Effect of Mixed Lactiplantibacillus plantarum- and Bacillus subtilis-Fermented Feed on Growth, Immunity, and Intestinal Health of Weaner Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Fermentation of Feed
2.2. Animals and Experimental Design
2.3. Number of Microorganisms in Feed
2.4. pH, Organic Acid, and Nutrient Content in Feed
2.5. Determination of Growth, Slaughtering, and Immunity Performance of Bamei Pigs
2.6. Extraction of DNA and Sequencing of 16S rDNA from Fecal Samples
2.7. High-Throughput-Sequencing Analysis of Fecal Samples
2.8. Statistical Analysis
3. Results
3.1. Effect of Adding Probiotics or Antibiotics on the Microbiota of Feeds
3.2. pH and Organic Acid Content of Different Bamei Pig Feeds
3.3. Differences in Nutrient Content in Different Feeds
3.4. Effect of Fermented Feeds on Growth and Slaughter Performance of Bamei Pigs
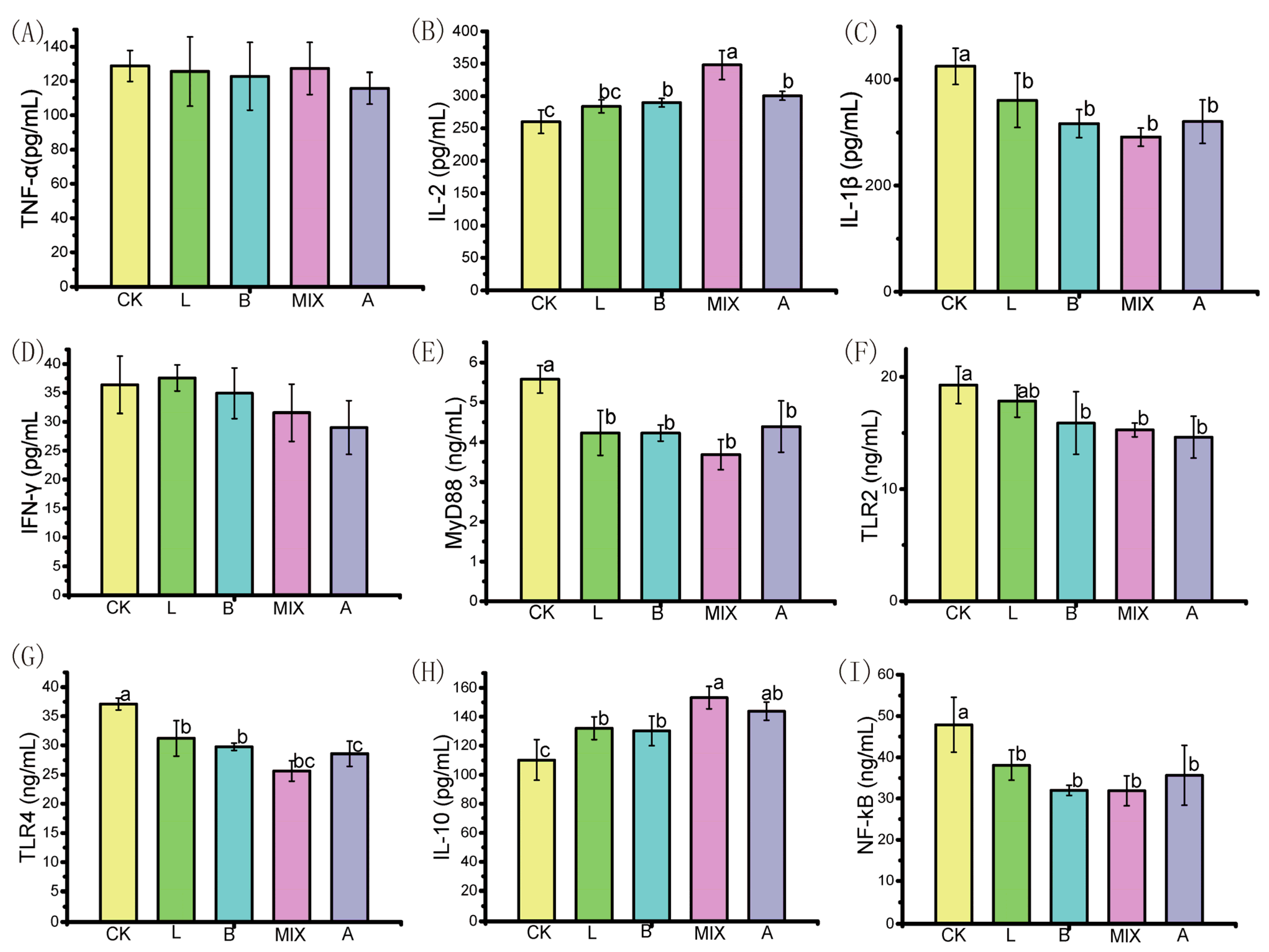
3.5. Effect of Fermented Feeds on the Immune Performance of Bamei Pigs
3.6. Effect of Fermented Feeds on Intestinal Microorganisms of Bamei Pigs
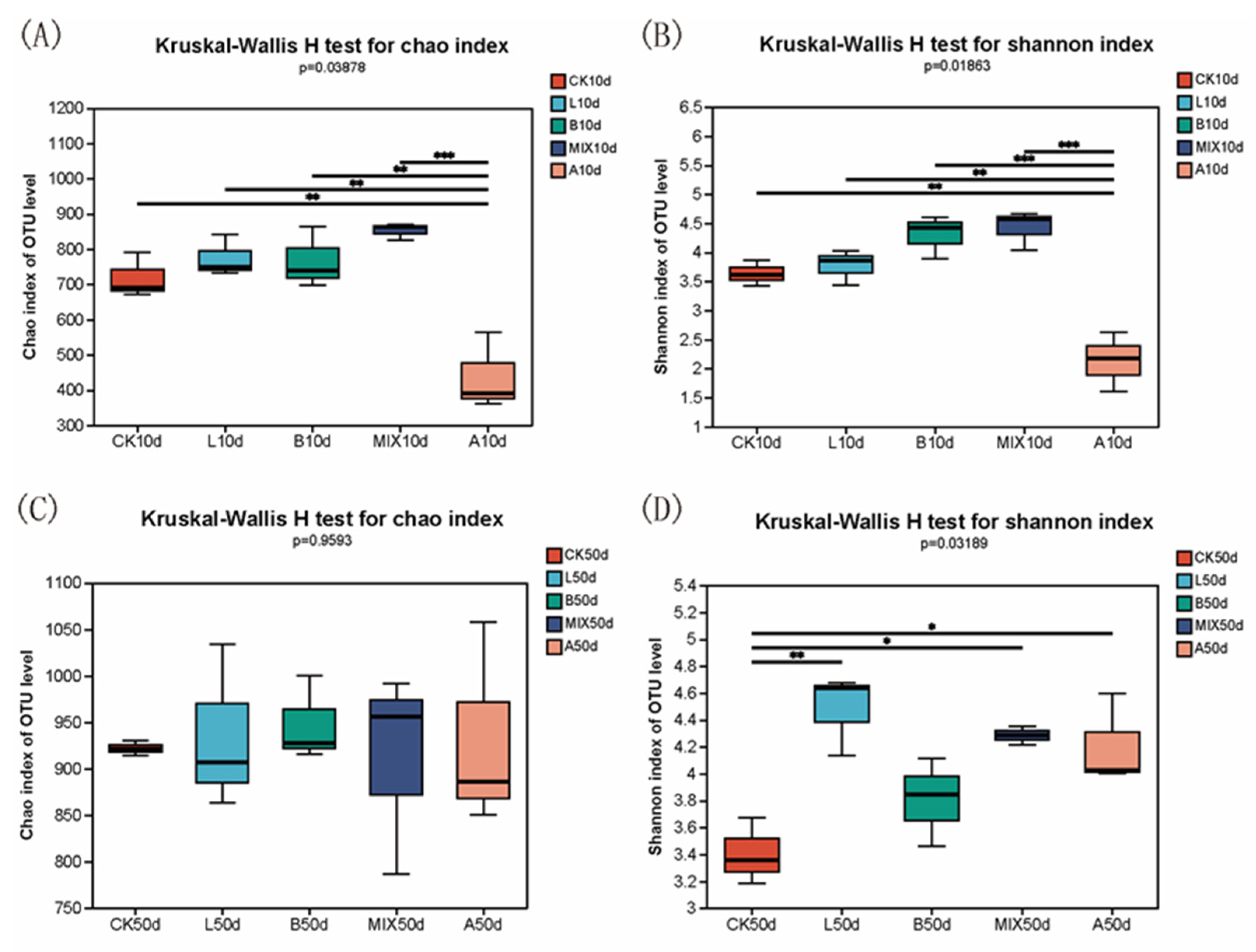
3.6.1. Effect of Fermented Feeds on the Diversity of Intestinal Bacteria in Bamei Pigs
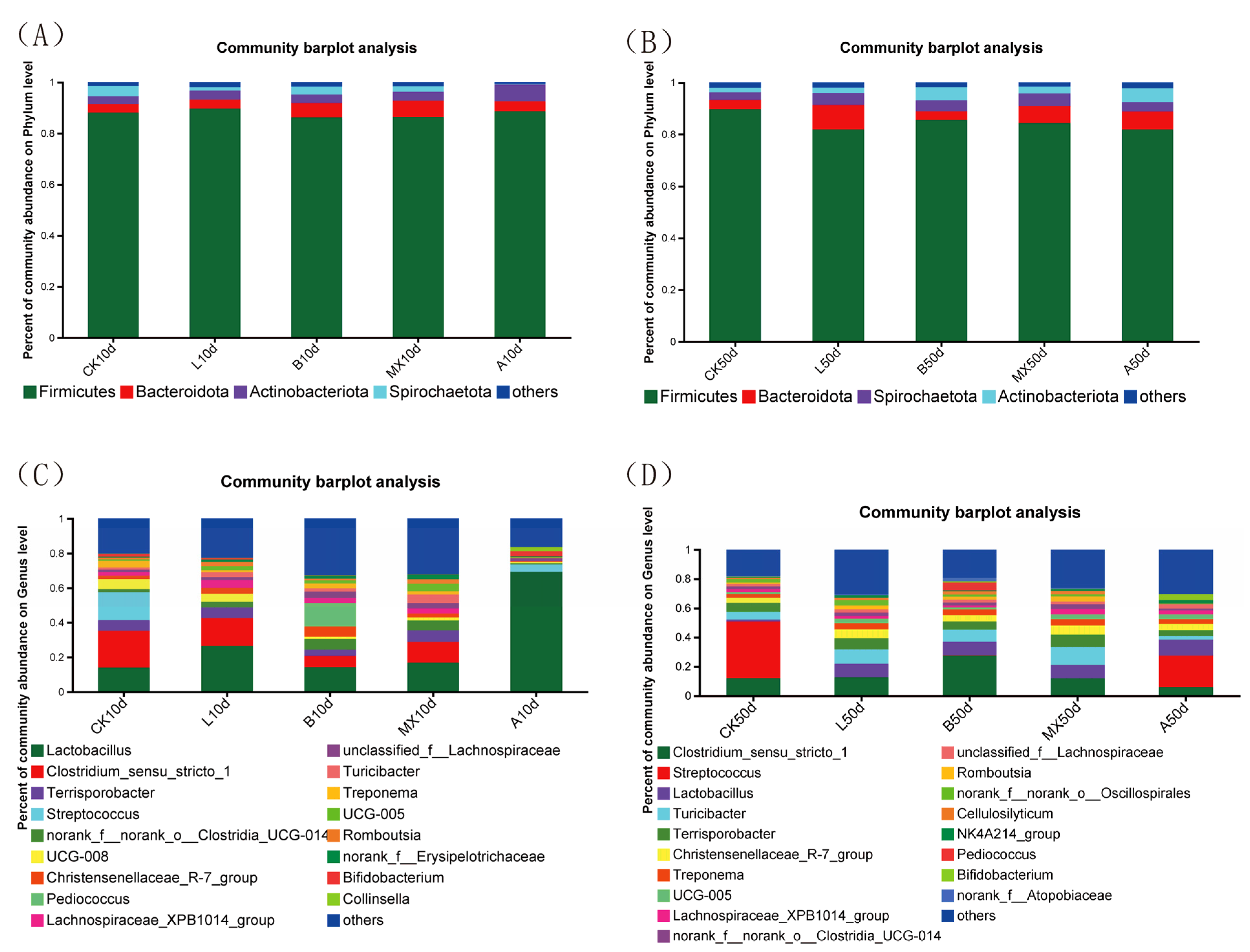
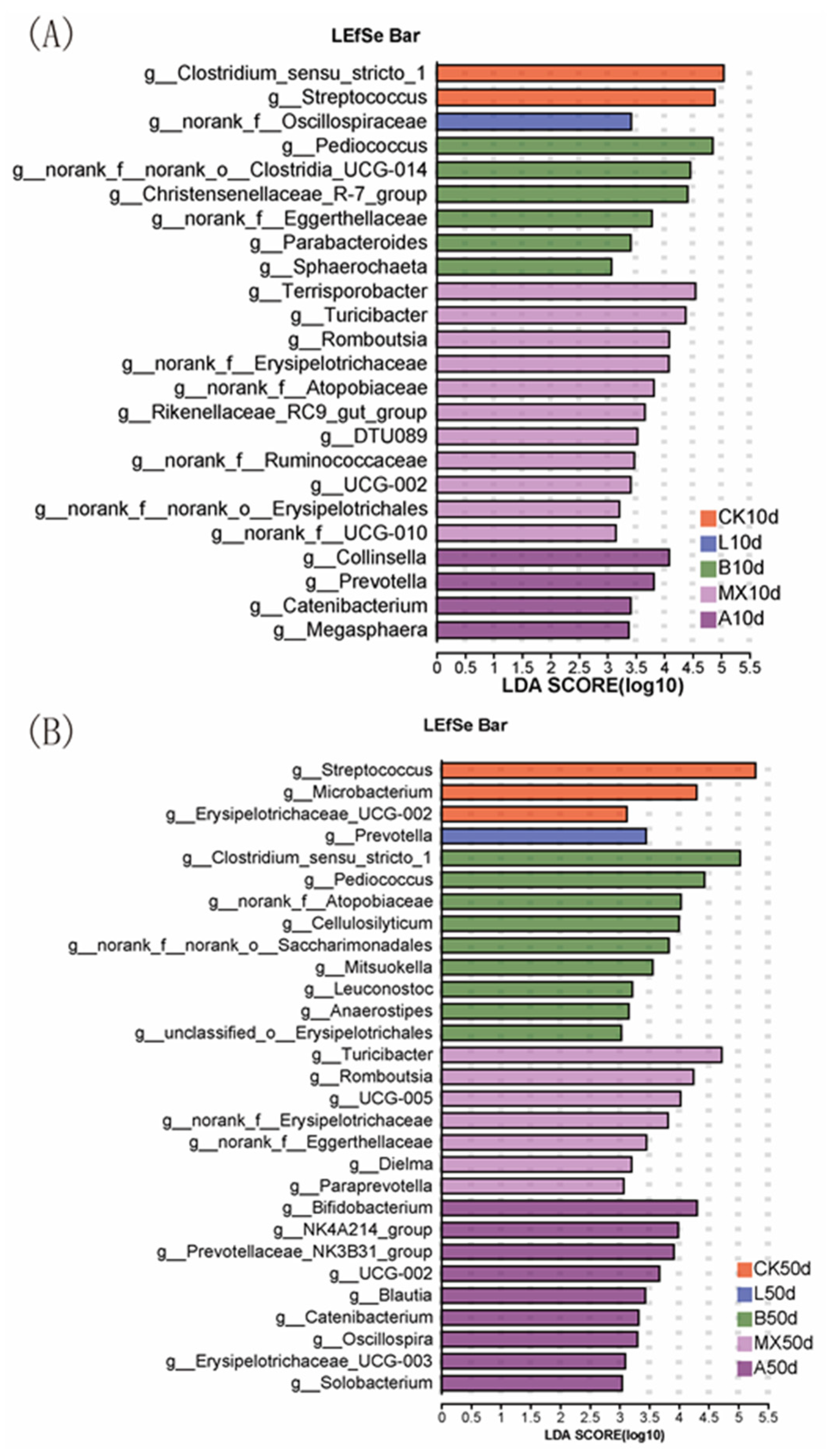
3.6.2. Comparison of the Composition of Intestinal Bacterial Community
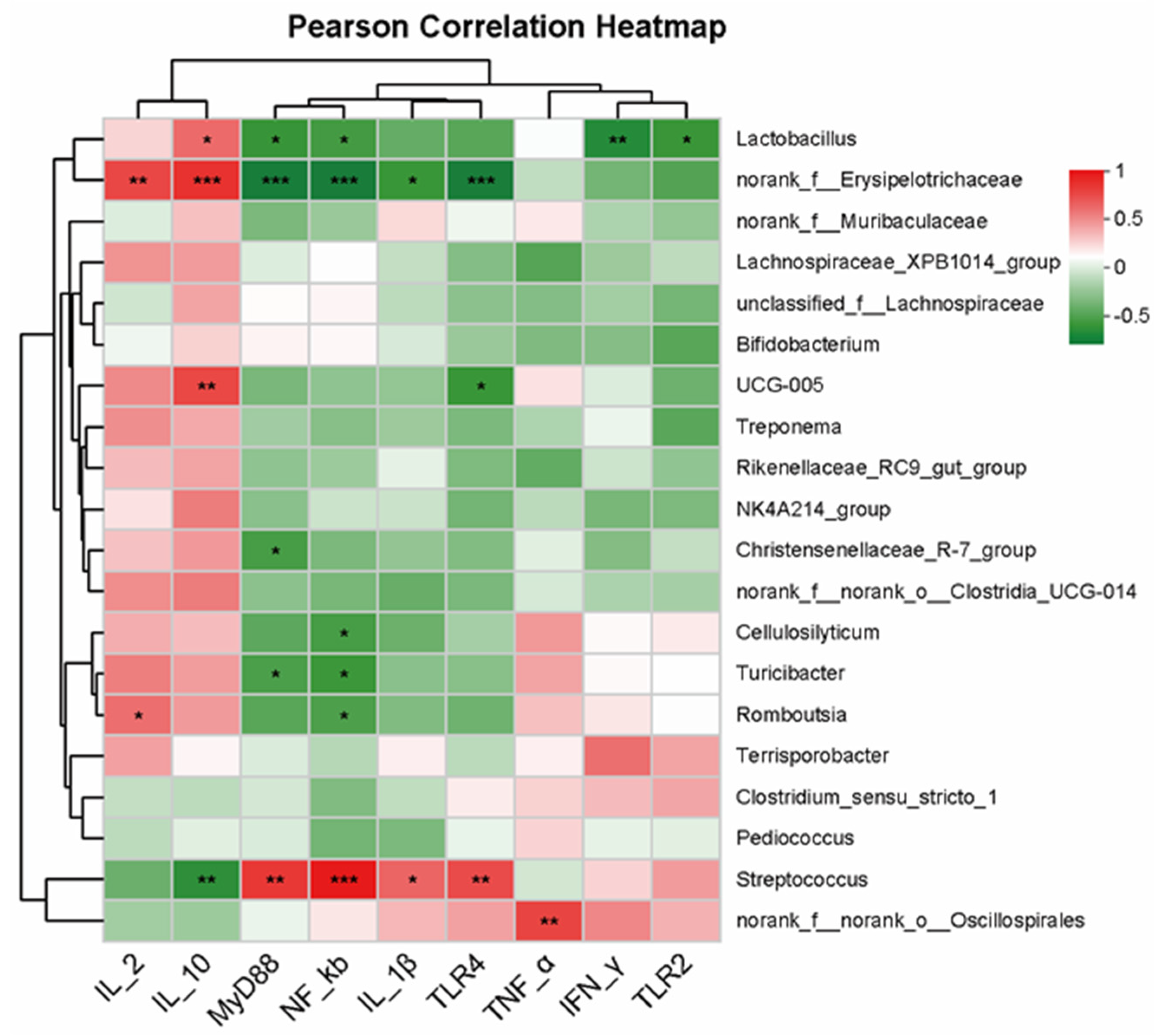
3.7. Analysis of the Correlation between Intestinal-Bacterial-Community Structure and Immune Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, P.R.; Evenson, A. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946, 165, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Thacker, P.A. Alternatives to antibiotics as growth promoters for use in swine production: A review. J. Anim. Sci. Biotechnol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Du, J.H.; Li, M.F.; He, J.Y.; Zhang, J.; Lian, W.W.; Huang, T. Influence of Microbiological Fermented Feed Made from Pineapple Leaf Residues on Growth Performance and Meat Quality of Growing and Fattening Pigs. Aer. Adv. Eng. Res. 2017, 135, 646–650. [Google Scholar]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poultry Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Barathikannan, K.; Chelliah, R.; Rubab, M.; Daliri, E.B.M.; Elahi, F.; Kim, D.H.; Agastian, P.; Oh, S.Y.; Oh, D.H. Gut Microbiome Modulation Based on Probiotic Application for Anti-Obesity: A Review on Efficacy and Validation. Microorganisms 2019, 7, 456. [Google Scholar] [CrossRef]
- Buesing, K.; Zeyner, A. Effects of oral Enterococcus faecium strain DSM 10663 NCIMB 10415 on diarrhoea patterns and performance of sucking piglets. Benef. Microbes 2015, 6, 41–44. [Google Scholar] [CrossRef]
- Yaqoob, M.U.; Wang, G.; Wang, M.Q. An updated review on probiotics as an alternative of antibiotics in poultry—A review. Anim. Biosci. 2022, 35, 1109–1120. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastro Hepat. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastro Hepat. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). Efsa J. 2020, 18, 5966. [Google Scholar] [CrossRef]
- Muhammad, Z.; Ramzan, R.; Abdelazez, A.; Amjad, A.; Afzaal, M.; Zhang, S.S.; Pan, S.Y. Assessment of the Antimicrobial Potentiality and Functionality of Lactobacillus plantarum Strains Isolated from the Conventional Inner Mongolian Fermented Cheese Against Foodborne Pathogens. Pathogens 2019, 8, 71. [Google Scholar] [CrossRef]
- Halimi, S.; Mirsalehian, A. Assessment and comparison of probiotic potential of four Lactobacillus species isolated from feces samples of Iranian infants. Microbiol. Immunol. 2016, 60, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tarrah, A.; Duarte, V.D.; de Castilhos, J.; Pakroo, S.; Junior, W.J.F.L.; Luchese, R.H.; Guerra, A.F.; Rossi, R.C.; Ziegler, D.R.; Corich, V.; et al. Probiotic potential and biofilm inhibitory activity of Lactobacillus casei group strains isolated from infant feces. J. Funct. Foods 2019, 54, 489–497. [Google Scholar] [CrossRef]
- Magnusson, J.; Schnürer, J. subsp.: Strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microb. 2001, 67, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Missotten, J.A.M.; Michiels, J.; Degroote, J.; De Smet, S. Fermented liquid feed for pigs: An ancient technique for the future. J. Anim. Sci. Biotechnol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Qiao, S.Y.; Li, D.F.; Piao, X.S.; Ren, J.P. Effects of Lactobacilli on the performance, diarrhea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian Austral J. Anim. 2004, 17, 401–409. [Google Scholar] [CrossRef]
- Santoso, U.; Tanaka, K.; Ohtani, S. Effect of Dried Bacillus-Subtilis Culture on Growth, Body-Composition and Hepatic Lipogenic Enzyme-Activity in Female Broiler Chicks. Brit J. Nutr. 1995, 74, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.H.; Ma, H.L.; He, R.H.; Huang, L.R.; Zhu, S.Y.; Ding, Q.Z.; Luo, L. Improvement of nutritional value and bioactivity of soybean meal by solid-state fermentation with Bacillus subtilis. LWT-Food Sci. Technol. 2017, 86, 1–7. [Google Scholar] [CrossRef]
- Delcenserie, V.; Martel, D.; Lamoureux, M.; Amiot, J.; Boutin, Y.; Roy, D. Immunomodulatory effects of probiotics in the intestinal tract. Curr. Issues Mol. Biol. 2008, 10, 37–53. [Google Scholar]
- Chen, J.; Pang, H.L.; Wang, L.; Ma, C.M.; Wu, G.F.; Liu, Y.; Guan, Y.F.; Zhang, M.; Qin, G.Y.; Tan, Z.F. Bacteriocin-Producing Lactic Acid Bacteria Strains with Antimicrobial Activity Screened from Bamei Pig Feces. Foods 2022, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.L.; Zhang, M.; Qin, G.Y.; Tan, Z.F.; Li, Z.W.; Wang, Y.P.; Cai, Y.M. Identification of lactic acid bacteria isolated from corn stovers. Anim. Sci. J. 2011, 82, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.L.; Yang, S.J.; Son, S.H.; Kim, W.S.; Lee, N.K.; Paik, H.D. Evaluation of probiotic P229 isolated from and its application in soybean fermentation. LWT-Food Sci. Technol. 2018, 97, 94–99. [Google Scholar] [CrossRef]
- Wu, G.F.; Tang, X.J.; Fan, C.; Wang, L.; Shen, W.J.; Ren, S.E.; Zhang, L.Z.; Zhang, Y.M. Gastrointestinal Tract and Dietary Fiber Driven Alterations of Gut Microbiota and Metabolites in Durco x Bamei Crossbred Pigs. Front. Nutr. 2022, 8, 806646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Wang, X.J.; Cui, M.Y.; Wang, Y.P.; Jiao, Z.; Tan, Z.F. Ensilage of oats and wheatgrass under natural alpine climatic conditions by indigenous lactic acid bacteria species isolated from high-cold areas. PLoS ONE 2018, 13, e0192368. [Google Scholar] [CrossRef]
- Zhao, S.S.; Wang, Y.P.; Yang, F.Y.; Wang, Y.; Zhang, H. Screening a Lactobacillus plantarum strain for good adaption in alfalfa ensiling and demonstrating its improvement of alfalfa silage quality. J. Appl. Microbiol. 2020, 129, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef]
- Thiex, N. Evaluation of Analytical Methods for the Determination of Moisture, Crude Protein, Crude Fat, and Crude Fiber in Distillers Dried Grains with Solubles. J. AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef]
- Vansoest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- NY/T 825-2004; Technical Specification for the Determination of Carcass Traits in Lean Pigs. China Standard Press: Beijing, China, 2004.
- Liu, Y.; Lv, H.; Xu, L.; Zhang, K.; Mei, Y.; Chen, J.; Wang, M.; Guan, Y.; Peng, H.; Wang, Y.; et al. The effect of dietary lactic acid bacteria on intestinal microbiota and immune responses of crucian carp (Carassius auratus) under water temperature decrease (vol 13, 847167, 2022). Front. Microbiol. 2022, 13, 976726. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Wang, W.D.; Lv, H.X.; Zhang, H.; Liu, Y.; Tan, Z.F. Thein vitroEffects of the Probiotic Strain, Lactobacillus casei ZX633 on Gut Microbiota Composition in Infants with Diarrhea. Front. Cell. Infect. Microbiol. 2020, 10, 576185. [Google Scholar] [CrossRef] [PubMed]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.A.; Whitehead, T.R.; Collins, M.D.; Lawson, P.A. Atopostipes suicloacale gen. nov., sp nov., isolated from an underground swine manure storage pit. Anaerobe 2004, 10, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Chen, X.B.; Di, P.P.; Wang, H.M.; Li, B.L.; Pan, Y.J.; Yan, S.L.; Wang, Y.J. Bacterial Community Associated with the Intestinal Tract of Chinese Mitten Crab (Eriocheir sinensis) Farmed in Lake Tai, China. PLoS ONE 2015, 10, e0123990. [Google Scholar] [CrossRef]
- Emborg, H.D.; Andersen, J.S.; Seyfarth, A.M.; Wegener, H.C. Relations between the consumption of antimicrobial growth promoters and the occurrence of resistance among Enterococcus faecium isolated from broilers. Epidemiol. Infect. 2004, 132, 95–105. [Google Scholar] [CrossRef]
- van Winsen, R.L.; Lipman, L.J.A.; Biesterveld, S.; Urlings, B.A.P.; Snijders, J.M.A.; van Knapen, F. Mechanism of Salmonella reduction in fermented pig feed. J. Sci. Food Agric. 2001, 81, 342–346. [Google Scholar] [CrossRef]
- Klewicki, R.; Klewicka, E. Antagonistic activity of lactic acid bacteria as probiotics against selected bacteria of the Enterobaceriacae family in the presence of polyols and their galactosyl derivatives. Biotechnol. Lett. 2004, 26, 317–320. [Google Scholar] [CrossRef]
- Tajima, K.; Ohmori, H.; Aminov, R.I.; Kobashi, Y.; Kawashima, T. Fermented liquid feed enhances bacterial diversity in piglet intestine. Anaerobe 2010, 16, 6–11. [Google Scholar] [CrossRef]
- Tabacco, E.; Piano, S.; Revello-Chion, A.; Borreani, G. Effect of LN4637 and LN40177 on the aerobic stability, fermentation products, and microbial populations of corn silage under farm conditions. J. Dairy Sci. 2011, 94, 5589–5598. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.T.; Wang, H.L.; Li, D.F.; Piao, X.S.; Lu, W.Q. Optimization of processing conditions for solid-state fermented soybean meal and its effects on growth performance and nutrient digestibility of weanling pigs. Livest. Sci. 2014, 170, 91–99. [Google Scholar] [CrossRef]
- Canibe, N.; Jensen, B.B. Fermented and nonfermented liquid feed to growing pigs: Effect on aspects of gastrointestinal ecology and growth performance. J. Anim. Sci. 2003, 81, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Jatkauskas, J.; Vrotniakiene, V. Effects of a Combined Pre- and Probiotics Product on Diarrhoea Patterns and Performance of Early Weaned Calves. Vet. Zootech.-Lith. 2009, 48, 17–23. [Google Scholar]
- van Winsen, R.L.; Urlings, B.A.P.; Lipman, L.J.A.; Snijders, J.M.A.; Keuzenkamp, D.; Verheijden, J.H.M.; van Knapen, F. Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Appl. Environ. Microb. 2001, 67, 3071–3076. [Google Scholar] [CrossRef]
- Kale-Pradhan, P.B.; Jassal, H.K.; Wilhelm, S.M. Role of Lactobacillus in the Prevention of Antibiotic-Associated Diarrhea: A Meta-analysis. Pharmacotherapy 2010, 30, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Demeckova, V.; Kelly, D.; Coutts, A.G.P.; Brooks, P.H.; Campbell, A. The effect of fermented liquid feeding on the faecal microbiology and colostrum quality of farrowing sows. Int. J. Food Microbiol. 2002, 79, 85–97. [Google Scholar] [CrossRef] [PubMed]
- van Winsen, R.L.; Keuzenkamp, D.; Urlings, B.A.P.; Lipman, L.J.A.; Snijders, J.A.M.; Verheijden, J.H.M.; van Knapen, F. Effect of fermented feed on shedding of Enterobacteriaceae by fattening pigs. Vet. Microbiol. 2002, 87, 267–276. [Google Scholar] [CrossRef]
- DeWitte, S.N.; Bekvalac, J. Oral Health and Frailty in the Medieval English Cemetery of St Mary Graces. Am. J. Phys. Anthropol. 2010, 142, 341–354. [Google Scholar] [CrossRef]
- Madrid, L.; Seale, A.C.; Kohli-Lynch, M.; Edmond, K.M.; Lawn, J.E.; Heath, P.T.; Madhi, S.A.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65, S160–S172. [Google Scholar] [CrossRef]
- Donkor, O.N.; Ravikumar, M.; Proudfoot, O.; Day, S.L.; Apostolopoulos, V.; Paukovics, G.; Vasiljevic, T.; Nutt, S.L.; Gill, H. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin. Exp. Immunol. 2012, 167, 282–295. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, K.A.; Targan, S.R. Tumor necrosis factor: Biology and therapeutic inhibitors. Gastroenterology 2000, 119, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Gonzalez-Rodriguez, I.; Gueimonde, M.; Margolles, A.; Suarez, A. Immune Response to Bifidobacterium bifidum Strains Support Treg/Th17 Plasticity. PLoS ONE 2011, 6, e24776. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroentero 2008, 14, 4280–4288. [Google Scholar] [CrossRef]
- De-Simone, F.I.; Sariyer, R.; Otalora, Y.L.; Yarandi, S.; Craigie, M.; Gordon, J.; Sariyer, I.K. IFN-Gamma Inhibits JC Virus Replication in Glial Cells by Suppressing T-Antigen Expression. PLoS ONE 2015, 10, e0129694. [Google Scholar] [CrossRef][Green Version]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]


| Material (w/w %) | Nutrition Level | ||
|---|---|---|---|
| Corn | 63.26 | Metabolizable energy (Kcal/kg) | 3095 |
| Wheat bran | 15 | Crude protein (%) | 14 |
| Soybean meal | 11.2 | Ca (%) | 0.55 |
| Alfalfa silage | 10 | Available phosphorus (%) | 0.21 |
| Rapeseed meal | 4 | Total phosphorus (%) | 0.46 |
| Soybean oil | 2.61 | Lysine (%) | 0.76 |
| Rape stalk | 0.85 | Methionine (%) | 0.21 |
| Stone powder | 1.1 | ||
| Dicalcium phosphate | 0.31 | ||
| Salt | 0.5 | ||
| Lysine | 0.17 | ||
| Premix | 1 |
| Group | Feed Formula | Concentration of Additive |
|---|---|---|
| CK | basal feed | - |
| L | basal feed + L. plantarum QP28-1a | 1 × 106 CFU/g |
| B | basal feed + B. subtilis QB8a | 1 × 106 CFU/g |
| MIX | basal feed + L. plantarum QP28-1a and B. subtilis QB8a | 1 × 106 CFU/g (1:1) |
| A | basal feed + gentamycin (antibiotic) | 50 mg/kg |
| Time (Day) | Microbial Colony Counts log cfu/g | Treatment Groups | SEM | p | ||||
|---|---|---|---|---|---|---|---|---|
| CK | L | B | MIX | A | ||||
| 2 | Yeast | ND | ND | 3.66 a | 4.05 a | ND | 0.18 | ** |
| Coliform bacteria | 6.57 a | 6.08 a | 6.24 a | 5.34 b | 6.09 a | 0.11 | ** | |
| Aerobic bacteria | 6.66 a | 6.07 c | 6.67 a | 5.67 d | 6.48 b | 0.04 | ** | |
| Clostridium | 5.54 a | 5.67 a | 5.71 a | 4.68 b | 5.41 a | 0.08 | ** | |
| Bacilli | 5.34 b | 5.39 a | 5.89 a | 5.64 a | 4.91 c | 0.09 | ** | |
| LAB | 5.56 c | 6.10 a | 5.77 b | 6.17 a | 5.65 bccc | 0.04 | ** | |
| 10 | Yeast | ND | ND | 4.15 a | 3.82 b | ND | 0.03 | ** |
| Coliform bacteria | 6.57 a | 4.29 d | 6.07 b | 4.61 c | 6.29 a | 0.04 | ** | |
| Aerobic bacteria | 6.61 a | 5.99 b | 6.75 a | 5.83 b | 6.52 a | 0.06 | ** | |
| Clostridium | 5.43 a | 5.14 a | 5.24 a | 4.21 b | 5.40 a | 0.08 | ** | |
| Bacilli | 5.57 a | 5.46 a | 5.47 a | 4.59 b | 5.21 ab | 0.13 | ** | |
| LAB | 5.43 c | 6.13 b | 7.71 a | 6.12 b | 5.91 b | 0.03 | ** | |
| 20 | Yeast | ND | ND | 4.65 | ND | ND | 0.01 | ** |
| Coliform bacteria | 6.56 a | 4.20 e | 4.63 c | 4.35 d | 6.20 b | 0.02 | ** | |
| Aerobic bacteria | 6.75 b | 4.94 d | 8.60 a | 4.58 e | 4.89 c | 0.04 | ** | |
| Clostridium | 5.31 a | 5.46 a | 4.94 b | 4.23 c | 4.89 b | 0.08 | ** | |
| Bacilli | 5.37 a | 4.76 c | 4.75 c | 4.98 b | 4.99 b | 0.05 | ** | |
| LAB | 5.25 c | 6.04 b | 8.03 a | 6.14 b | 5.20 c | 0.07 | ** | |
| 30 | Yeast | ND | ND | 4.06 | ND | ND | 0.02 | ** |
| Coliform bacteria | 6.63 a | 4.64 b | 4.42 b | 4.12 b | 6.17 a | 0.18 | ** | |
| Aerobic bacteria | 6.57 b | 4.71 c | 8.26 a | 4.48 c | 6.40 b | 0.07 | ** | |
| Clostridium | 5.51 a | 4.93 b | 5.53 a | 3.54 c | 5.04 b | 0.06 | ** | |
| Bacilli | 5.31 b | 3.95 c | 5.63 a | 3.75 c | 5.24 b | 0.07 | ** | |
| LAB | 5.42 c | 6.11 b | 8.81 a | 5.96 b | 5.31 c | 0.10 | ** | |
| 60 | Yeast | ND | ND | ND | ND | ND | - | - |
| Coliform bacteria | 6.40 a | 4.05 b | 6.01 a | 4.14 b | 6.05 a | 0.10 | ** | |
| Aerobic bacteria | 6.72 b | 4.59 d | 8.46 a | 4.40 e | 6.52 c | 0.04 | ** | |
| Clostridium | 4.77 b | 3.82 c | 5.88 a | 4.03 c | 4.91 b | 0.14 | ** | |
| Bacilli | 5.01 b | 3.95 c | 5.66 a | 4.69 b | 4.97 b | 0.15 | ** | |
| LAB | 5.43 b | 4.37 c | 8.66 a | 4.32 c | 5.37 b | 0.08 | ** | |
| Nutrient Composition | Treatment Groups | Fermented Days (Day) | Average Value | SEM | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 10 | 20 | 30 | 40 | 60 | T | D | T × D | ||||
| DM (%) | CK | 88.30 a | 88.07 a | 87.81 a | 87.27 a | 87.59 a | 88.31 a | 87.89a | 0.03 | ** | ** | ** |
| L | 54.39 b | 54.77 bc | 55.28 bc | 55.31 c | 55.59 c | 55.70 c | 55.17 c | |||||
| B | 54.77 bC | 55.53 bBC | 56.73 bB | 58.30 bA | 58.95 bA | 58.51 bA | 57.13 b | |||||
| MIX | 53.47 bB | 53.59 cB | 53.83 cB | 54.77 cAB | 55.65 cA | 54.93 cAB | 54.37 d | |||||
| A | 87.80 a | 88.20 a | 87.57 a | 86.57 a | 87.73 a | 88.17 a | 87.67 a | |||||
| CP (%DM) | CK | 12.90 | 12.31 c | 13.33 ab | 13.20 ab | 13.19 ab | 12.84 b | 12.96 b | 0.13 | ** | ** | ** |
| L | 12.28 B | 14.21 aA | 13.01 aA | 13.44 aA | 13.71 aA | 13.88 aA | 13.58 a | |||||
| B | 12.50 B | 13.62 abA | 12.80 bAB | 12.93 abAB | 12.81 bAB | 12.56 bB | 12.87 b | |||||
| MIX | 12.52 AB | 13.31 bA | 12.72 bAB | 12.36 bB | 12.41 bAB | 12.99 bAB | 12.71 b | |||||
| A | 12.31 | 12.75 bc | 12.74 b | 13.24 a | 12.85 ab | 12.69 b | 12.77 b | |||||
| NDF (%DM) | CK | 30.64 | 30.63 b | 31.07 a | 31.55 ab | 31.17 a | 31.48 a | 31.09 a | 0.38 | ** | ** | ** |
| L | 31.87 AB | 33.25 aA | 31.03 aAB | 30.75 abAB | 30.56 abB | 29.81 abB | 31.03 a | |||||
| B | 30.70 AB | 32.20 abA | 28.40 bBC | 28.01 cC | 28.84 bcBC | 28.40 bcBC | 29.43 b | |||||
| MIX | 31.59 A | 30.42 bA | 29.53 abAB | 29.09 bcABC | 26.88 cC | 27.12 cBC | 29.11 b | |||||
| A | 31.73 | 31.32 ab | 31.82 a | 31.92 a | 31.80 a | 31.76 a | 31.72 a | |||||
| ADF (%DM) | CK | 13.54 a | 13.48 a | 13.95 a | 13.79 a | 13.66 a | 13.55 a | 13.66 a | 0.09 | ** | * | ** |
| L | 12.82 bcA | 12.77 bA | 12.07 bB | 12.46 bAB | 12.09 bB | 12.29 bAB | 12.42 c | |||||
| B | 13.28 abA | 13.51 aA | 12.56 bB | 12.66 bB | 13.23 aAB | 13.29 aA | 13.09 b | |||||
| MIX | 12.66 c | 12.45 b | 12.40 b | 12.25 b | 12.29 b | 12.06 b | 12.35 c | |||||
| A | 13.57 a | 13.56 a | 13.65 a | 13.81 a | 13.69 a | 13.52 a | 13.64 a | |||||
| Hemicellulose (%DM) | CK | 18.09 ab | 17.36 b | 17.12 ab | 17.76 a | 17.52 a | 17.93 a | 18.03 a | 0.35 | ** | ** | ** |
| L | 19.05 a | 19.48 a | 19.08 a | 18.30 a | 18.47 a | 17.51 a | 18.62 a | |||||
| B | 16.58 bA | 17.83 bA | 15.04 bB | 14.53 bB | 14.77 bB | 14.27 bB | 15.50 c | |||||
| MIX | 18.92 aA | 17.97 bA | 17.13 abA | 16.84 aA | 14.59 bB | 15.06 bB | 16.75 b | |||||
| A | 18.16 ab | 17.75 b | 18.17 a | 18.11 a | 18.11 a | 18.22 a | 18.09 a | |||||
| Item | Dietary Treatments | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CK | L | B | MIX | A | |||
| Initial weight (kg) | 9.12 | 8.88 | 9.04 | 8.87 | 8.83 | 0.11 | NS |
| Final weight (kg) | 29.37 | 28.65 | 29.74 | 31.83 | 30.06 | 1.07 | NS |
| ADG (kg/d) | 0.337 | 0.329 | 0.345 | 0.383 | 0.354 | 0.02 | NS |
| ADFI (kg/d) | 1.05 ab | 0.99 b | 1.13 a | 1.14 a | 1.09 ab | 0.02 | * |
| FCR | 3.10 b | 3.02 bc | 3.21 a | 2.99 c | 3.07 bc | 0.02 | * |
| Left ketone weight (kg) | 15.72 | 14.18 | 13.59 | 15.04 | 15.13 | 0.17 | NS |
| Backfat thickness(mm) | 16.7 | 16.03 | 16.59 | 17.52 | 16.64 | 0.29 | NS |
| Eye-muscle area (cm2) | 26.45 a | 20.57 b | 18.14 b | 25.01 a | 21.45 b | 0.57 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wu, G.; Pang, H.; Hua, J.; Guan, Y.; Zhang, M.; Duan, Y.; Qin, G.; Wang, L.; Cai, Y.; et al. Effect of Mixed Lactiplantibacillus plantarum- and Bacillus subtilis-Fermented Feed on Growth, Immunity, and Intestinal Health of Weaner Pigs. Fermentation 2023, 9, 1005. https://doi.org/10.3390/fermentation9121005
Chen J, Wu G, Pang H, Hua J, Guan Y, Zhang M, Duan Y, Qin G, Wang L, Cai Y, et al. Effect of Mixed Lactiplantibacillus plantarum- and Bacillus subtilis-Fermented Feed on Growth, Immunity, and Intestinal Health of Weaner Pigs. Fermentation. 2023; 9(12):1005. https://doi.org/10.3390/fermentation9121005
Chicago/Turabian StyleChen, Jun, Guofang Wu, Huili Pang, Jiyun Hua, Yifei Guan, Miao Zhang, Yaoke Duan, Guangyong Qin, Lei Wang, Yimin Cai, and et al. 2023. "Effect of Mixed Lactiplantibacillus plantarum- and Bacillus subtilis-Fermented Feed on Growth, Immunity, and Intestinal Health of Weaner Pigs" Fermentation 9, no. 12: 1005. https://doi.org/10.3390/fermentation9121005
APA StyleChen, J., Wu, G., Pang, H., Hua, J., Guan, Y., Zhang, M., Duan, Y., Qin, G., Wang, L., Cai, Y., & Tan, Z. (2023). Effect of Mixed Lactiplantibacillus plantarum- and Bacillus subtilis-Fermented Feed on Growth, Immunity, and Intestinal Health of Weaner Pigs. Fermentation, 9(12), 1005. https://doi.org/10.3390/fermentation9121005







