- da Costa Cardoso, L.A.; Feitosa Kanno, K.Y.; Karp, S.G. Microbial production of carotenoids—A review. African J. Biotechnol. 2017, 16, 139–146. [Google Scholar]
- Barbosa-Filho, J.M.; Alencar, A.A.; Nunes, X.P.; de Andrade Tomaz, A.C.; Sena-Filho, J.G.; Athayde-Filho, P.F.; Silva, M.S.; de Souza, M.F.V.; da-Cunha, E.V.L. Sources of alpha-, beta-, gamma-, delta- and epsilon-carotenes: A twentieth century review. Rev. Bras. Farmacogn. 2008, 18, 135–154. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Carle, R. Carotenoid Production by Bacteria, Microalgae, and Fungi. In Carotenoids: Nutrition, Analysis and Technology; Kaczor, A., Baranska, M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 217–240. [Google Scholar]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Technological aspects of β-carotene production. Food Bioprocess. Technol. 2011, 4, 693–701. [Google Scholar] [CrossRef]
- Daraseliia, G.I.; Khachapuridze, D.O.; Gagelidze, N.A.; Amiranashvili, L.L. The kinetics of Mycobacterium rubrum growth under the conditions of batch cultivation. Mikrobiologiia 1989, 58, 596–601. (In Russian) [Google Scholar]
- Zhao, A.; Zhang, X.; Li, Y.; Wang, Z.; Lv, Y.; Liu, J.; Alam, M.A.; Xiong, W.; Xu, J. Mycolicibacterium cell factory for the production of steroid-based drug intermediates. Biotechnol. Adv. 2021, 53, 107860. [Google Scholar] [CrossRef]
- Wang, N.; Peng, H.; Yang, C.; Guo, W.; Wang, M.; Li, G.; Liu, D. Metabolic engineering of model microorganisms for the production of xanthophyll. Microorganisms 2023, 11, 1252. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef]
- Elamin, O.; Marwa, C.; Koraichi, S.I.; Iraqui, H.M. Lycopene production in Mycobacterium smegmatis by expression of crt genes from Mycobacterium aurum and protective effect of lycopene in vivo and in vitro against UV radiation. Int. J. Pharm. Pharm. Sci. 2018, 10, 49–53. [Google Scholar] [CrossRef]
- Khaliq, S.; Akhtar, K.; Afzal Ghauri, M.; Iqbal, R.; Mukhtar Khalid, A.; Muddassar, M. Change in colony morphology and kinetics of tylosin production after UV and gamma irradiation mutagenesis of Streptomyces fradiae NRRL-2702. Microbiol. Res. 2009, 164, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Brabender, M.; Hussain, M.S.; Rodriguez, G.; Blenner, M.A. Urea and urine are a viable and cost-effective nitrogen source for Yarrowia lipolytica biomass and lipid accumulation. Appl. Microbiol. Biotechnol. 2018, 102, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hu, R.; Chen, D.; Chen, J.; He, W.; Huang, L.; Lin, C.; Chen, H.; Chen, Y.; Zhu, J.; et al. Lipid and carotenoid production by the Rhodosporidium toruloides mutant in cane molasses. Bioresour. Technol. 2021, 326, 124816. [Google Scholar] [CrossRef] [PubMed]
- Siziya, I.N.; Yoon, D.J.; Kim, M.; Seo, M.J. Enhanced production of C30 carotenoid 4,4′-diaponeurosporene by optimizing culture conditions of Lactiplantibacillus plantarum subsp. plantarum KCCP11226T. J. Microbiol. Biotechnol. 2022, 32, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Kapoor, S.; Mishra, S. Carotenoid production by a novel isolate of Microbacterium paraoxydans. Indian. J. Microbiol. 2018, 58, 118–122. [Google Scholar] [CrossRef]
- Suwaleerat, T.; Thanapimmetha, A.; Srisaiyoot, M.; Chisti, Y.; Srinophakun, P. Enhanced production of carotenoids and lipids by Rhodococcus opacus PD630. J. Chem. Technol. Biotechnol. 2018, 93, 2160–2169. [Google Scholar] [CrossRef]
- Ni, H.; Chen, Q.H.; Ruan, H.; Yang, Y.F.; Li, L.J.; Wu, G.B.; Hu, Y.; He, G.Q. Studies on optimization of nitrogen sources for astaxanthin production by Phaffia rhodozyma. J. Zhejiang Univ. Sci. B 2007, 8, 365–370. [Google Scholar] [CrossRef]
- El-Banna, A.A.; El-Razek, A.M.A.; El-Mahdy, A.R. Some factors affecting the production of carotenoids by Rhodotorula glutinis var. glutinis. Food Nutr. Sci 2012, 3, 64–71. [Google Scholar]
- Baughn, A.D.; Rhee, K.Y. Metabolomics of central carbon metabolism in Mycobacterium tuberculosis. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Paniagua-Michel, J.; Olmos-Soto, J.; Ruiz, M.A. Pathways of carotenoid biosynthesis in bacteria and microalgae. Methods Mol. Biol. 2012, 892, 1–12. [Google Scholar] [PubMed]
- Janisch, N.; Levendosky, K.; Budell, W.C.; Quadri, L.E.N. Genetic underpinnings of carotenogenesis and light-induced transcriptome remodeling in the opportunistic pathogen Mycobacterium kansasii. Pathogens 2023, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Rekha, P.D.; Young, C.C.; Hameed, A.; Lin, S.Y.; Arun, A.B. Zeaxanthin production by novel marine isolates from coastal sand of India and its antioxidant properties. Appl. Biochem. Biotechnol. 2013, 171, 817–831. [Google Scholar] [CrossRef]
- Takaichi, S.; Ishidsu, J. Influence of growth temperature on compositions of carotenoids and fatty acids from carotenoid glucoside ester and from cellular lipids in Rhodococcus rhodochrous RNMSI. Biosci. Biotechnol. Biochem. 1993, 57, 1886–1889. [Google Scholar] [CrossRef]
- Xiong, L.B.; Liu, H.H.; Song, L.; Dong, M.M.; Ke, J.; Liu, Y.J.; Liu, K.; Zhao, M.; Wang, F.Q.; Wei, D.Z. Improving the biotransformation efficiency of soybean phytosterols in Mycolicibacterium neoaurum by the combined deletion of fbpC3 and embC in cell envelope synthesis. Synth. Syst. Biotechnol. 2021, 7, 453–459. [Google Scholar] [CrossRef]
- Thanapimmetha, A.; Suwaleerat, T.; Saisriyoot, M.; Chisti, Y.; Srinophakun, P. Production of carotenoids and lipids by Rhodococcus opacus PD630 in batch and fed-batch culture. Bioprocess. Biosyst. Eng. 2017, 40, 133–143. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| Medium volume (with allowance for a steam condensate) | 1.5 L |
| Temperature | 35 ± 1 °C |
| Aeration | 0.1 L/L/min |
| Stirring rate | 250 rpm |
| pO2 level | 100% of saturation |
| Pressure within a fermenter | 0.03–0.05 MPa |
| Medium pH | 6.8–7.2 |
| Eluent (Mobile Phase) | Biomass Extract | Reference Samples | ||
|---|---|---|---|---|
| β-Carotene | Lycopene | β-Carotene | Lycopene | |
| Hexane:benzene (5:1) | 0.98 ± 0.01 | – | 0.98 ± 0.01 | 0.80 ± 0.01 |
| Hexane:benzene (9:1) | 0.74 ± 0.01 | – | 0.74 ± 0.01 | 0.64 ± 0.01 |
| Benzene:acetone (25:1) | 0.98 ± 0.01 | – | 0.98 ± 0.01 | 0.81 ± 0.01 |
| Hexane:acetone (98:2) | 0.54 ± 0.01 | – | 0.54 ± 0.01 | 0.45 ± 0.01 |
| Cultivation Temperature, °C | Growth Period, h | Biomass Accumulation, g/L | Growth Rate, g/L/h | β-Carotene Content, mg/kg |
|---|---|---|---|---|
| 25 | 65 | 8.5 ± 0.5 | 0.13 | 113.8 ± 1.5 |
| 30 | 65 | 12.2 ± 1.2 | 0.17 | 122.3 ± 0.75 |
| 35 | 50 | 15.2 ± 0.7 | 0.28 | 136.2 ± 1.5 |
| 40 | 50 | 12.4 ± 1.1 | 0.23 | 128.5 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaderets, V.; Karpova, N.; Glagoleva, E.; Shibaeva, A.; Dzhavakhiya, V. Enhanced β-Carotene Production in Mycolicibacterium neoaurum Ac-501/22 by Combining Mutagenesis, Strain Selection, and Subsequent Fermentation Optimization. Fermentation 2023, 9, 1007. https://doi.org/10.3390/fermentation9121007
Yaderets V, Karpova N, Glagoleva E, Shibaeva A, Dzhavakhiya V. Enhanced β-Carotene Production in Mycolicibacterium neoaurum Ac-501/22 by Combining Mutagenesis, Strain Selection, and Subsequent Fermentation Optimization. Fermentation. 2023; 9(12):1007. https://doi.org/10.3390/fermentation9121007
Chicago/Turabian StyleYaderets, Vera, Nataliya Karpova, Elena Glagoleva, Alexandra Shibaeva, and Vakhtang Dzhavakhiya. 2023. "Enhanced β-Carotene Production in Mycolicibacterium neoaurum Ac-501/22 by Combining Mutagenesis, Strain Selection, and Subsequent Fermentation Optimization" Fermentation 9, no. 12: 1007. https://doi.org/10.3390/fermentation9121007
APA StyleYaderets, V., Karpova, N., Glagoleva, E., Shibaeva, A., & Dzhavakhiya, V. (2023). Enhanced β-Carotene Production in Mycolicibacterium neoaurum Ac-501/22 by Combining Mutagenesis, Strain Selection, and Subsequent Fermentation Optimization. Fermentation, 9(12), 1007. https://doi.org/10.3390/fermentation9121007






