Strategies for the Biotransformation of Tung Leaves in Bioethanol Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Test Method
2.4. Energy Conversion Efficiency Analysis
2.5. Kinetic Analysis
3. Results and Discussion
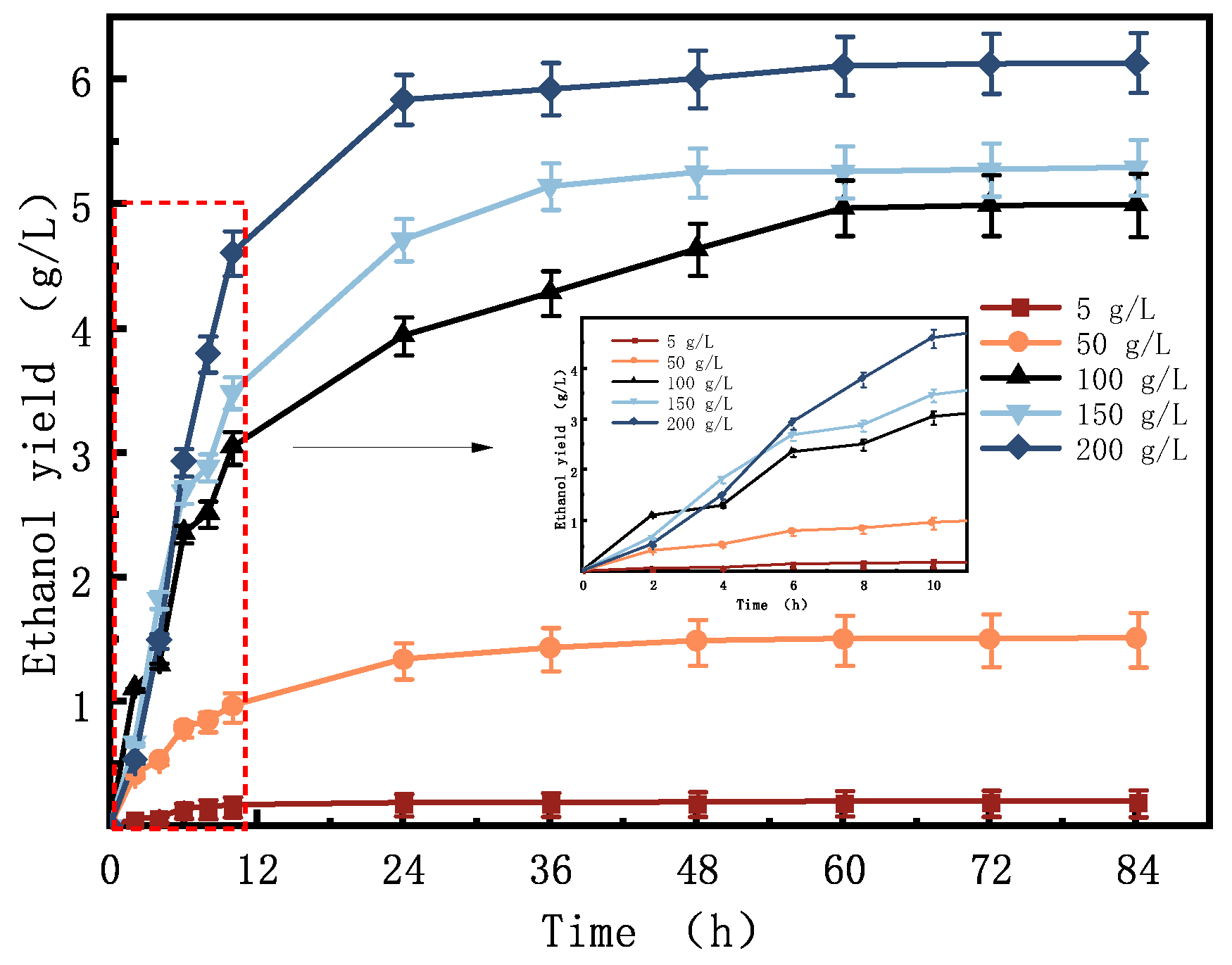
3.1. Effect of Substrate Concentration on the Gas Characteristics of Bioethanol Production
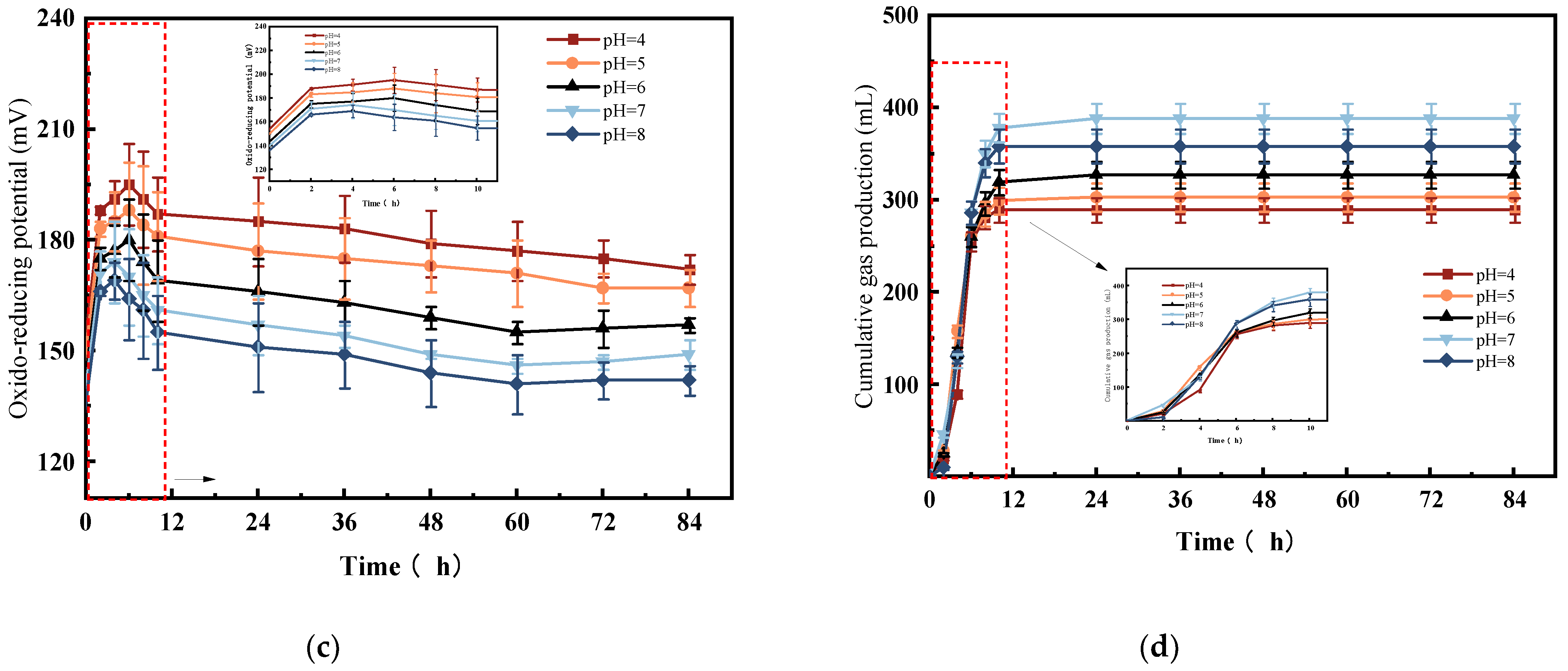
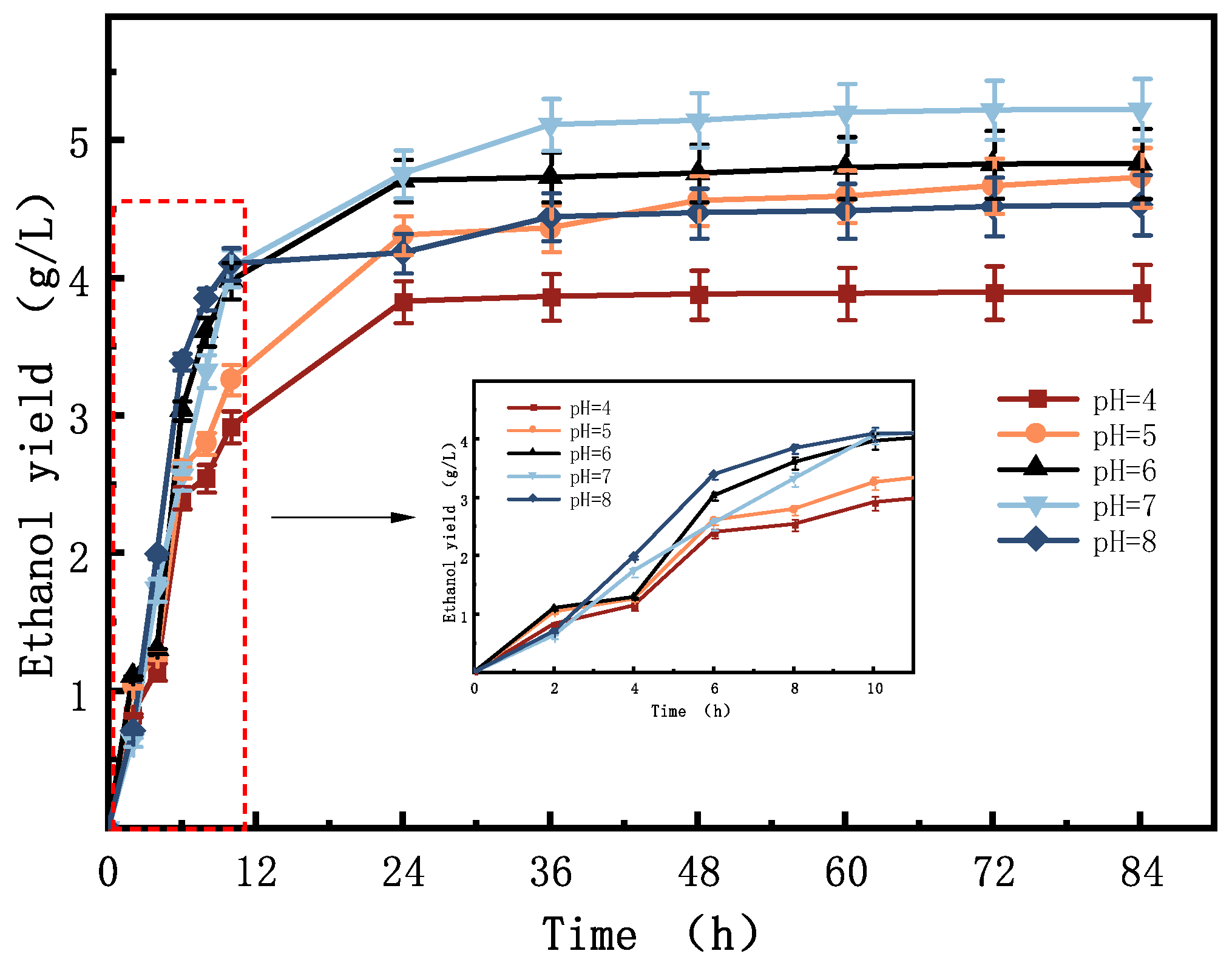
3.2. Analysis of the Effect of Initial pH on Bioethanol Results
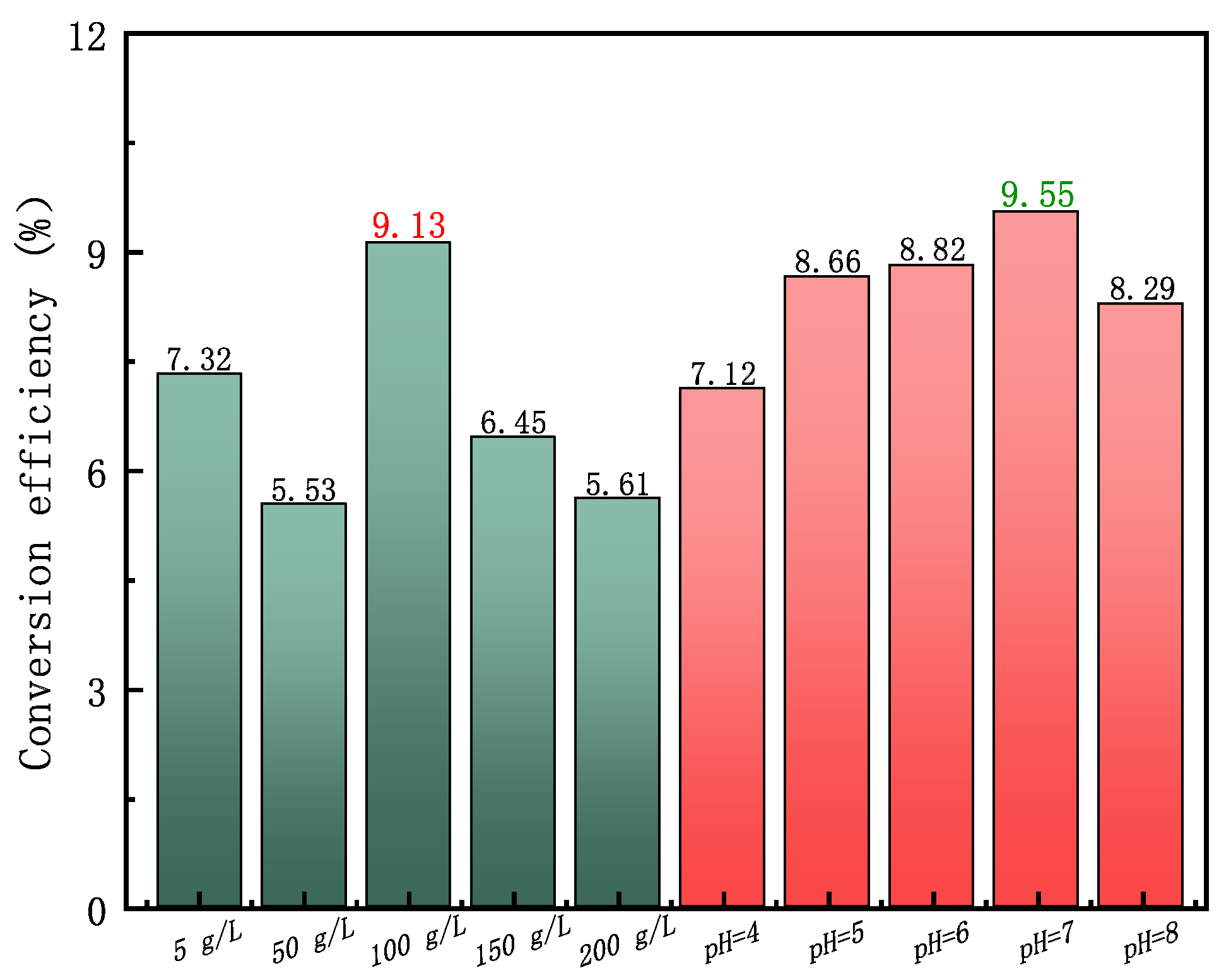
3.3. Analysis of Energy Conversion Efficiency
3.4. Analysis of Fermentation Kinetics under Different Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, H.B.; Chu, L.Y. Resource evaluation of typical energy plants and possible functional zone planning in China. Biomass Bioenergy 2008, 32, 283–288. [Google Scholar] [CrossRef]
- Zhan, Z.; Wang, Y.; Shockey, J.; Chen, Y.; Zhou, Z.; Yao, X.; Ren, H. Breeding status of tung tree (Vernicia sp.) in China, a multipurpose oilseed crop with industrial uses. Silvae Genet. 2012, 61, 265–270. [Google Scholar] [CrossRef]
- Li, Z.; Shi, K.; Zhang, F.; Zhang, L.; Long, H.; Zeng, Y.; Liu, Z.; Niu, G.; Tan, X. Growth, Physiological, and Biochemical Responses of Tung Tree (Vernicia fordii) Seedlings to Different Light Intensities. Hortscience 2019, 54, 1361–1369. [Google Scholar] [CrossRef]
- Lu, C.; Wang, G.; Zhang, Q.; Yang, X.; Yu, J.; Liu, T.; Petracchini, F.; Zhang, Z.; Sun, Y.; Jiang, D.; et al. Comparison of biorefinery characteristics: Photo-fermentation biohydrogen, dark fermentation biohydrogen, biomethane, and bioethanol production. Appl. Energy 2023, 347, 121463. [Google Scholar] [CrossRef]
- Lu, C.; Li, W.; Zhang, Q.; Liu, L.; Zhang, N.; Qu, B.; Yang, X.; Xu, R.; Chen, J.; Sun, Y. Enhancing photo-fermentation biohydrogen production by strengthening the beneficial metabolic products with catalysts. J. Clean. Prod. 2021, 317, 128437. [Google Scholar] [CrossRef]
- Ben Atitallah, I.; Antonopoulou, G.; Ntaikou, I.; Soto Beobide, A.; Dracopoulos, V.; Mechichi, T.; Lyberatos, G. A Comparative Study of Various Pretreatment Approaches for Bio-Ethanol Production from Willow Sawdust, Using Co-Cultures and Mono-Cultures of Different Yeast Strains. Molecules 2022, 27, 1344. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Y.-W.; Dai, Y.-H.; Song, C.; Zhu, Q.-L.; Qin, H.; Tan, F.-R.; Chen, H.-C.; Dai, L.-C.; Hu, G.-Q.; et al. Current status and future prospective of bio-ethanol industry in China. Renew. Sustain. Energy Rev. 2021, 145, 111079. [Google Scholar] [CrossRef]
- Johnston, D.B.; Nghiem, N.P. Mixed fermentation of corn and pretreated corn stover for fuel ethanol production. Cereal Chem. 2021, 98, 926–934. [Google Scholar] [CrossRef]
- Li, H.; Xiao, W.; Liu, J.; Ran, Q.; Wang, R.; Yu, C.; Zhang, X.; Song, H.; Jiang, Z. Combination strategy of laccase pretreatment and rhamnolipid addition enhance ethanol production in simultaneous saccharification and fermentation of corn stover. Bioresour. Technol. 2022, 345, 126414. [Google Scholar] [CrossRef]
- Tin Diep Trung, L.; Vi Phuong Nguyen, T.; My Thi Tra, N.; Kim, T.H.; Oh, K.K. Pretreatment of Corn Stover Using an Extremely Low-Liquid Ammonia (ELLA) Method for the Effective Utilization of Sugars in Simultaneous Saccharification and Fermentation (SSF) of Ethanol. Fermentation 2021, 7, 191. [Google Scholar] [CrossRef]
- Molaverdi, M.; Karimi, K.; Mirmohamadsadeghi, S.; Galbe, M. High efficient ethanol production from corn stover by modified mild alkaline pretreatment. Renew. Energy 2021, 170, 714–723. [Google Scholar] [CrossRef]
- Stenberg, K.; Bollok, M.; Reczey, K.; Galbe, M.; Zacchi, G. Effect of substrate and cellulase concentration on simultaneous saccharification and fermentation of steam-pretreated softwood for ethanol production. Biotechnol. Bioeng. 2000, 68, 204–210. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Y.; Du, M.; Zhang, J. Characterization of the volatiles and active components in ethanol extracts of fruits of Litsea cubeba (Lour.) by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O). J. Med. Plants Res. 2011, 5, 3298–3303. [Google Scholar]
- Zhu, J.; Zhu, Y.; Jiang, F.; Xu, Y.; Ouyang, J.; Yu, S. An integrated process to produce ethanol, vanillin, and xylooligosaccharides from Camellia oleifera shell. Carbohydr. Res. 2013, 382, 52–57. [Google Scholar] [CrossRef]
- Yan, C.; Liu, Y.; Cao, L.; Xia, M.; Zhang, Q.; Li, C.; Ruan, R. Oligosaccharide preparation from microwave-ethanol pretreated Camellia oleifera seed shell by enzymolysis of Agrocybe aegerita. Ind. Crops Prod. 2021, 161, 113155. [Google Scholar] [CrossRef]
- Liu, H.; Ru, G.; Zhang, Z.; Li, Y.; Xia, C.; Lu, C.; Zhang, Q. Experimental study on optimization of initial pH for photo-fermentation bio-hydrogen under different enzymatic hydrolysis of chlorella vulgaris. Bioresour. Technol. 2021, 338, 125571. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Ge, X.; Wang, Y.; Zhou, X.; You, X.; Liu, H.; Zhang, Q. Bio-hydrogen production from apple waste by photosynthetic bacteria HAU-M1. Int. J. Hydrog. Energy 2016, 41, 13399–13407. [Google Scholar] [CrossRef]
- Xiang, G.; Zhang, Q.; Li, Y.; Zhang, X.; Liu, H.; Lu, C.; Zhang, H. Enhancement on photobiological hydrogen production from corn stalk via reducing hydrogen pressure in bioreactors by way of phased decompression schemes. Bioresour. Technol. 2023, 385, 129377. [Google Scholar] [CrossRef]
- He, D.P.; Chen, X.L.; Lu, M.S.; Shi, S.; Cao, L.M.; Yu, H.T.; Lin, H.; Jia, X.W.; Han, L.J.; Xiao, W.H. High-solids saccharification and fermentation of ball-milled corn stover enabling high titer bioethanol production. Renew. Energy 2023, 202, 336–346. [Google Scholar] [CrossRef]
- Liu, C.-G.; Xiao, Y.; Xia, X.-X.; Zhao, X.-Q.; Peng, L.; Srinophakun, P.; Bai, F.-W. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Song, Y.; Miu, X.; Zhang, Q.; Qu, J.; Sun, Y. Enhanced enzymatic saccharification and ethanol production of corn stover via pretreatment with urea and steam explosion. Bioresour. Technol. 2023, 376, 128856. [Google Scholar] [CrossRef] [PubMed]
- Bhuyar, P.; Shen, M.-Y.; Trejo, M.; Unpaprom, Y.; Ramaraj, R. Improvement of fermentable sugar for enhanced bioethanol production from Amorphophallus spp. tuber obtained from northern Thailand. Environ. Dev. Sustain. 2022, 24, 8351–8362. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The Role of Yeasts and Lactic Acid Bacteria on the Metabolism of Organic Acids during Winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef]
- Shuang, X.; Yaping, W.; Xuewu, G.; Zhiyang, W.; Zhongguan, S.; Dongguang, X. Effects of fermentation conditions on ethanol fermentation and ester production of five ester-producing yeasts. China Brew. 2018, 37, 24–28. [Google Scholar]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Plotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. TrAC Trends Anal. Chem. 2019, 120, 115630. [Google Scholar] [CrossRef]
- Scutarasu, E.C.; Teliban, I.V.; Zamfir, C.I.; Luchian, C.E.; Colibaba, L.C.; Niculaua, M.; Cotea, V.V. Effect of Different Winemaking Conditions on Organic Acids Compounds of White Wines. Foods 2021, 10, 2569. [Google Scholar] [CrossRef]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Rozès, N.; Mas, A.; Guillamòn, J.M. Effect of organic acids and nitrogen source on alcoholic fermentation:: Study of their buffering capacity. J. Agric. Food Chem. 2003, 51, 916–922. [Google Scholar] [CrossRef]
- Liu, C.-G.; Xue, C.; Lin, Y.-H.; Bai, F.-W. Redox potential control and applications in microaerobic and anaerobic fermentations. Biotechnol. Adv. 2013, 31, 257–265. [Google Scholar] [CrossRef]
- Farina, L.; Medina, K.; Urruty, M.; Boido, E.; Dellacassa, E.; Carrau, F. Redox effect on volatile compound formation in wine during fermentation by Saccharomyces cerevisiae. Food Chem. 2012, 134, 933–939. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chien, W.-S.; Duan, K.-J. Correlations between reduction–oxidation potential profiles and growth patterns of Saccharomyces cerevisiae during very-high-gravity fermentation. Process Biochem. 2010, 45, 765–770. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Xie, G.; Yang, Y.; Liu, B.; Ren, Z.J.; Xing, D. Electro-fermentation enhances H2 and ethanol co-production by regulating electron transfer and substrate transmembrane transport. Chem. Eng. J. 2022, 429, 132223. [Google Scholar] [CrossRef]
- Vassilev, I.; Averesch, N.J.H.; Ledezma, P.; Kokko, M. Anodic electro-fermentation: Empowering anaerobic production processes via anodic respiration. Biotechnol. Adv. 2021, 48, 107728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Meng, J.; Sun, K.; Yan, H. A neutral red mediated electro-fermentation system of Clostridium beijerinckii for effective co-production of butanol and hydrogen. Bioresour. Technol. 2021, 332, 125097. [Google Scholar] [CrossRef] [PubMed]
- Vinayaka, B.S.; Nisha, S.; Manasa, B.; Manasa, N.; Mascarenhas, L.N.; Goveas, L.C.; Vaman Rao, C.; Ujwal, P.; Sandesh, K.; Aparna, A. Acid hydrolysis optimization of cocoa pod shell using response surface methodology approach toward ethanol production. Agric. Nat. Resour. 2018, 52, 581–587. [Google Scholar] [CrossRef]
- Muktham, R.; Ball, A.S.; Bhargava, S.K.; Bankupalli, S. Bioethanol production from non-edible de-oiled Pongamia pinnata seed residue-optimization of acid hydrolysis followed by fermentation. Ind. Crops Prod. 2016, 94, 490–497. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.R.; Powell, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. Fems Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef] [PubMed]
- Waseem, W.; Noor, R.S.; Umair, M. The anaerobic transformation of agricultural waste for bioethanol production. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; de Souza Vandenberghe, L.P.; Soccol, C.R. A biorefinery approach for pectin extraction and second-generation bioethanol production from cocoa pod husk. Bioresour. Technol. 2022, 346, 126635. [Google Scholar] [CrossRef]
- Murata, Y.; Nwuche, C.O.; Nweze, J.E.; Ndubuisi, I.A.; Ogbonna, J.C. Potentials of multi-stress tolerant yeasts, Saccharomyces cerevisiae and Pichia kudriavzevii for fuel ethanol production from industrial cassava wastes. Process Biochem. 2021, 111, 305–314. [Google Scholar] [CrossRef]
- Lu, C.; Jiang, D.; Jing, Y.; Zhang, Z.; Liang, X.; Yue, J.; Li, Y.; Zhang, H.; Zhang, Y.; Wang, K.; et al. Enhancing photo-fermentation biohydrogen production from corn stalk by iron ion. Bioresour. Technol. 2022, 345, 126457. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, I.; Boskou, D.; Kanellaki, M.; Koutinas, A.A. Low-temperature alcoholic fermentation by delignified cellulosic material supported cells of kefir yeast. J. Agric. Food Chem. 1999, 47, 4474–4477. [Google Scholar] [CrossRef] [PubMed]
- Eardley, J.; Timson, D.J. Yeast Cellular Stress: Impacts on Bioethanol Production. Fermentation 2020, 6, 109. [Google Scholar] [CrossRef]
- Liu, X.; Jia, B.; Sun, X.; Ai, J.; Wang, L.; Wang, C.; Zhao, F.; Zhan, J.; Huang, W. Effect of Initial PH on Growth Characteristics and Fermentation Properties of Saccharomyces cerevisiae. J. Food Sci. 2015, 80, M800–M808. [Google Scholar] [CrossRef]
- Liu, R.; Shen, F. Impacts of main factors on bioethanol fermentation from stalk juice of sweet sorghum by immobilized Saccharomyces cerevisiae (CICC 1308). Bioresour. Technol. 2008, 99, 847–854. [Google Scholar] [CrossRef]
- Srinivas, D.; Jagannadha Rao, K.; Theodore, K.; Panda, T. Direct conversion of cellulosic material to ethanol by the intergeneric fusant Trichoderma reesei QM 9414/Saccharomyces cerevisiae NCIM 3288. Enzym. Microb. Technol. 1995, 17, 418–423. [Google Scholar] [CrossRef]
- Ratnam, B.V.V.; Rao, S.S.; Rao, M.D.; Rao, M.N.; Ayyanna, C. Optimization of medium constituents and fermentation conditions for the production of ethanol from palmyra jaggery using response surface methodology. World J. Microbiol. Biotechnol. 2005, 21, 399–404. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, H.; He, C.; Zhang, Q. Potential use and the energy conversion efficiency analysis of fermentation effluents from photo and dark fermentative bio-hydrogen production. Bioresour. Technol. 2017, 245, 884–889. [Google Scholar] [CrossRef]


| Pm (g/L) | Rm (g/L/h) | λ (h) | R2 | |
|---|---|---|---|---|
| Substrate mass concentration | ||||
| 5 g/L | 0.20 | 0.02 | 0.01 | 0.98 |
| 50 g/L | 1.42 | 0.12 | 0.22 | 0.98 |
| 100 g/L | 4.30 | 0.35 | 0.22 | 0.98 |
| 150 g/L | 5.02 | 0.43 | 0.50 | 0.97 |
| 200 g/L | 5.91 | 0.64 | 1.63 | 0.99 |
| Initial pH value | ||||
| pH = 4 | 3.87 | 0.36 | 0.24 | 0.98 |
| pH = 5 | 4.54 | 0.39 | 0.21 | 0.98 |
| pH = 6 | 4.78 | 0.54 | 0.74 | 0.98 |
| pH = 7 | 5.11 | 0.50 | 0.85 | 0.99 |
| pH = 8 | 4.43 | 0.74 | 1.17 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Lu, C.; Liang, X.; Dai, K.; Sun, P.; Li, H.; Zheng, T.; Hu, J.; Wang, D.; Liu, J.; et al. Strategies for the Biotransformation of Tung Leaves in Bioethanol Fermentation. Fermentation 2023, 9, 986. https://doi.org/10.3390/fermentation9110986
Wang G, Lu C, Liang X, Dai K, Sun P, Li H, Zheng T, Hu J, Wang D, Liu J, et al. Strategies for the Biotransformation of Tung Leaves in Bioethanol Fermentation. Fermentation. 2023; 9(11):986. https://doi.org/10.3390/fermentation9110986
Chicago/Turabian StyleWang, Guangtao, Chaoyang Lu, Xiaoyu Liang, Kangshuai Dai, Peng Sun, Hui Li, Tianyu Zheng, Jianwei Hu, Decheng Wang, Jialu Liu, and et al. 2023. "Strategies for the Biotransformation of Tung Leaves in Bioethanol Fermentation" Fermentation 9, no. 11: 986. https://doi.org/10.3390/fermentation9110986
APA StyleWang, G., Lu, C., Liang, X., Dai, K., Sun, P., Li, H., Zheng, T., Hu, J., Wang, D., Liu, J., & Wang, G. (2023). Strategies for the Biotransformation of Tung Leaves in Bioethanol Fermentation. Fermentation, 9(11), 986. https://doi.org/10.3390/fermentation9110986






