Purified Acidic Sophorolipid Biosurfactants in Skincare Applications: An Assessment of Cytotoxic Effects in Comparison with Synthetic Surfactants Using a 3D In Vitro Human Skin Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Characterisation of Acidic SL
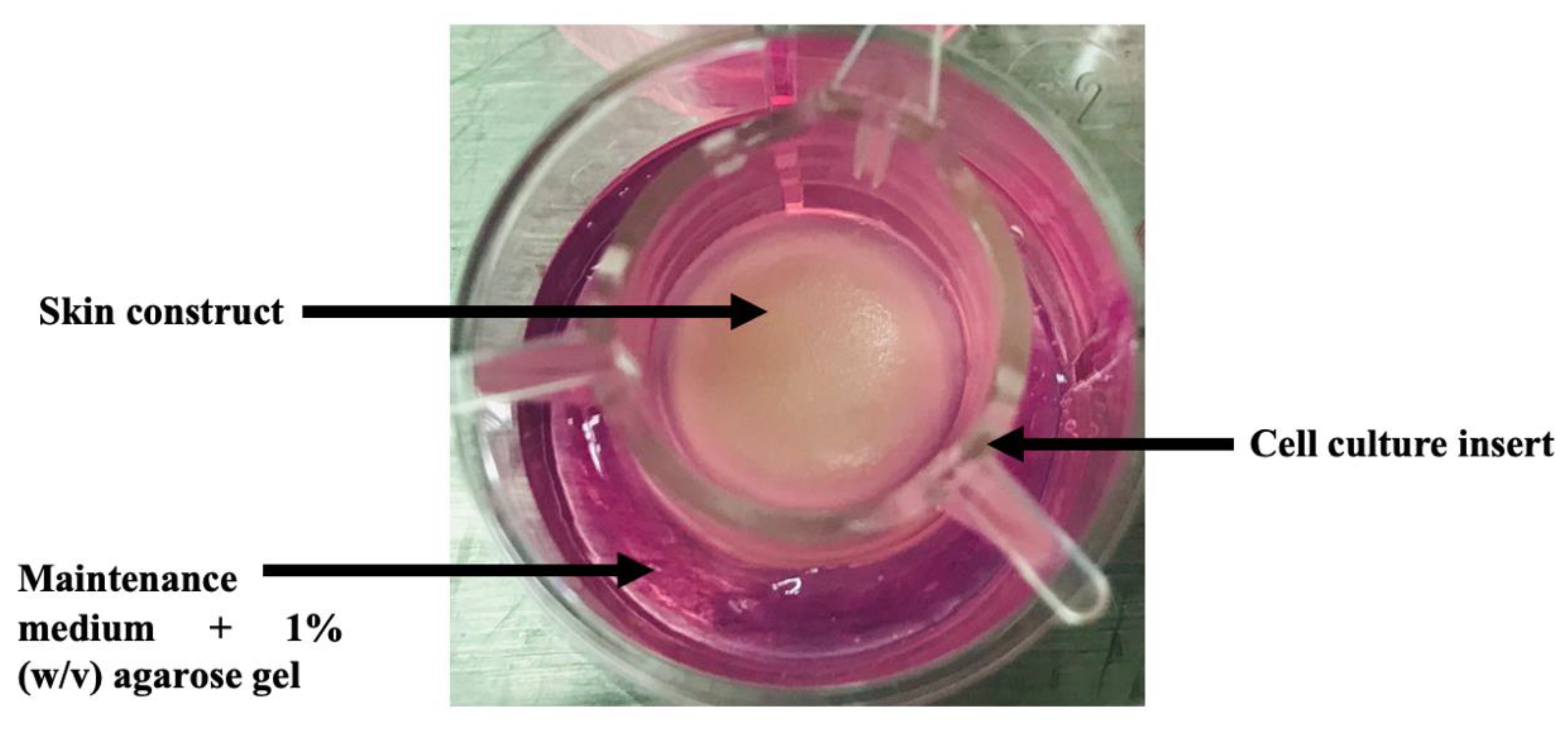
2.2. 3D In Vitro Skin Model Preparation
2.3. Colonisation of Skin Constructs with Staphylococcus Epidermidis
2.4. Surfactant Treatments
2.5. Assessment of Bacterial Viability
2.6. Formalin Fixation, Tissue Processing, and Sectioning
2.7. Haematoxylin and Eosin (H & E) Staining
2.8. Immunohistochemistry (IHC)
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. RNA Extraction
2.11. cDNA Synthesis
2.12. Quantitative PCR (qPCR)
2.13. Statistical Analysis
3. Results
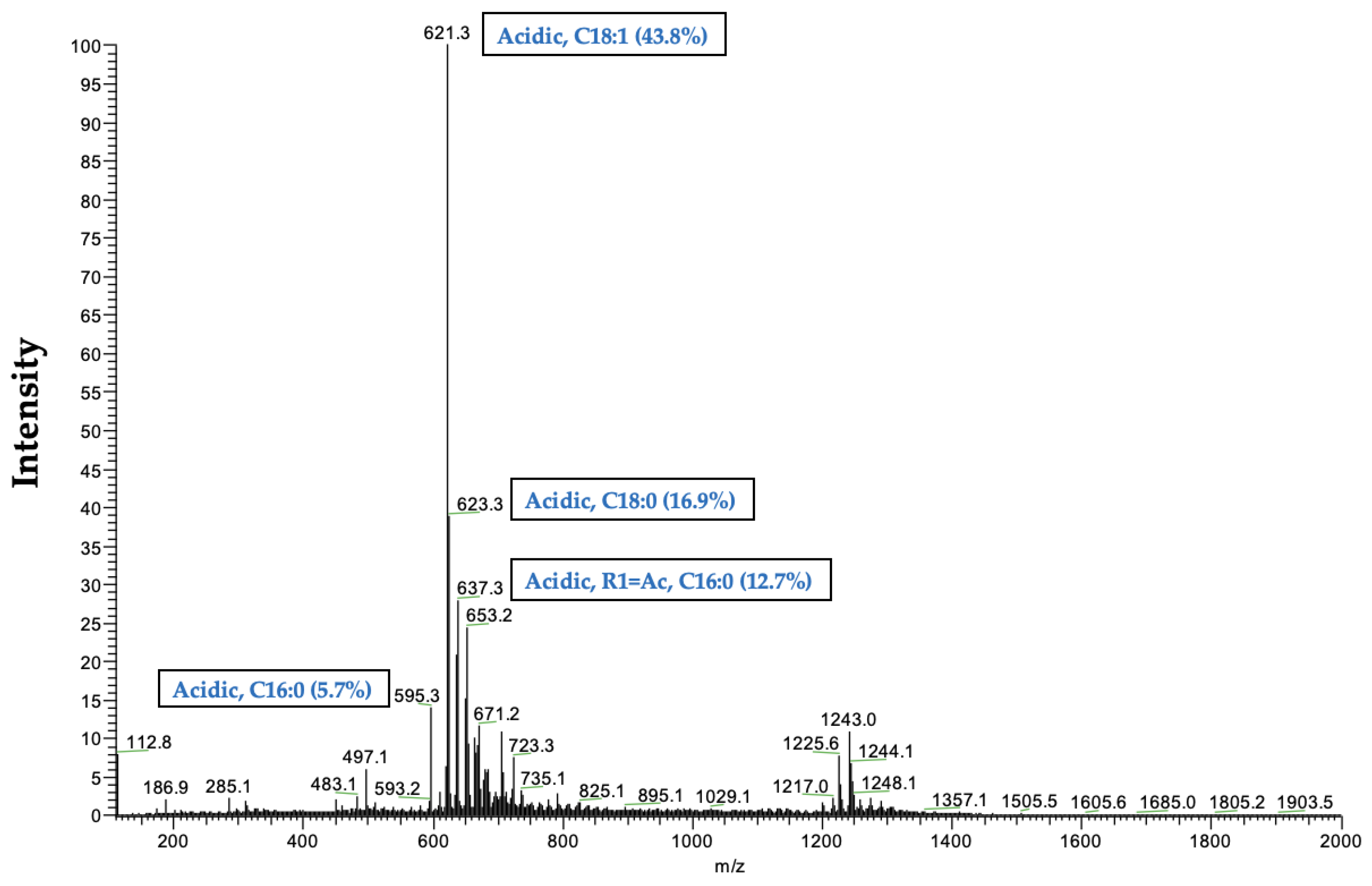
3.1. Chemical Characterisation of Acidic SL
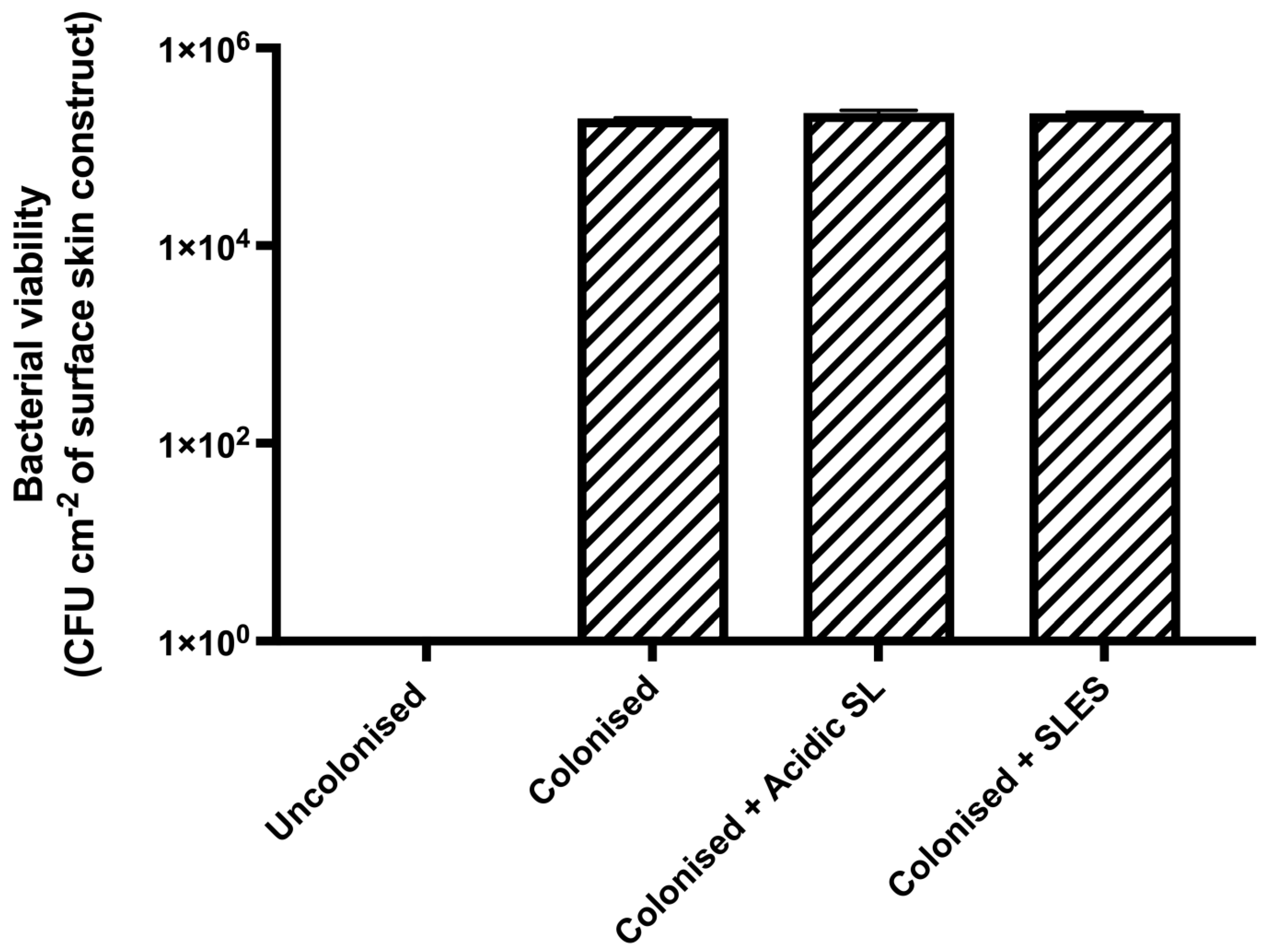
3.2. Effects of Acidic SL and SLES on the Viability of S. epidermidis Colonised on Skin Constructs
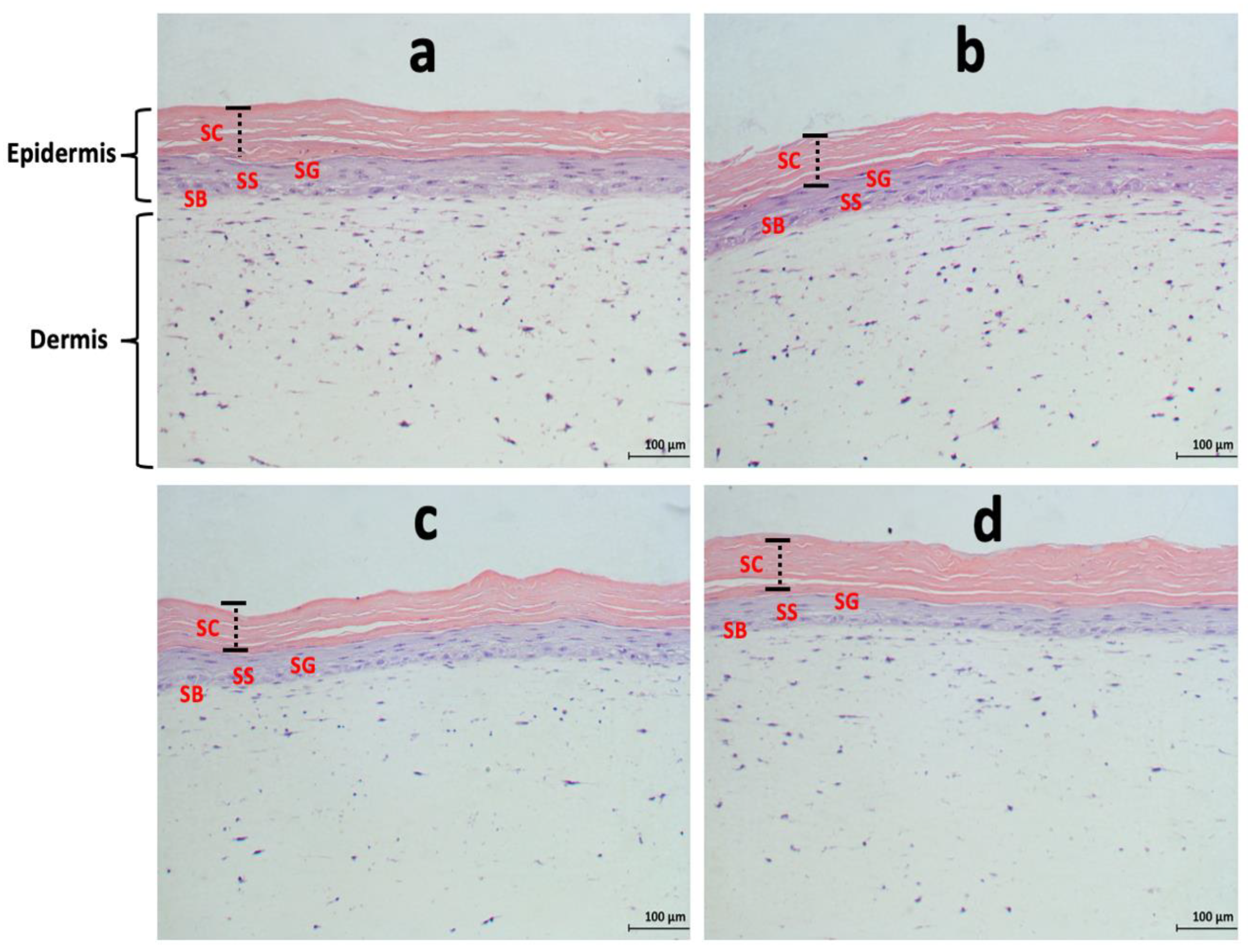
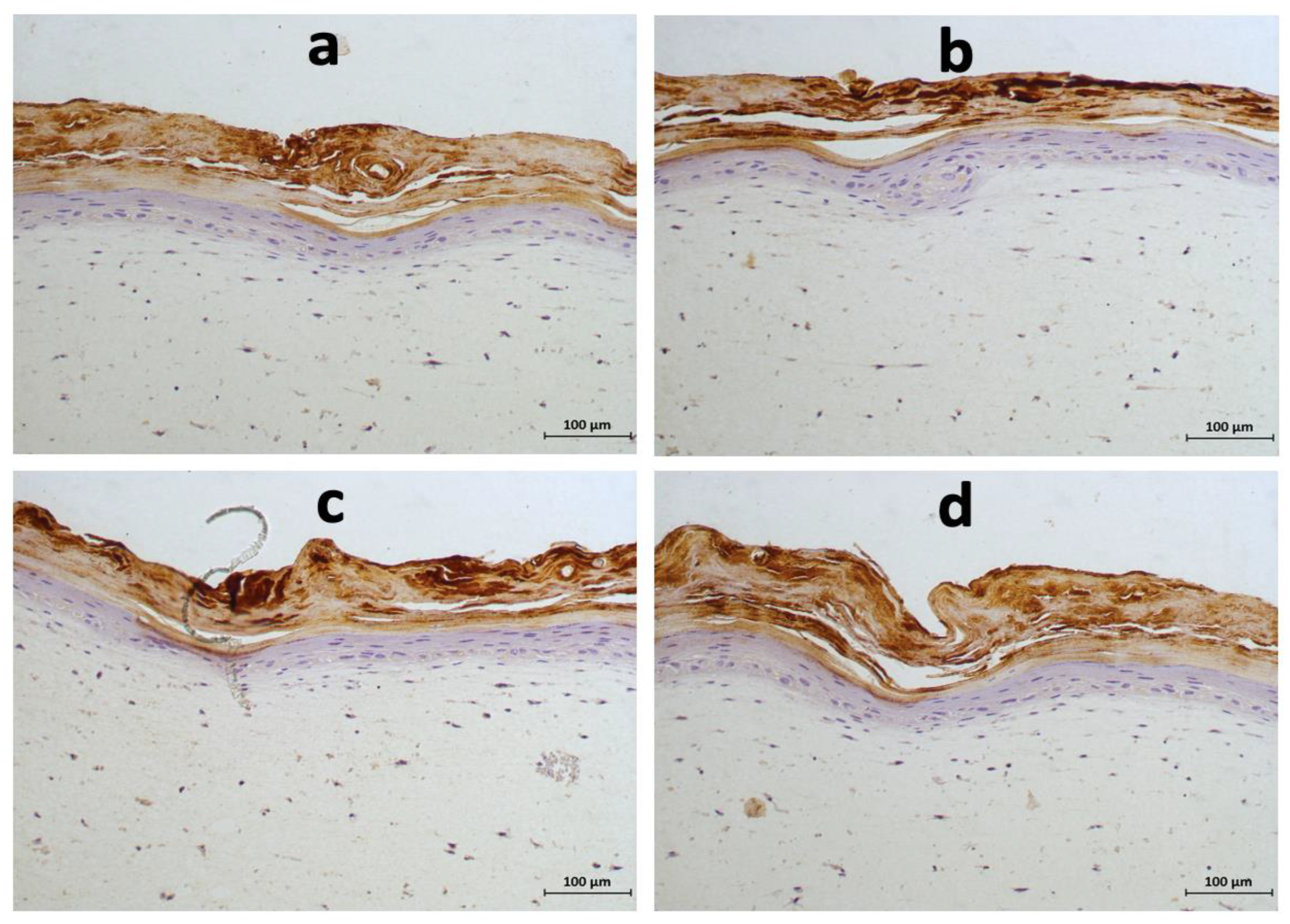
3.3. Effects of Acidic SL and SLES on the Morphology of Pre-Colonised Skin Constructs
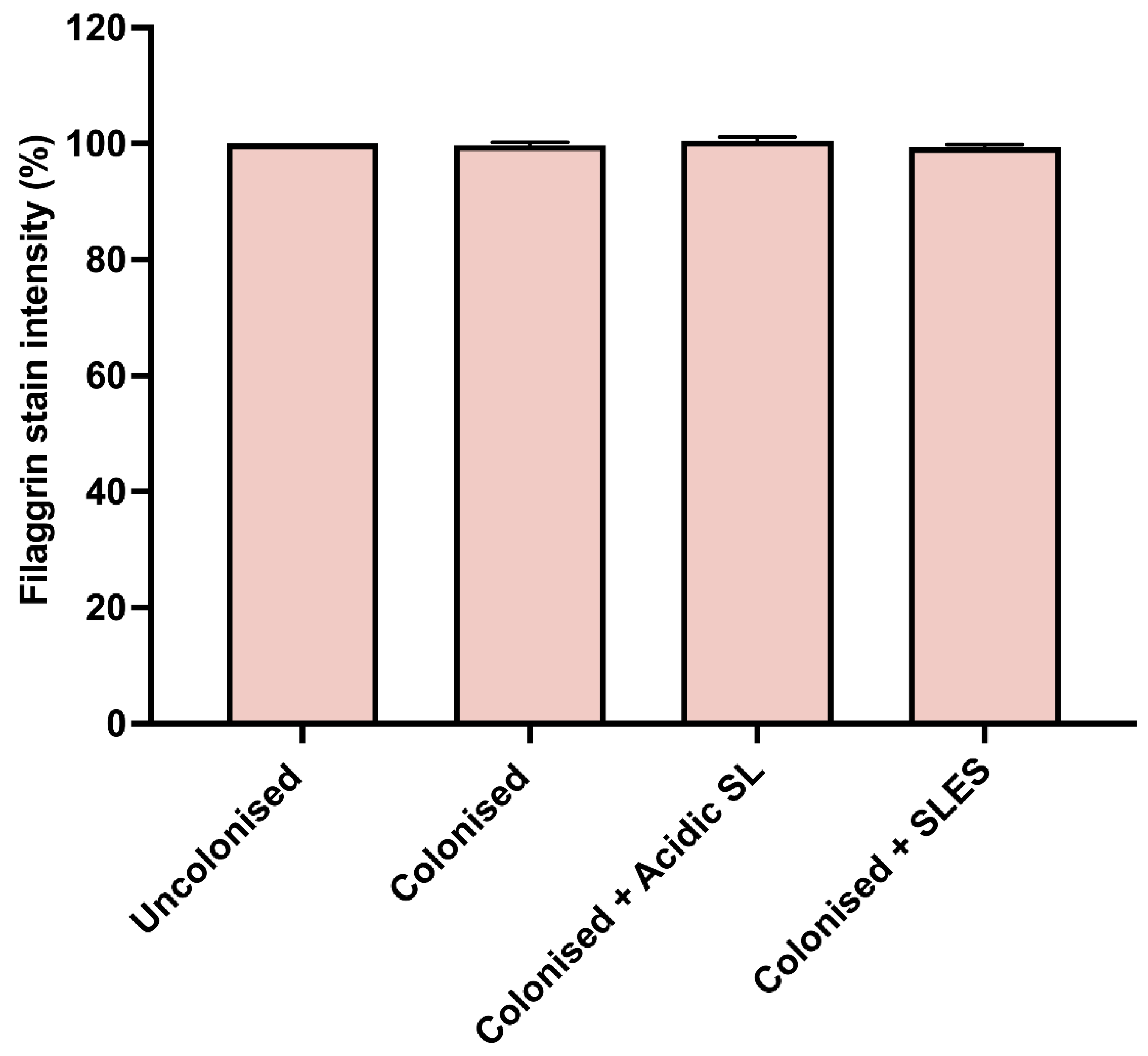
3.4. Effects of Acidic SL and SLES on Filaggrin Expression in the Epidermis of Skin Constructs
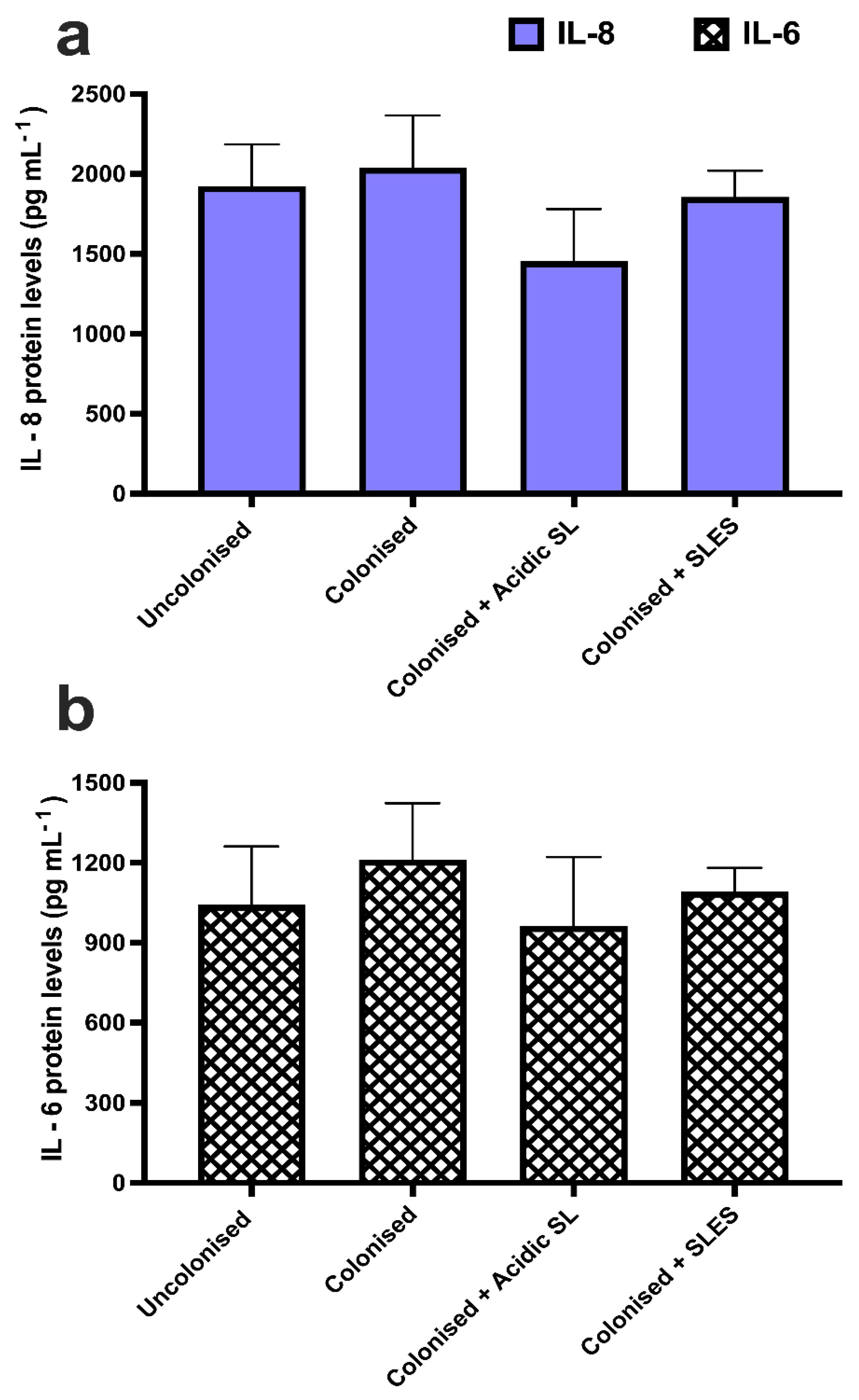
3.5. Effects of Acidic SL and SLES on Pro-Inflammatory Cytokine Production in Skin Constructs
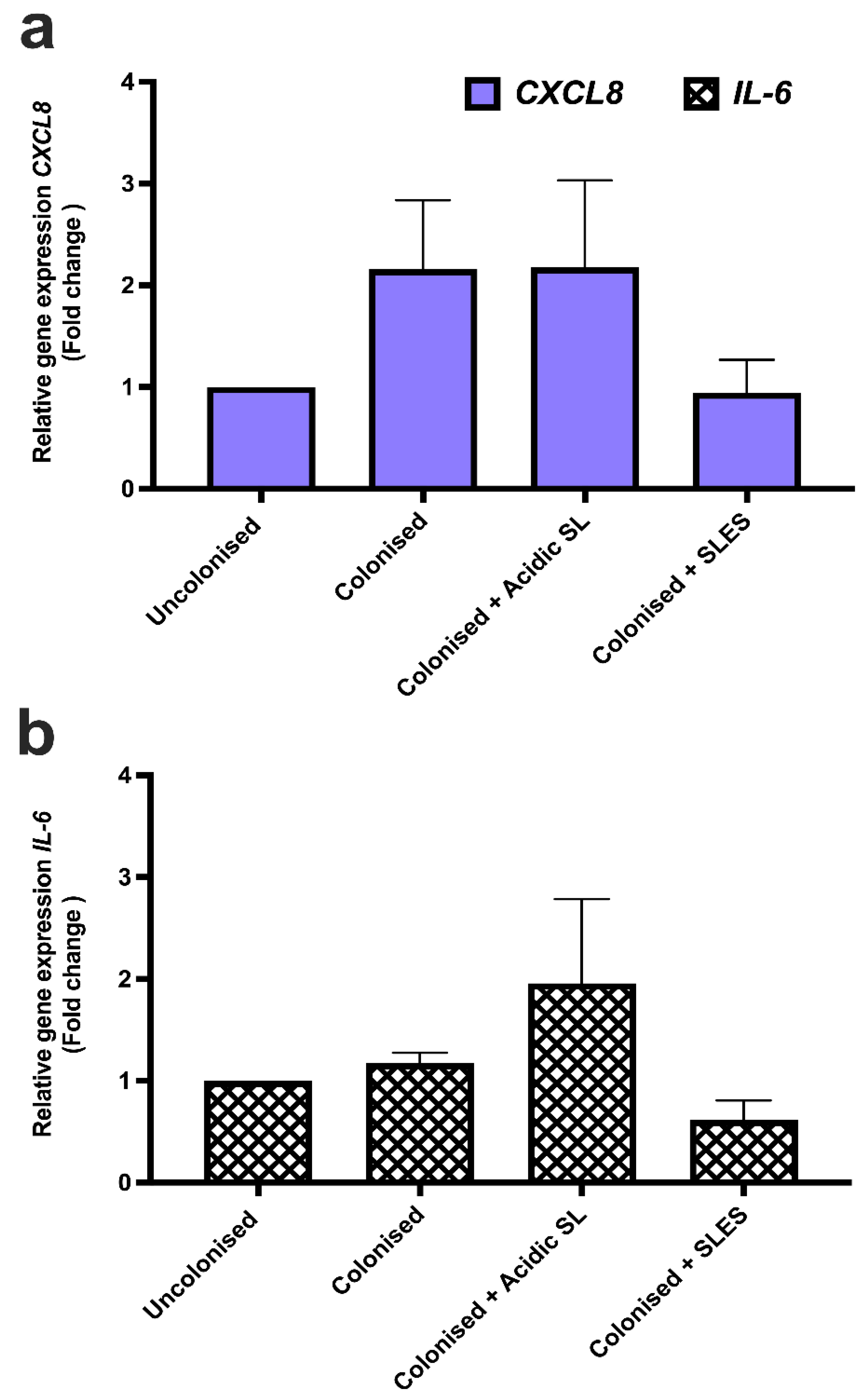
3.6. Effects of Acidic SL and SLES on Pro-Inflammatory Cytokine Gene Expression in LabskinTM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial Biosurfactant as an Alternate to Chemical Surfactants for Application in Cosmetics Industries in Personal and Skin Care Products: A Critical Review. Biomed Res. Int. 2023, 2023, 2375223. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Vecino, X.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Novel cosmetic formulations containing a biosurfactant from Lactobacillus paracasei. Colloids Surf. B Biointerfaces 2017, 155, 522–529. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and Topical Probiotics and Postbiotics in Skincare and Dermatological Therapy: A Concise Review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Glycolipid Biosurfactants in Skincare Applications: Challenges and Recommendations for Future Exploitation. Molecules 2023, 28, 4463. [Google Scholar] [CrossRef]
- Ahmadi-Ashtiani, H.R.; Baldisserotto, A.; Cesa, E.; Manfredini, S.; Zadeh, H.S.; Gorab, M.G.; Khanahmadi, M.; Zakizadeh, S.; Buso, P.; Vertuani, S. Microbial biosurfactants as key multifunctional ingredients for sustainable cosmetics. Cosmetics 2020, 7, 46. [Google Scholar] [CrossRef]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Characterisation of cytotoxicity and immunomodulatory effects of glycolipid biosurfactants on human keratinocytes. Appl. Microbiol. Biotechnol. 2022, 107, 137–152. [Google Scholar] [CrossRef]
- Maeng, Y.; Kim, K.T.; Zhou, X.; Jin, L.; Kim, K.S.; Kim, Y.H.; Lee, S.; Park, J.H.; Chen, X.; Kong, M.; et al. A novel microbial technique for producing high-quality sophorolipids from horse oil suitable for cosmetic applications. Microb. Biotechnol. 2018, 11, 917–929. [Google Scholar] [CrossRef]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef]
- Shu, Q.; Lou, H.; Wei, T.; Liu, X.; Chen, Q. Contributions of Glycolipid Biosurfactants and Glycolipid-Modified Materials to Antimicrobial Strategy: A Review. Pharmaceutics 2021, 13, 227. [Google Scholar] [CrossRef]
- Cho, W.Y.; Ng, J.F.; Yap, W.H.; Goh, B.H. Sophorolipids—Bio-Based Antimicrobial Formulating Agents for Applications in Food and Health. Molecules 2022, 27, 5556. [Google Scholar] [CrossRef] [PubMed]
- Lydon, H.L.; Baccile, N.; Callaghan, B.; Marchant, R.; Mitchell, C.A.; Banat, I.M. Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing. Antimicrob. Agents Chemother. 2017, 61, e02547-16. [Google Scholar] [CrossRef]
- Dardouri, M.; Mendes, R.M.; Frenzel, J.; Costa, J.; Ribeiro, I.A.C. Seeking faster, alternative methods for glycolipid biosurfactant characterization and purification. Anal. Bioanal. Chem. 2021, 413, 4311–4320. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, A.; Yeung, P.; Agu, R.; Teimouri, A.; Yeung, P.; Agu, R. 2D vs. 3D Cell Culture Models for In Vitro Topical (Dermatological) Medication Testing. In Cell Culture; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910. [Google Scholar] [CrossRef]
- Holland, D.B.; Bojar, R.A.; Farrar, M.D.; Holland, K.T. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol. Lett. 2009, 290, 149–155. [Google Scholar] [CrossRef]
- Ali, N.; Hosseini, M.; Vainio, S.; Taïeb, A.; Cario-André, M.; Rezvani, H.R. Skin equivalents: Skin from reconstructions as models to study skin development and diseases. Br. J. Dermatol. 2015, 173, 391–403. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Donaldson, M.; Francese, S.; Clench, M.R. MALDI MSI analysis of lipid changes in living skin equivalents in response to emollient creams containing palmitoylethanolamide. Methods 2016, 104, 93–100. [Google Scholar] [CrossRef]
- Silva, R.J.; Tamburic, S. A State-of-the-Art Review on the Alternatives to Animal Testing for the Safety Assessment of Cosmetics. Cosmetics 2022, 9, 90. [Google Scholar] [CrossRef]
- Ma, X.; Wang, F.; Wang, B. Application of an in vitro reconstructed human skin on cosmetics in skin irritation tests. J. Cosmet. Dermatol. 2021, 20, 1933–1941. [Google Scholar] [CrossRef]
- Duffy, E.; De Guzman, K.; Wallace, R.; Murphy, R.; Morrin, A. Non-Invasive Assessment of Skin Barrier Properties: Investigating Emerging Tools for In Vitro and In Vivo Applications. Cosmetics 2017, 4, 44. [Google Scholar] [CrossRef]
- Rademacher, F.; Simanski, M.; Gläser, R.; Harder, J. Skin microbiota and human 3D skin models. Exp. Dermatol. 2018, 27, 489–494. [Google Scholar] [CrossRef]
- De Wever, B.; Petersohn, D.; Mewes, K.R. Overview of human three-dimensional (3D) skin models used for dermal toxicity assessment. Househ. Pers. Care Today 2013, 8, 18–23. [Google Scholar]
- Klicks, J.; von Molitor, E.; Ertongur-Fauth, T.; Rudolf, R.; Hafner, M. In vitro skin three-dimensional models and their applications. J. Cell. Biotechnol. 2017, 3, 21–39. [Google Scholar] [CrossRef]
- Bojar, R.A. Studying the Human Skin Microbiome Using 3D In Vitro Skin Models. Appl. Vitr. Toxicol. 2015, 1, 165–171. [Google Scholar] [CrossRef]
- Larson, P.; Chong, D.; Fleming, E.; Oh, J. Challenges Developing a Human Model System for Skin Microbiome Research HHS Public Access. J. Investig. Dermatol. 2021, 141, 228–231. [Google Scholar] [CrossRef]
- Lewis, E.E.L.; Barrett, M.R.T.; Freeman-Parry, L.; Bojar, R.A.; Clench, M.R. Examination of the skin barrier repair/wound healing process using a living skin equivalent model and matrix-assisted laser desorption-ionization-mass spectrometry imaging. Int. J. Cosmet. Sci. 2018, 40, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure. Pharmaceutics 2022, 14, 360. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Mõcsai, G.; Gáspár, K.; Nagy, G.; Irinyi, B.; Kapitány, A.; Bírõ, T.; Gyimesi, E.; Tõth, B.; Marõdi, L.; Szegedi, A. Severe skin inflammation and filaggrin mutation similarly alter the skin barrier in patients with atopic dermatitis. Br. J. Dermatol. 2014, 170, 617–624. [Google Scholar] [CrossRef][Green Version]
- Therkildsen, P.; Hædersdal, M.; Lock-Andersen, J.; Olivarius, F.D.F.; Poulsen, T.; Wulf, H.C. Epidermal thickness measured by light microscopy: A methodological study. Ski. Res. Technol. 1998, 4, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Maussion, G.; Thomas, R.A.; Demirova, I.; Gu, G.; Cai, E.; Chen, C.X.Q.; Abdian, N.; Strauss, T.J.P.; Kelaï, S.; Nauleau-Javaudin, A.; et al. Auto-qPCR; a python-based web app for automated and reproducible analysis of qPCR data. Sci. Rep. 2021, 11, 21293. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.M.; Horswill, A.R. Staphylococcus epidermidis-Skin friend or foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef] [PubMed]
- OECD Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. Available online: https://www.oecd-ilibrary.org/environment/test-no-439-in-vitro-skin-irritation-reconstructed-human-epidermis-test-method_9789264242845-en (accessed on 23 August 2022).
- Sleiman, J.N.; Kohlhoff, S.A.; Roblin, P.M.; Wallner, S.; Gross, R.; Hammerschlag, M.R.; Zenilman, M.E.; Bluth, M.H. Sophorolipids as Antibacterial Agents. Ann. Clin. Lab. 2009, 39, 60–63. [Google Scholar]
- Díaz De Rienzo, M.A.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Lydon, H.; Roelants, S.L.K.W.; Van Bogaert, I.N.A.; Marchant, R.; Banat, I.M.; Mitchell, C.A. Lactonic sophorolipids increase tumor burden in Apcmin+/− mice. PLoS ONE 2016, 11, e0156845. [Google Scholar] [CrossRef]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Bogaert, I.N.A. Van Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Badia, D.; Ratsep, P. Sophorolipids Having Enhanced Antibacterial Activity. Antimicrob. Agents Chemother. 2007, 51, 397. [Google Scholar] [CrossRef]
- Urzedo, C.A.; Freitas, Q.; Akemi, V.; Silveira, I.; Pedrine, M.A.; Celligoi, C. Adv Biotech & Micro Antimicrobial Applications of Sophorolipid from Candida bombicola: A Promising Alternative to Conventional Drugs. Adv. Biotechnol. Microbiol. 2018, 9, 555753. [Google Scholar]
- Shao, L.; Song, X.; Ma, X.; Li, H.; Qu, Y. Bioactivities of Sophorolipid with Different Structures Against Human Esophageal Cancer Cells. J. Surg. Res. 2012, 173, 286–291. [Google Scholar] [CrossRef]
- Boyce, J.A.; Finkelman, F.; Shearer, W.T.; Mcaleer, M.A.; Irvine, A.D. The multifunctional role of filaggrin in allergic skin disease. J. Allergy Clin. Immunol. 2013, 131, 280–291. [Google Scholar] [CrossRef]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. 2019, 124, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pendaries, V.; Malaisse, J.; Pellerin, L.; Le Lamer, M.; Nachat, R.; Kezic, S.; Schmitt, A.M.; Paul, C.; Poumay, Y.; Serre, G.; et al. Knockdown of Filaggrin in a Three-Dimensional Reconstructed Human Epidermis Impairs Keratinocyte Differentiation. J. Investig. Dermatol. 2014, 134, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Moosbrugger-Martinz, V.; Leprince, C.; Méchin, M.C.; Simon, M.; Blunder, S.; Gruber, R.; Dubrac, S. Revisiting the Roles of Filaggrin in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 5318. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Kezic, S.; Hellerup, B.; Denmark, A. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Friedlander, S.F.; Del Rosso, J.Q. Atopic Dermatitis and the Stratum Corneum: Part 1: The Role of Filaggrin in the Stratum Corneum Barrier and Atopic Skin. J. Clin. Aesthet. Dermatol. 2013, 6, 16. [Google Scholar] [PubMed]
- Tö Rmä, H.; Lindberg, M.; Berne, B. Skin Barrier Disruption by Sodium Lauryl Sulfate-Exposure Alters the Expressions of Involucrin, Transglutaminase 1, Profilaggrin, and Kallikreins during the Repair Phase in Human Skin In Vivo. J. Investig. Dermatol. 2008, 128, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 14, 360. [Google Scholar] [CrossRef] [PubMed]
| Probable Structure | Observed m/z | Peak Area | % Relative Abundance |
|---|---|---|---|
| % Abundance of Acidic SL congeners—96.41 | |||
| Acidic, C16:0 | 595.3 | 158,650,232.27 | 5.74 |
| Acidic, C18:2 | 619.2 | 70,540,287.99 | 2.55 |
| Acidic, C18:1 | 621.3 | 1,210,090,321.46 | 43.81 |
| Acidic, C18:0 | 623.3 | 465,744,019.84 | 16.86 |
| Acidic, R1 = Ac, C16:0 | 637.3 | 350,794,715.78 | 12.70 |
| Acidic, 1Ac, C18:0 | 665.5 | 37,939,611.09 | 1.37 |
| Acidic, R1 = Ac, C18:1 or Acidic, R2 = Ac, C18:1 | 663.3 | 102,670,879.63 | 3.72 |
| Acidic, R1 + R2 = Ac, C16:0 | 679.2 | 29,300,132.66 | 1.06 |
| Acidic, R1 + R2 = Ac, C18:2 | 703.1 | 17,417,772.37 | 0.63 |
| Acidic, R1 + R2 = Ac, C18:1 | 705.3 | 126,826,959.83 | 4.59 |
| Acidic, R1 + R2 = Ac, C18:0 | 707.3 | 57,366,159.94 | 2.08 |
| Acidic, R1 = Ac, C22:0 | 721.2 | 15,293,285.62 | 0.55 |
| Acidic, R1 + R2 = Ac, C24:0 | 791.3 | 20,615,084.25 | 0.75 |
| % Abundance of Lactonic SL congeners—3.59 | |||
| Lactonic, R1 + R2 = Ac, C18:2 or Lactonic, R1 + R2 = Ac, C18:2 | 685.1 | 69,269,763.09 | 2.51 |
| Lactonic, R1 + R2-Ac, C18:1 or Lactonic, R1 + R2-Ac, C18:1 or Lactonic, R1 + R2 = Ac, C18:1 | 687.1 | 29,782,951.00 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Purified Acidic Sophorolipid Biosurfactants in Skincare Applications: An Assessment of Cytotoxic Effects in Comparison with Synthetic Surfactants Using a 3D In Vitro Human Skin Model. Fermentation 2023, 9, 985. https://doi.org/10.3390/fermentation9110985
Adu SA, Twigg MS, Naughton PJ, Marchant R, Banat IM. Purified Acidic Sophorolipid Biosurfactants in Skincare Applications: An Assessment of Cytotoxic Effects in Comparison with Synthetic Surfactants Using a 3D In Vitro Human Skin Model. Fermentation. 2023; 9(11):985. https://doi.org/10.3390/fermentation9110985
Chicago/Turabian StyleAdu, Simms A., Matthew S. Twigg, Patrick J. Naughton, Roger Marchant, and Ibrahim M. Banat. 2023. "Purified Acidic Sophorolipid Biosurfactants in Skincare Applications: An Assessment of Cytotoxic Effects in Comparison with Synthetic Surfactants Using a 3D In Vitro Human Skin Model" Fermentation 9, no. 11: 985. https://doi.org/10.3390/fermentation9110985
APA StyleAdu, S. A., Twigg, M. S., Naughton, P. J., Marchant, R., & Banat, I. M. (2023). Purified Acidic Sophorolipid Biosurfactants in Skincare Applications: An Assessment of Cytotoxic Effects in Comparison with Synthetic Surfactants Using a 3D In Vitro Human Skin Model. Fermentation, 9(11), 985. https://doi.org/10.3390/fermentation9110985







