Metabolic Engineering of Saccharomyces cerevisiae for Conversion of Formate and Acetate into Free Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Plasmid Constructions
2.2. Fermentation Conditions
2.3. Fed-Batch Fermentation
2.4. Analytical Methods
2.5. Statistics
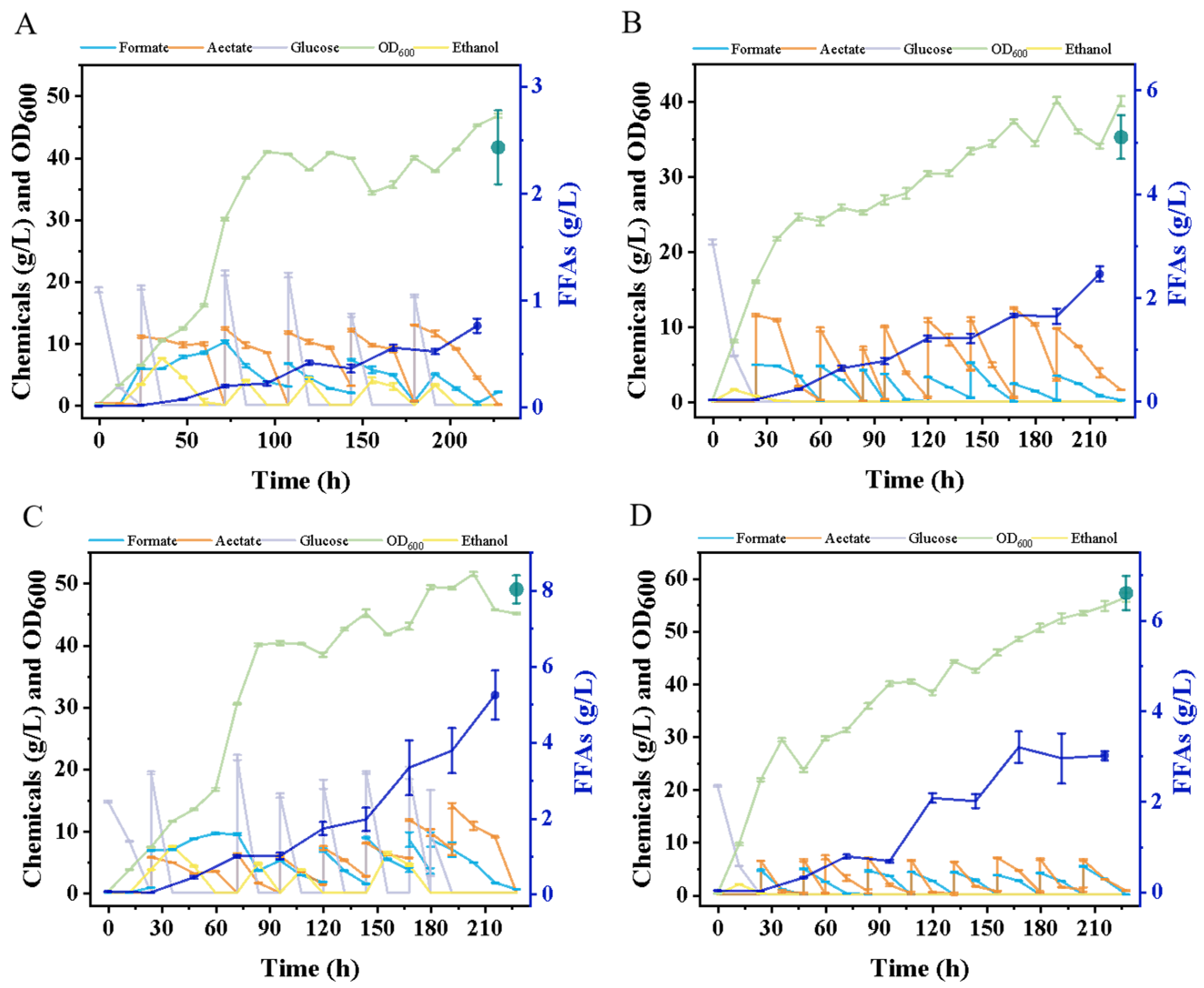
3. Results and Discussion
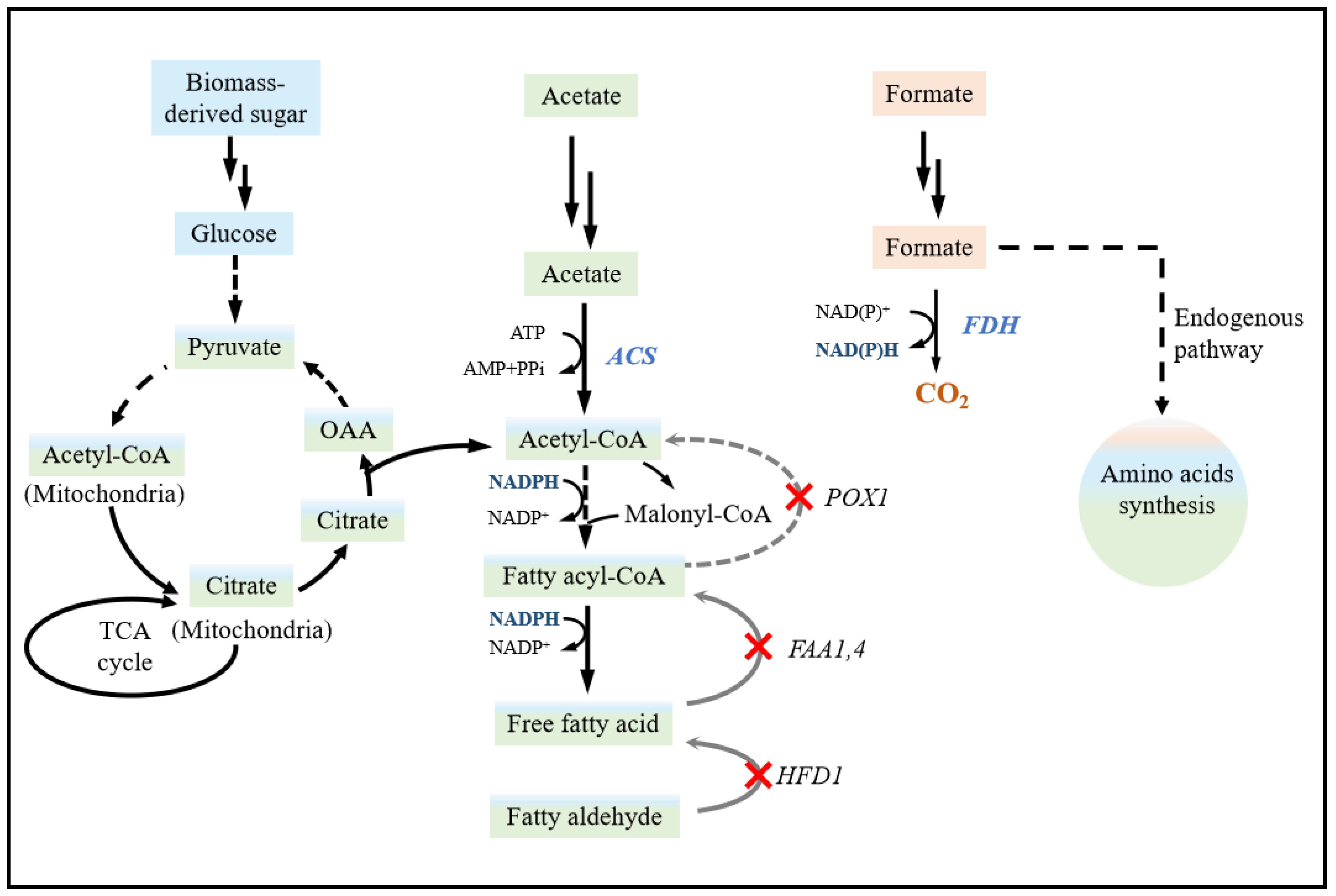
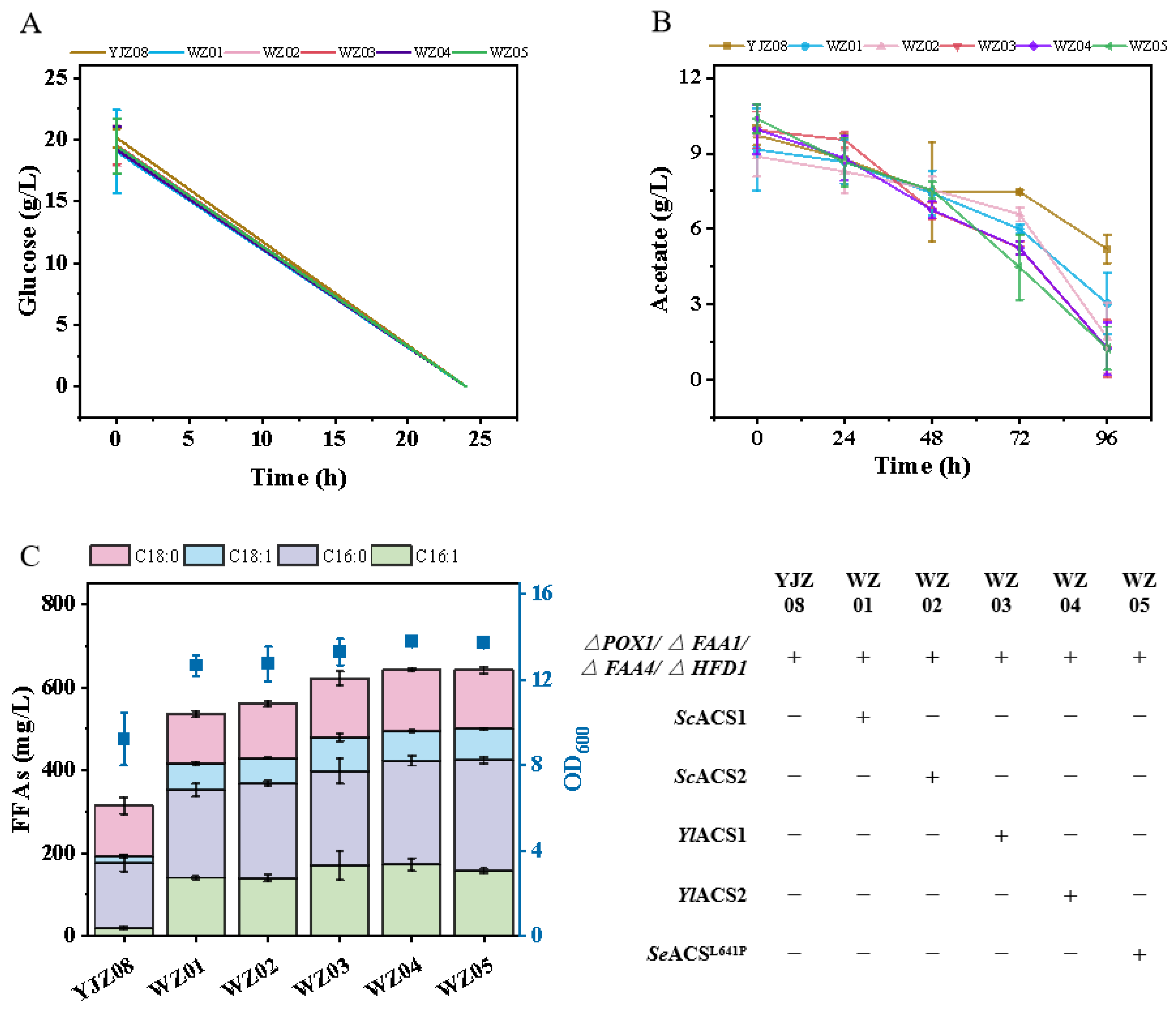
3.1. Metabolic Engineering Enables Acetate Conversion in Yeast
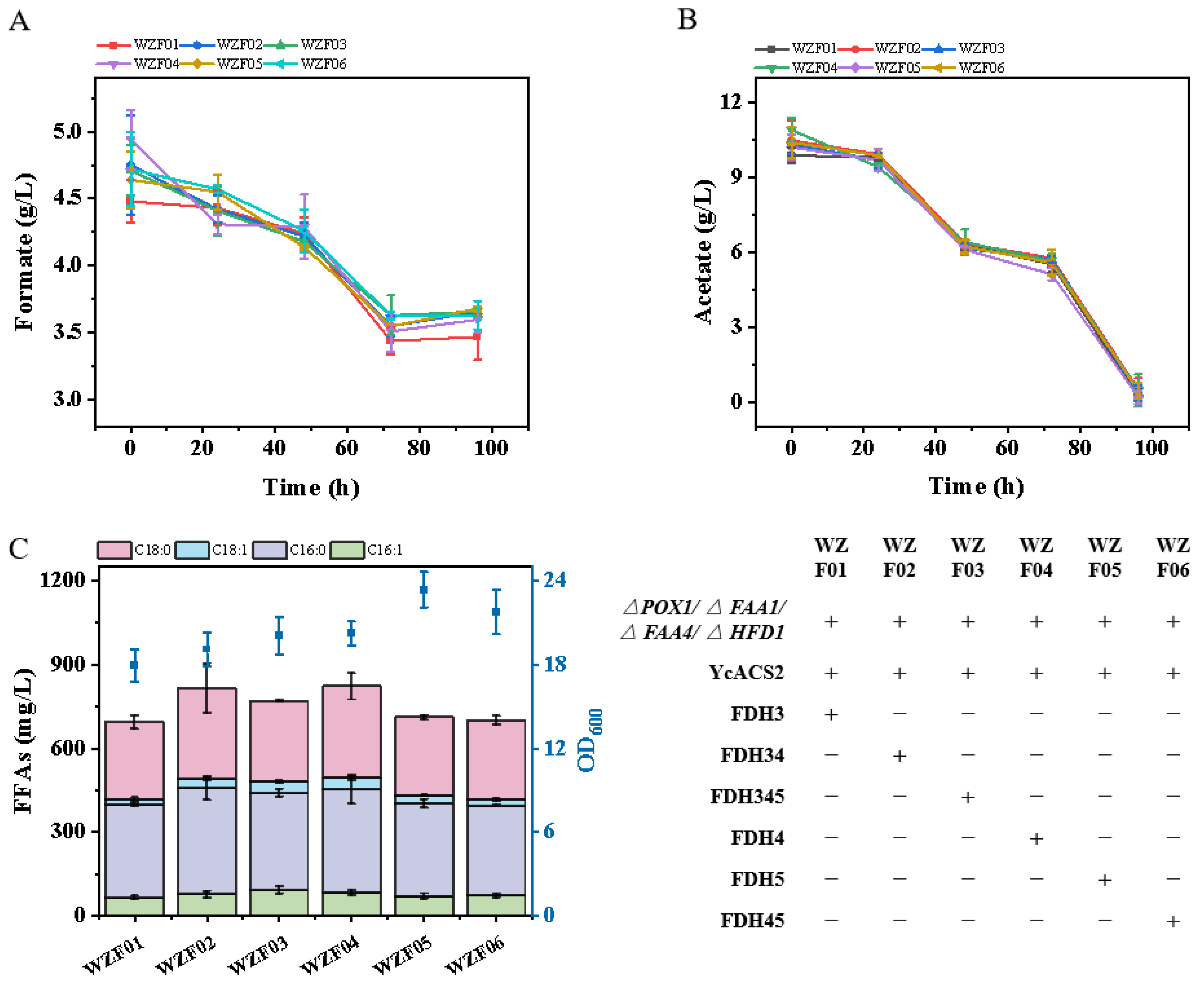
3.2. Introducing the Formate Pathway for Energy Supply: A Promising Approach
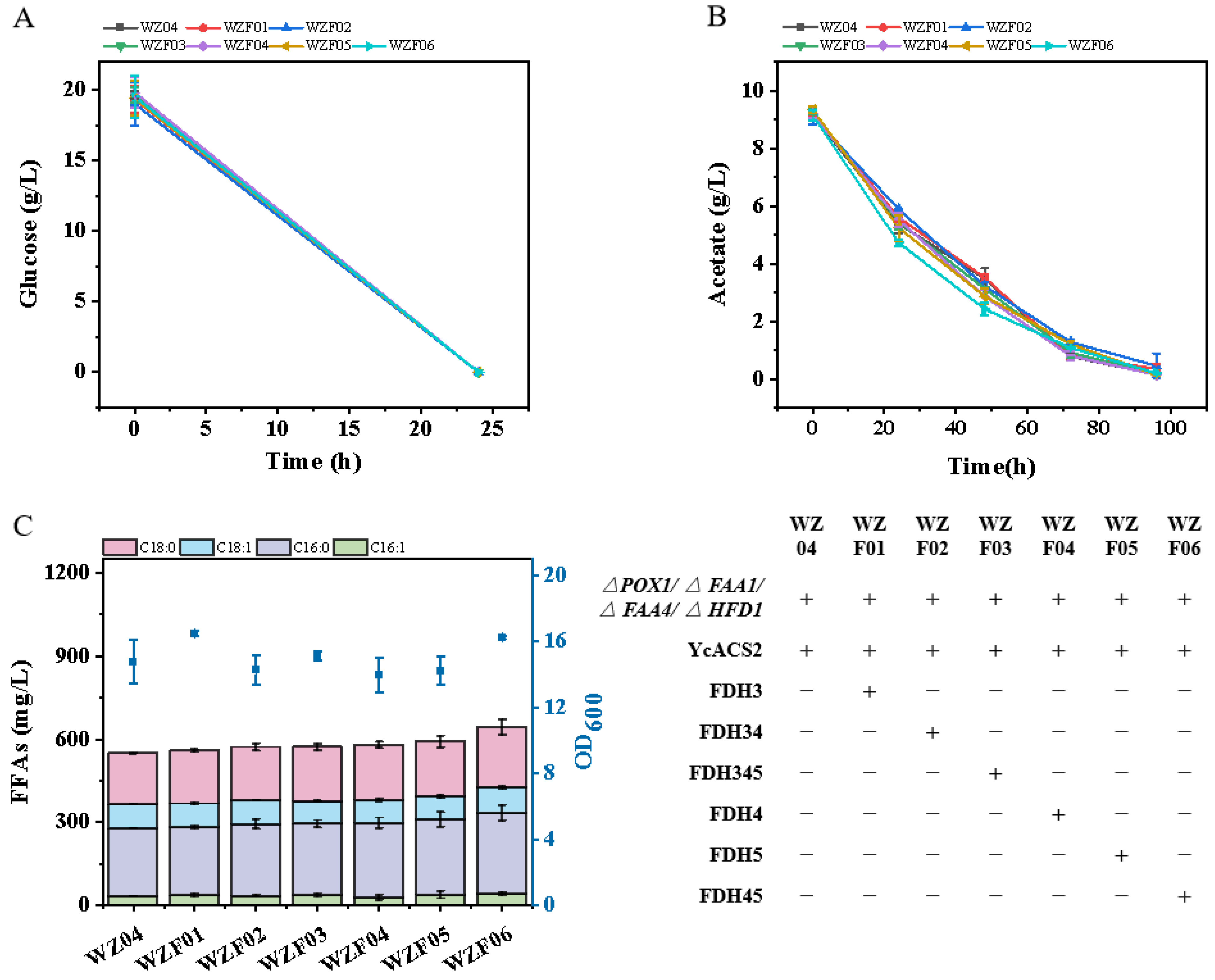
3.3. Scaling up Fermentation: Achievements in a 5 L Fermenter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, S.R.; Rorrer, N.A.; Uekert, T.; Avery, G.; Carpenter, A.C.; Beckham, G.T. Manufacturing Energy and Greenhouse Gas Emissions Associated with United States Consumption of Organic Petrochemicals. ACS Sustain. Chem. Eng. 2023, 11, 2198–2208. [Google Scholar] [CrossRef]
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: A systematic review of two decades of research from 1995 to 2017. Sci. Total Environ. 2019, 649, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morales, P.; Yin, K.; Landera, A.; Cort, J.R.; Young, R.P.; Kyle, J.E.; Bertrand, R.; Iavarone, A.T.; Acharya, S.; Cowan, A.; et al. Biosynthesis of polycyclopropanated high energy biofuels. Joule 2022, 6, 1590–1605. [Google Scholar] [CrossRef]
- Schuchmann, K.; Muller, V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science 2013, 342, 1382–1385. [Google Scholar] [CrossRef]
- Wei, D.; Sang, R.; Sponholz, P.; Junge, H.; Beller, M. Reversible hydrogenation of carbon dioxide to formic acid using a Mn-pincer complex in the presence of lysine. Nat. Energy 2022, 7, 438–447. [Google Scholar] [CrossRef]
- Moret, S.; Dyson, P.J.; Laurenczy, G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat. Commun. 2014, 5, 4017. [Google Scholar] [CrossRef]
- Lee, B.; Choe, C.; Kim, H.; Kim, A.; Shin, Y.U.; Haider, J.; Lim, H. Economic Parity Analysis of Green Methanol Synthesis Using Water Electrolysis Based on Renewable Energy. ACS Sustain. Chem. Eng. 2021, 9, 15807–15818. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sharma, T.; Nguyen, D.D.; Cheng, C.K.; Lam, S.S.; Xia, C.; Nadda, A.K. Integrated catalytic insights into methanol production: Sustainable framework for CO2 conversion. J. Environ. Manag. 2021, 289, 112468. [Google Scholar] [CrossRef]
- Kothandaraman, J.; Goeppert, A.; Czaun, M.; Olah, G.A.; Prakash, G.S. Conversion of CO2 from Air into Methanol Using a Polyamine and a Homogeneous Ruthenium Catalyst. J. Am. Chem. Soc. 2016, 138, 778–781. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, M.; Wu, L.; Guo, S.; Liu, X.; Zhao, J.; Xue, W.; Li, J.; Liu, C.; Li, X.; et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 2022, 5, 388–396. [Google Scholar] [CrossRef]
- Tian, W.; Li, N.; Chen, D.; Xu, Q.; Li, H.; Yan, C.; Lu, J. Vibration-driven Reduction of CO2 to Acetate with 100% Selectivity by SnS Nanobelt Piezocatalysts. Angew. Chem. Int. Ed. 2023, 62, e202306964. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Niu, Y.; Liu, X.; Xu, C.; Chen, C.; Meyer, T.J.; Chen, Z. Selective CO2 Photoreduction to Acetate at Asymmetric Ternary Bridging Sites. ACS Nano 2023, 17, 4922–4932. [Google Scholar] [CrossRef]
- Abdinejad, M.; Yuan, T.; Tang, K.; Duangdangchote, S.; Farzi, A.; van Montfort, H.P.I.; Li, M.; Middelkoop, J.; Wolff, M.; Seifitokaldani, A.; et al. Electroreduction of Carbon Dioxide to Acetate using Heterogenized Hydrophilic Manganese Porphyrins. Chem. Eur. J. 2023, 29, e202203977. [Google Scholar] [CrossRef] [PubMed]
- Boecker, S.; Espinel-Rios, S.; Bettenbrock, K.; Klamt, S. Enabling anaerobic growth of Escherichia coli on glycerol in defined minimal medium using acetate as redox sink. Metab. Eng. 2022, 73, 50–57. [Google Scholar] [CrossRef]
- Seong, W.; Han, G.H.; Lim, H.S.; Baek, J.I.; Kim, S.J.; Kim, D.; Kim, S.K.; Lee, H.; Kim, H.; Lee, S.G.; et al. Adaptive laboratory evolution of Escherichia coli lacking cellular byproduct formation for enhanced acetate utilization through compensatory ATP consumption. Metab. Eng. 2020, 62, 249–259. [Google Scholar] [CrossRef]
- Kleman, G.L.; Strohl, W.R. Acetate metabolism by Escherichia coli in high-cell-density fermentation. Appl. Environ. Microbiol. 1994, 60, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zeng, W.; Zhou, J.; Xu, S. O-Acetyl-L-homoserine production enhanced by pathway strengthening and acetate supplementation in Corynebacterium glutamicum. Biotechnol. Biofuels Bioprod. 2022, 15, 27. [Google Scholar] [CrossRef]
- Kim, Y.; Lama, S.; Agrawal, D.; Kumar, V.; Park, S. Acetate as a potential feedstock for the production of value-added chemicals: Metabolism and applications. Biotechnol. Adv. 2021, 49, 107736. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y.; Cheng, S.; Li, M.; Wang, L.; Cheng, M.; Li, F.; Cao, Y.; Song, H. Enhanced acetate utilization for value-added chemicals production in Yarrowia lipolytica by integration of metabolic engineering and microbial electrosynthesis. Biotechnol. Bioeng. 2023, 120, 3013–3024. [Google Scholar] [CrossRef]
- Bi, H.; Wang, K.; Xu, C.; Wang, M.; Chen, B.; Fang, Y.; Tan, X.; Zeng, J.; Tan, T. Biofuel synthesis from carbon dioxide via a bio-electrocatalysis system. Chem Catal. 2023, 3, 100557. [Google Scholar] [CrossRef]
- Claassens, N.J.; Sanchez-Andrea, I.; Sousa, D.Z.; Bar-Even, A. Towards sustainable feedstocks: A guide to electron donors for microbial carbon fixation. Curr. Opin. Biotechnol. 2018, 50, 195–205. [Google Scholar] [CrossRef]
- Yishai, O.; Lindner, S.N.; de la Cruz, J.G.; Tenenboim, H.; Bar-Even, A. The formate bio-economy. Curr. Opin. Chem. Biol. 2016, 35, 1–9. [Google Scholar] [CrossRef]
- Wang, K.; Da, Y.; Bi, H.; Liu, Y.; Chen, B.; Wang, M.; Liu, Z.; Nielsen, J.; Tan, T. A one-carbon chemicals conversion strategy to produce precursor of biofuels with Saccharomyces cerevisiae. Renew. Energy 2023, 208, 331–340. [Google Scholar] [CrossRef]
- Cotton, C.A.; Claassens, N.J.; Benito-Vaquerizo, S.; Bar-Even, A. Renewable methanol and formate as microbial feedstocks. Curr. Opin. Biotechnol. 2019, 62, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Yu, W.; Zhou, Y.J. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol. Nat. Metab. 2022, 4, 932–943. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, M.; Qin, N.; Nielsen, J.; Liu, Z. Expressing a cytosolic pyruvate dehydrogenase complex to increase free fatty acid production in Saccharomyces cerevisiae. Microb. Cell Fact. 2020, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Shin, K.S.; Jung, H.W.; Lee, Y.; Sathesh-Prabu, C.; Lee, S.K. Combinatorial Metabolic Engineering Strategies for the Enhanced Production of Free Fatty Acids in Escherichia coli. J. Agric. Food Chem. 2022, 70, 13913–13921. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Vora, H.; Khosla, C. Overproduction of free fatty acids in E. coli: Implications for biodiesel production. Metab. Eng. 2008, 10, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Fan, J.; Luo, S.; Chen, Y.; Wang, C.; Cao, Y.; Song, H. Genome-scale target identification in Escherichia coli for high-titer production of free fatty acids. Nat. Commun. 2021, 12, 4976. [Google Scholar] [CrossRef]
- Yu, T.; Liu, Q.; Wang, X.; Liu, X.; Chen, Y.; Nielsen, J. Metabolic reconfiguration enables synthetic reductive metabolism in yeast. Nat. Metab. 2022, 4, 1551–1559. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, Y.; Teixeira, P.G.; Pereira, R.; Chen, Y.; Siewers, V.; Nielsen, J. Multidimensional engineering of Saccharomyces cerevisiae for efficient synthesis of medium-chain fatty acids. Nat. Catal. 2020, 3, 64–74. [Google Scholar] [CrossRef]
- Qin, N.; Li, L.; Ji, X.; Pereira, R.; Chen, Y.; Yin, S.; Li, C.; Wan, X.; Qiu, D.; Jiang, J.; et al. Flux regulation through glycolysis and respiration is balanced by inositol pyrophosphates in yeast. Cell 2023, 186, 748–763.e15. [Google Scholar] [CrossRef]
- Zhai, X.; Gao, J.; Li, Y.; Grininger, M.; Zhou, Y.J. Peroxisomal metabolic coupling improves fatty alcohol production from sole methanol in yeast. Proc. Natl. Acad. Sci. USA 2023, 120, e2220816120. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, X.; Yu, W.; Feng, D.; Shah, A.A.; Gao, J.; Zhou, Y.J. Production of free fatty acids from various carbon sources by Ogataea polymorpha. Bioresour. Bioprocess. 2022, 9, 78. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Buijs, N.A.; Zhu, Z.; Qin, J.; Siewers, V.; Nielsen, J. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat. Commun. 2016, 7, 11709. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Wu, Z.; Wu, Y.; Bi, H.; Liu, Y.; Wang, M.; Chen, B.; Nielsen, J.; Liu, Z.; et al. Investigating formate tolerance mechanisms in Saccharomyces cerevisiae and its application. Green. Carbon. 2023, 1, 65–74. [Google Scholar] [CrossRef]
- van den Berg, M.A.; de Jong-Gubbels, P.; Kortland, C.J.; van Dijken, J.P.; Pronk, J.T.; Steensma, H.Y. The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae differ with respect to kinetic properties and transcriptional regulation. J. Biol. Chem. 1996, 271, 28953–28959. [Google Scholar] [CrossRef]
- Shiba, Y.; Paradise, E.M.; Kirby, J.; Ro, D.K.; Keasling, J.D. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae for high-level production of isoprenoids. Metab. Eng. 2007, 9, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, Y.; Wang, Z.; Chen, T. Metabolic engineering of Halomonas bluephagenesis for high-level mevalonate production from glucose and acetate mixture. Metab. Eng. 2023, 79, 203–213. [Google Scholar] [CrossRef]
- Li, Y.; Huang, B.; Wu, H.; Li, Z.; Ye, Q.; Zhang, Y.P. Production of Succinate from Acetate by Metabolically Engineered Escherichia coli. ACS Synth. Biol. 2016, 5, 1299–1307. [Google Scholar] [CrossRef]
- Hu, G.; Li, Z.; Ma, D.; Ye, C.; Zhang, L.; Gao, C.; Liu, L.; Chen, X. Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals. Nat. Catal. 2021, 4, 395–406. [Google Scholar] [CrossRef]
| Host | C1/C2 Substrate | Co-Substrates | Products | Titer (g L−1) | Ref. |
|---|---|---|---|---|---|
| H. bluephagenesis | Acetate | Glucose | Mevalonate | 121 | [39] |
| E. coli | Acetate | --- | Succinate | 7.29 | [40] |
| Formate and CO2 | Glucose | Malate | 18.6 | [41] | |
| Acetate | Glycerol | Ethanol | ~4 | [14] | |
| Y. lipolytica | Acetate | Glucose | Fatty alcohols | 0.437 | [19] |
| Formate and acetate | Formate | β-farnesene | 14.8 | [20] | |
| S. cerevisiae | Formate | Glucose | Free fatty acids | 10.1 | [23] |
| Acetate | Glucose and formate | Free fatty acids | 6.6 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wu, Z.; Du, J.; Liu, Y.; Zhu, Z.; Feng, P.; Bi, H.; Zhang, Y.; Liu, Y.; Chen, B.; et al. Metabolic Engineering of Saccharomyces cerevisiae for Conversion of Formate and Acetate into Free Fatty Acids. Fermentation 2023, 9, 984. https://doi.org/10.3390/fermentation9110984
Wang K, Wu Z, Du J, Liu Y, Zhu Z, Feng P, Bi H, Zhang Y, Liu Y, Chen B, et al. Metabolic Engineering of Saccharomyces cerevisiae for Conversion of Formate and Acetate into Free Fatty Acids. Fermentation. 2023; 9(11):984. https://doi.org/10.3390/fermentation9110984
Chicago/Turabian StyleWang, Kai, Zhuoheng Wu, Jingping Du, Yining Liu, Zehao Zhu, Pan Feng, Haoran Bi, Yang Zhang, Yanhui Liu, Biqiang Chen, and et al. 2023. "Metabolic Engineering of Saccharomyces cerevisiae for Conversion of Formate and Acetate into Free Fatty Acids" Fermentation 9, no. 11: 984. https://doi.org/10.3390/fermentation9110984
APA StyleWang, K., Wu, Z., Du, J., Liu, Y., Zhu, Z., Feng, P., Bi, H., Zhang, Y., Liu, Y., Chen, B., Wang, M., & Tan, T. (2023). Metabolic Engineering of Saccharomyces cerevisiae for Conversion of Formate and Acetate into Free Fatty Acids. Fermentation, 9(11), 984. https://doi.org/10.3390/fermentation9110984





