Barley Vinegar Relieves Loperamide-Induced Constipation in Mice via the Modulation of the Gut Microbiota and Serum Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Animals
2.2. Design of Animal Experiments
2.3. Animal Sample Collection
2.4. Bowel Movements and Gastric Emptying Tests
2.5. Small Bowel Motility Experiments
2.6. Histopathological Testing of the Colon
2.7. Intestinal Neurotransmitter Assay
2.8. Microbial Diversity Analysis
2.9. Determination of Short-Chain Fatty Acid Content
2.10. UHLC-Q-TOF-MS Analysis
2.11. Data Analysis and Processing
2.11.1. Pearson Correlation Analysis
2.11.2. Data Statistical Analysis
3. Results
3.1. Changes in Mice Status and Body Weight
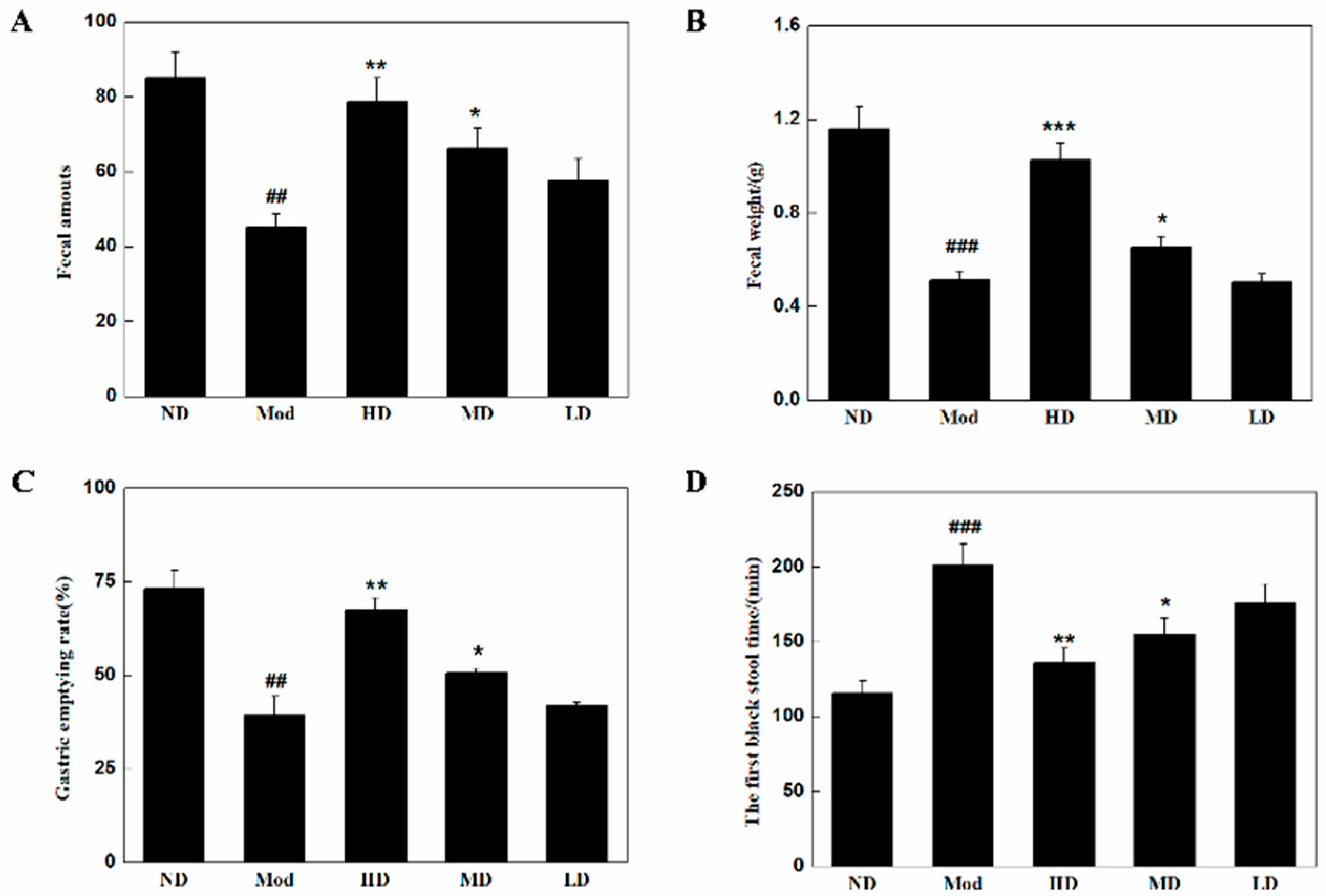
3.2. Changes in Defecation and Gastric Emptying in Mice
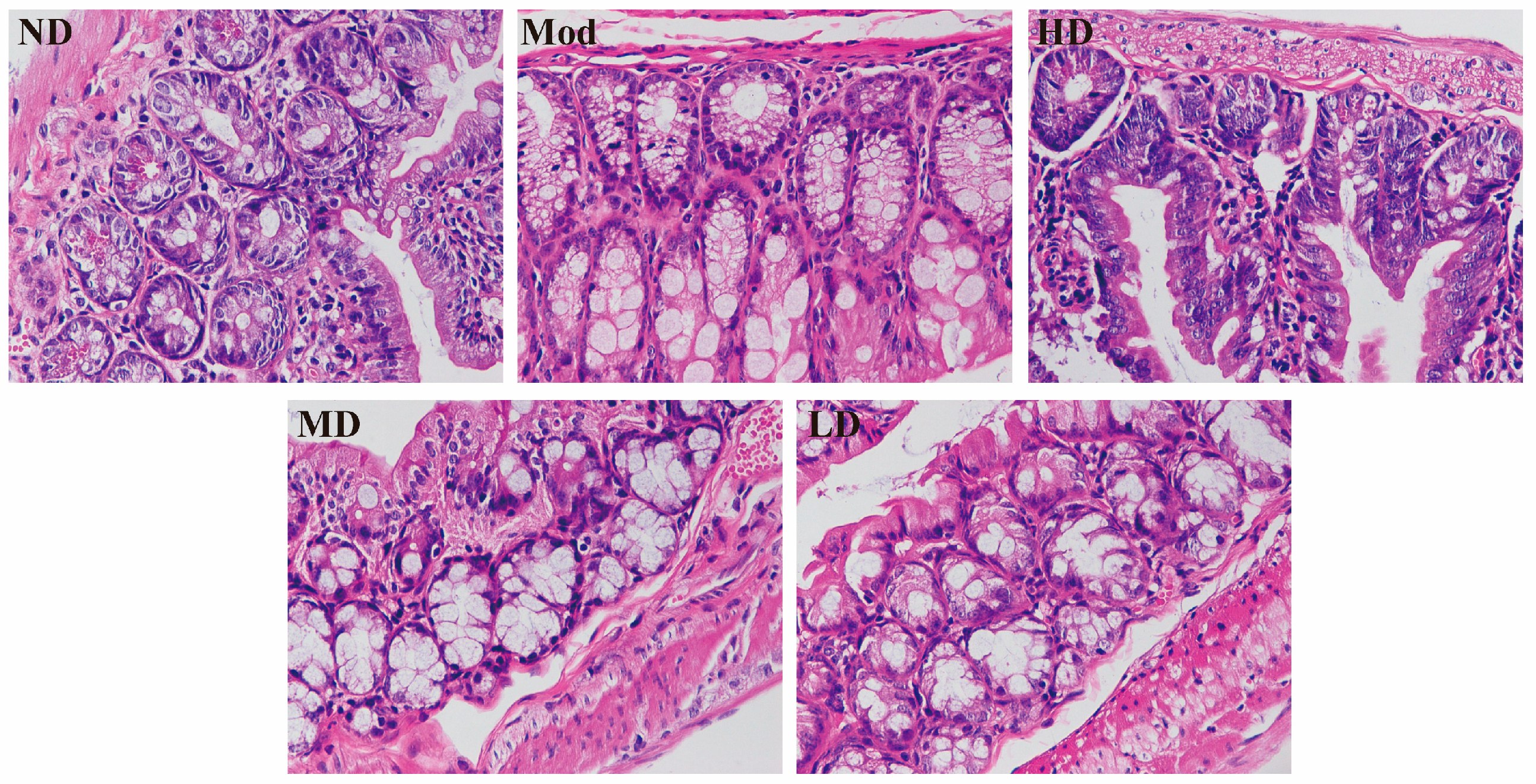
3.3. Ameliorating Effect on Intestinal Barrier Damage in Mice
3.4. Effects on Small Bowel Motility in Mice
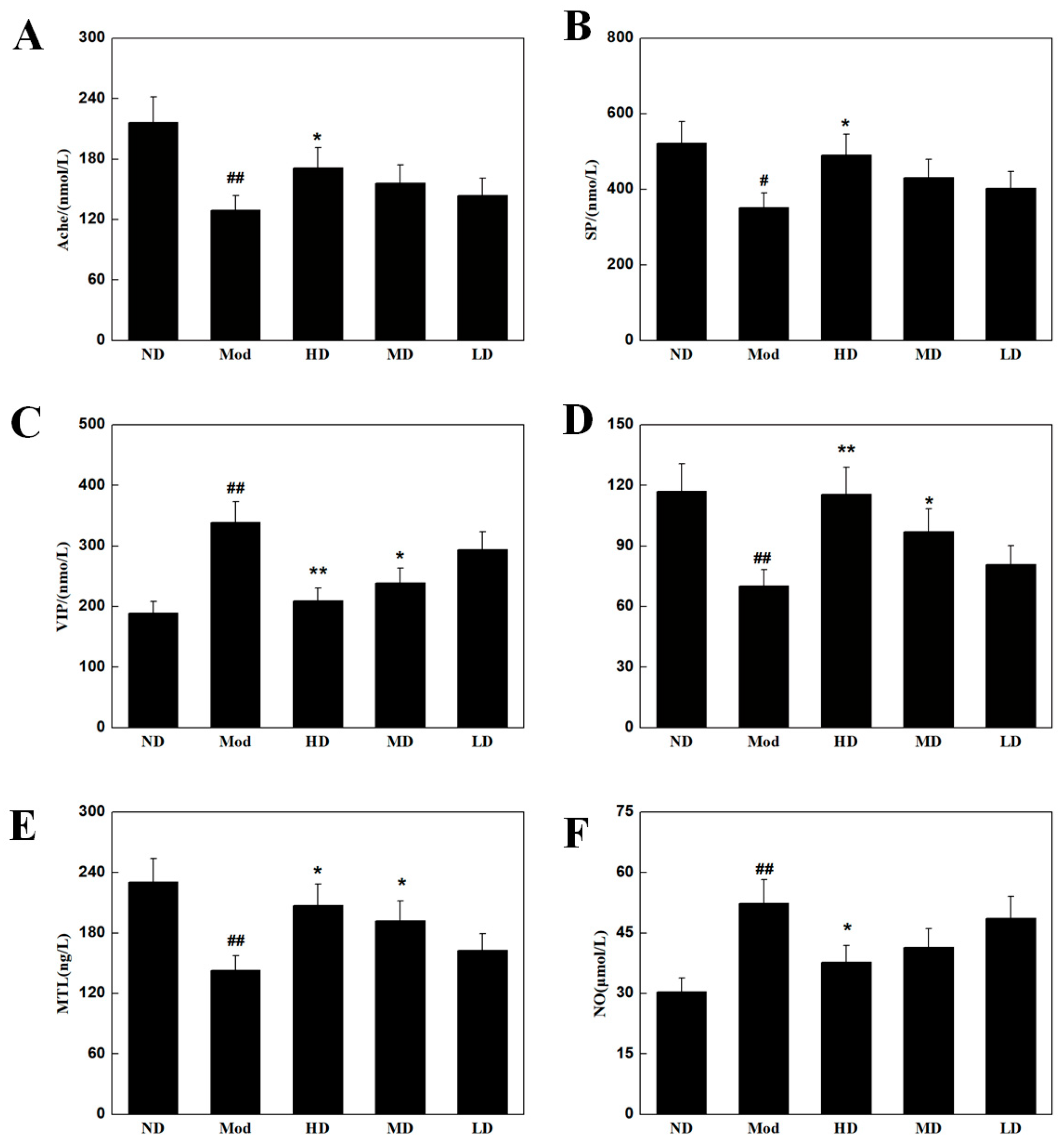
3.5. Effects on Intestinal Neurotransmitters in Mice
3.6. Effects on the Diversity of Intestinal Flora in Mice
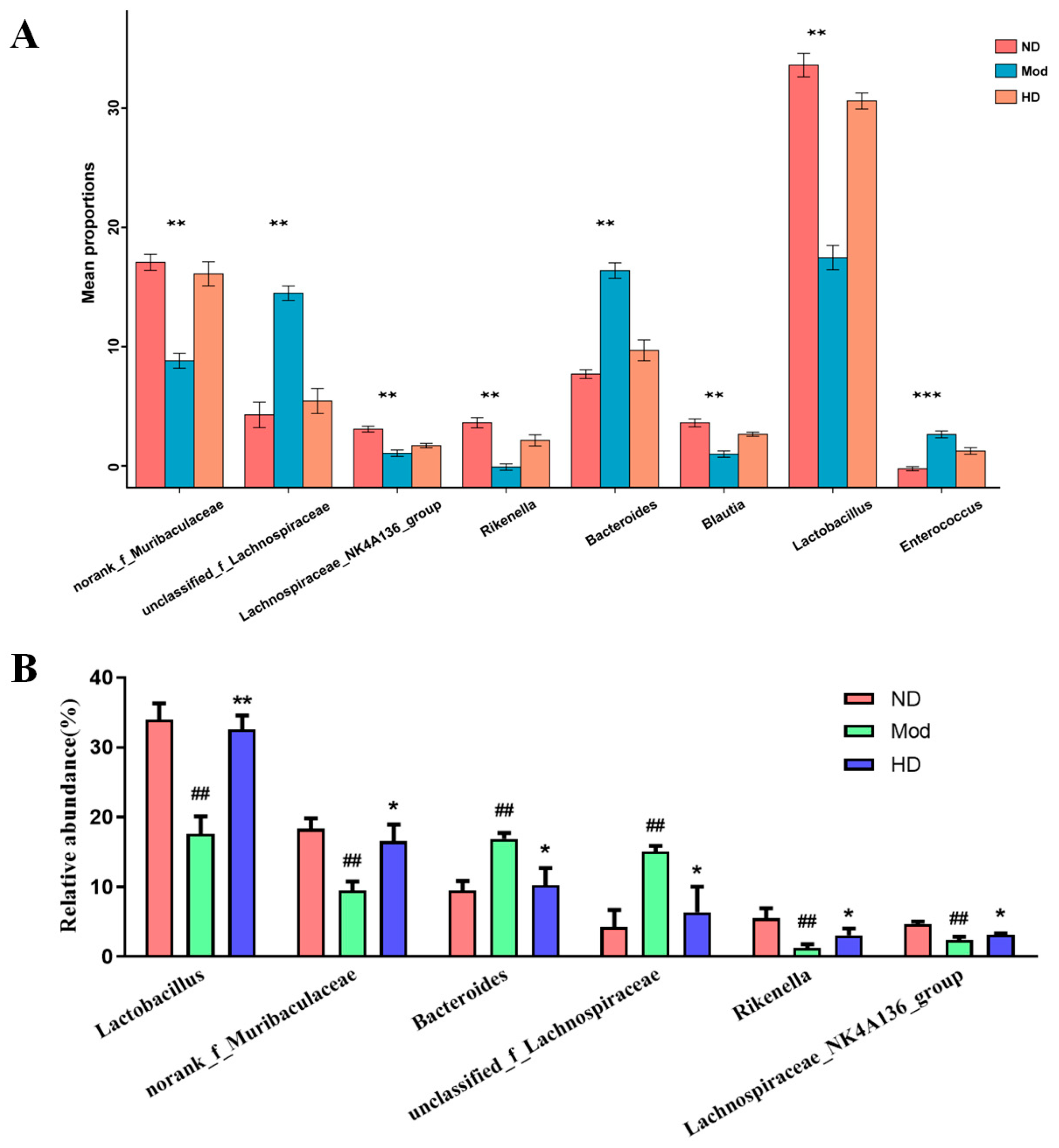
3.7. Changes in the Abundance of Intestinal Flora in Mice
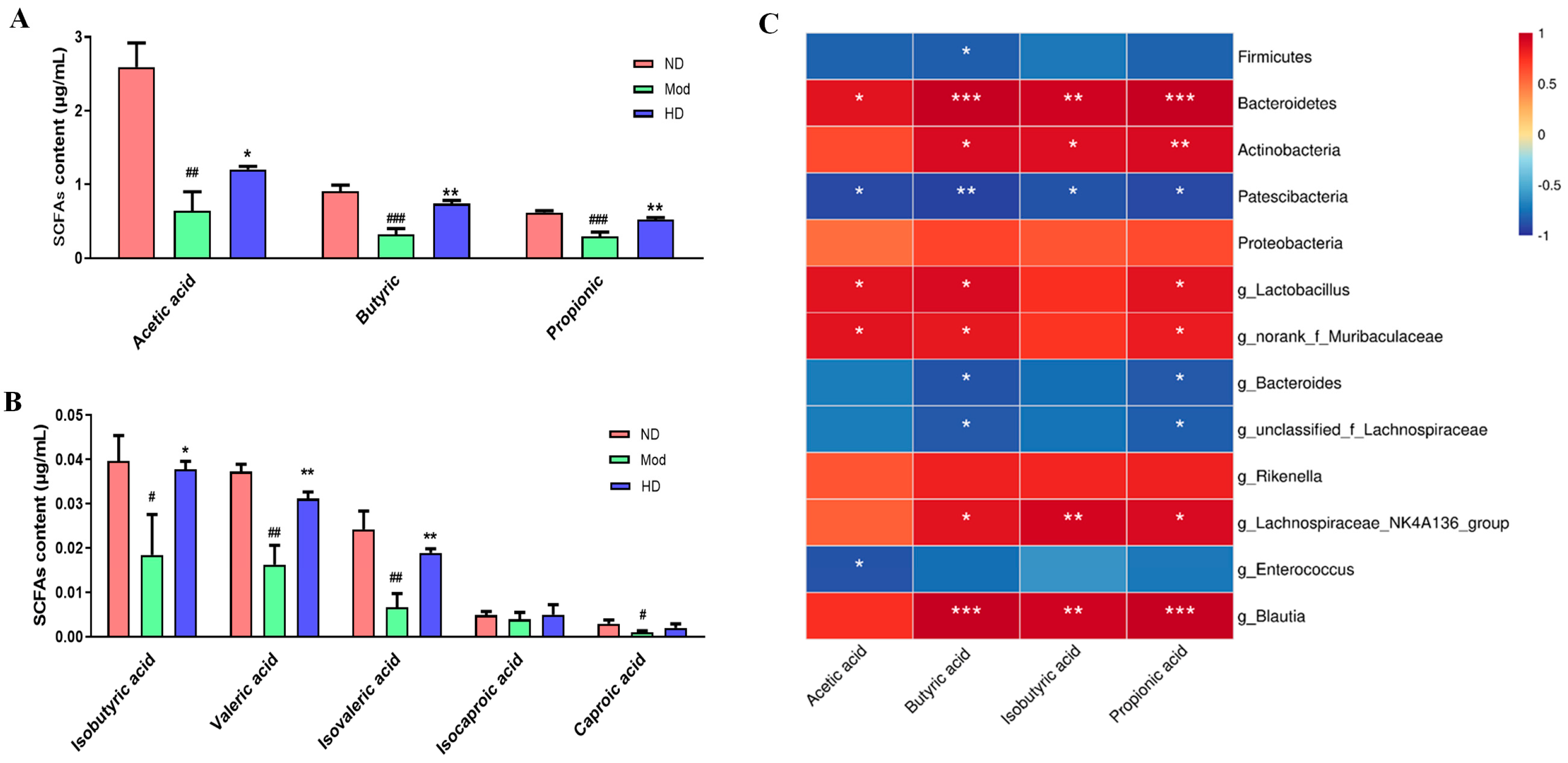
3.8. Effects on Species Differences in Mice Gut Microbiota
3.9. Fecal Short-Chain Fatty Acid Changes in Mice
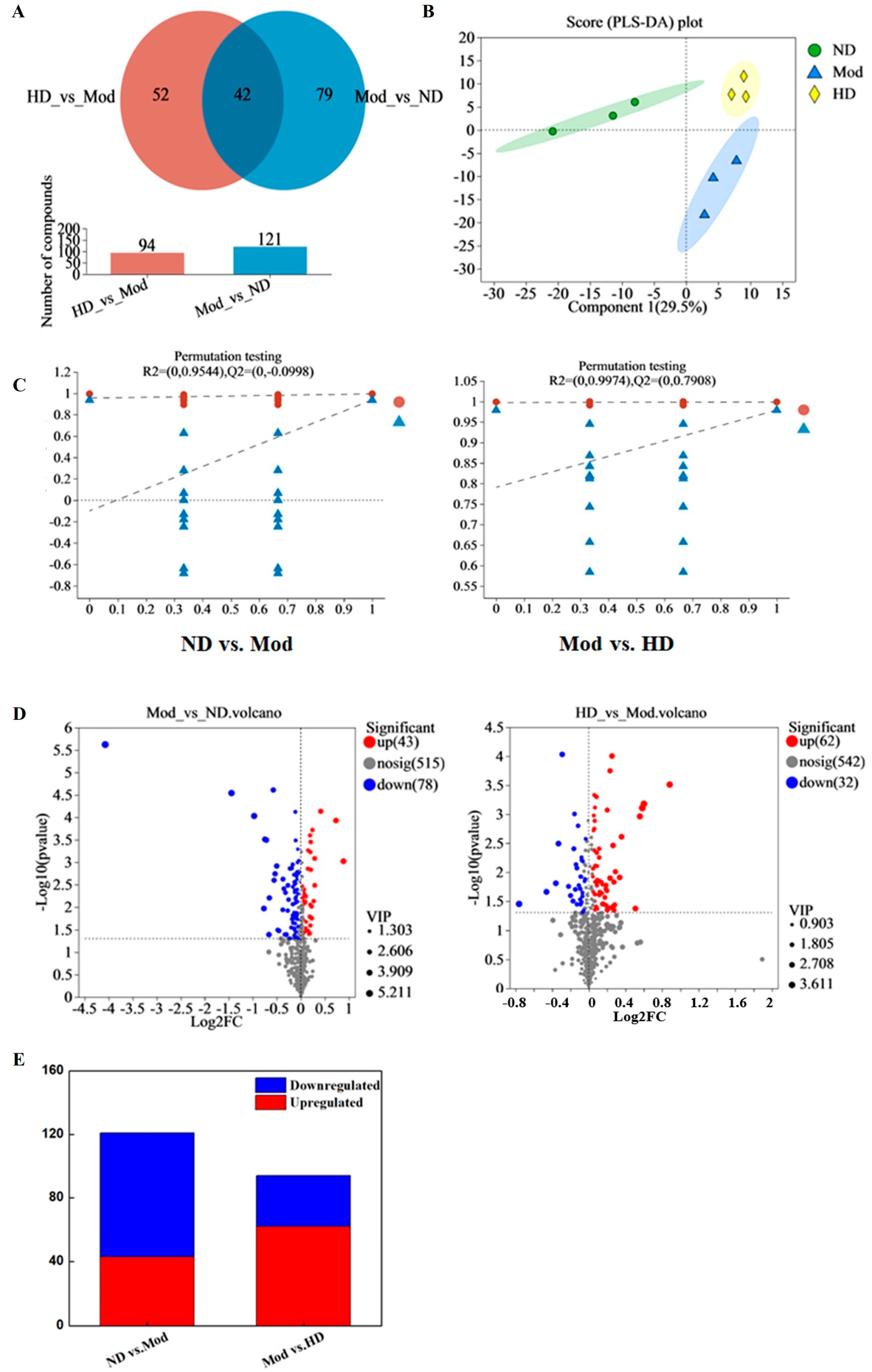
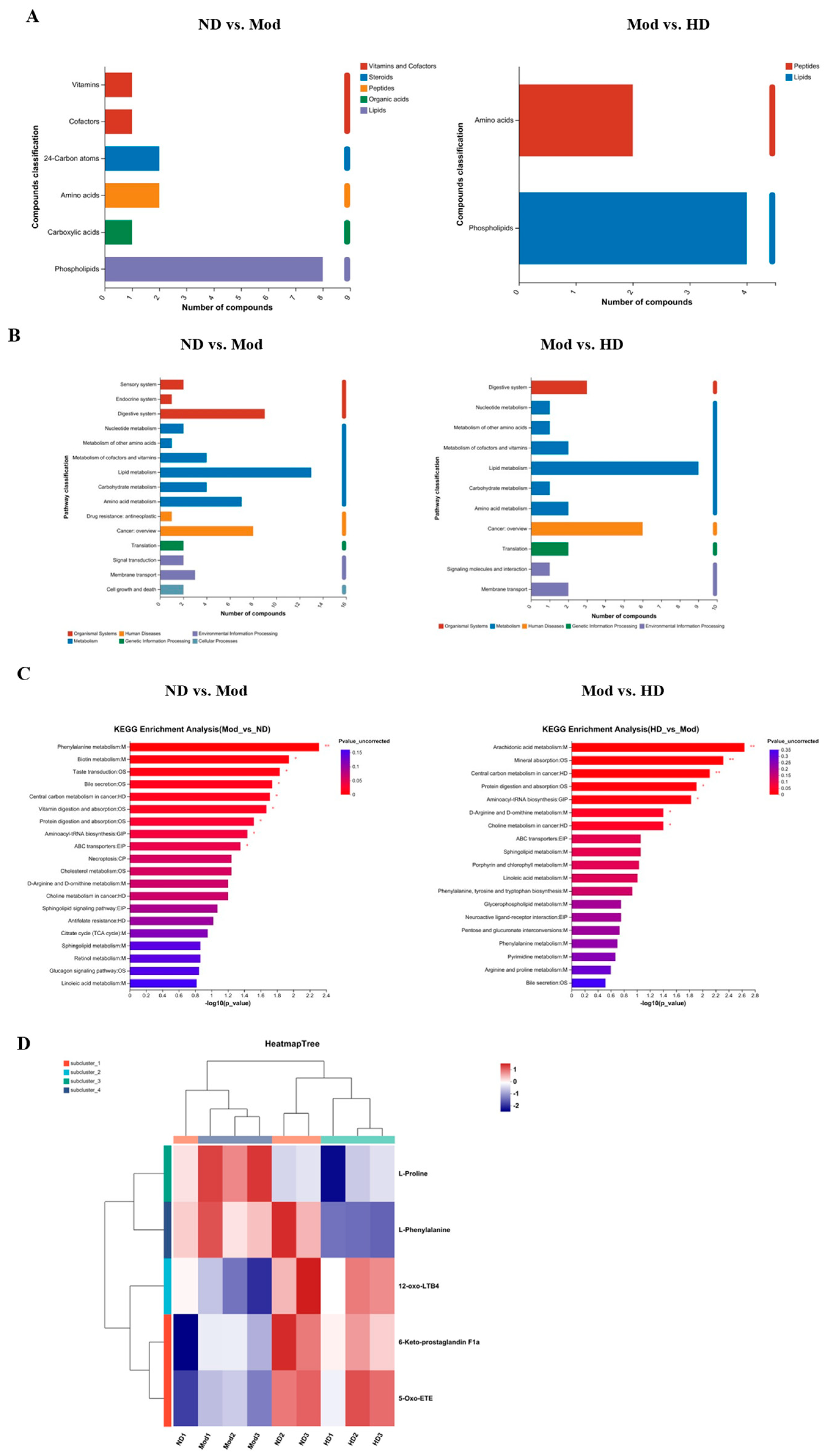
3.10. Effects on Intestinal Metabolic Profile in Mice
3.10.1. Principal Component Analysis and Statistical Analysis of Differences between Groups
3.10.2. Functional Pathway Statistics and Potential Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bharucha, A.E.; Lacy, B.E. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020, 158, 1232–1249.e3. [Google Scholar] [CrossRef]
- Drossman, D.A. The Functional Gastrointestinal Disorders and the Rome III Process. Gastroenterology 2019, 130, 1377–1390. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, D.; Xing, T.; Liu, Q.; Chi, Y.; Zhang, H.; Qian, H. The impact of visceral obesity on chronic constipation, inflammation, immune function and cognitive function in patients with inflammatory bowel disease. Aging 2021, 13, 6702–6711. [Google Scholar] [CrossRef]
- Ishiyama, Y.; Hoshide, S.; Mizuno, H.; Kario, K. Constipation-induced pressor effects as triggers for cardiovascular events. J. Clin. Hypertens. 2019, 21, 421–425. [Google Scholar] [CrossRef]
- Dimidi, E.; Scott, S.M.; Whelan, K. Probiotics and constipation: Mechanisms of action, evidence for effectiveness and utilisation by patients and healthcare professionals. Proc. Nutr. Soc. 2019, 79, 147–157. [Google Scholar] [CrossRef]
- Obata, Y.; Pachnis, V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Xu, Q.; Yin, B.; Fang, D.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis Exerts Strain-Specific Effects on Constipation Induced by Loperamide in BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 318. [Google Scholar] [CrossRef]
- Qu, C.; Yang, G.-H.; Zheng, R.-B.; Yu, X.-T.; Peng, S.-Z.; Xie, J.-H.; Chen, J.-N.; Wang, X.-F.; Su, Z.-R.; Zhang, X.-J. The immune-regulating effect of Xiao’er Qixingcha in constipated mice induced by high-heat and high-protein diet. BMC Complement. Altern. Med. 2017, 17, 185. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T.; Francis, T. Gut microbes formation of short chain fatty acids by the gut microbiota and their impact on human metabolism formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2017, 7, 189–200. [Google Scholar] [CrossRef]
- Brownlee, I. The impact of dietary fibre intake on the physiology and health of the stomach and upper gastrointestinal tract. Bioact. Carbohydr. Diet. Fibre 2014, 4, 155–169. [Google Scholar] [CrossRef]
- Vu, V.; Muthuramalingam, K.; Singh, V.; Choi, C.; Kim, Y.M.; Unno, T.; Cho, M. Schizophyllum commune-derived β-glucan improves intestinal health demonstrating protective effects against constipation and common metabolic disorders. Appl. Biol. Chem. 2022, 65, 9. [Google Scholar] [CrossRef]
- Zhuang, Z.; Chen, M.; Niu, J.; Qu, N.; Ji, B.; Duan, X.; Liu, Z.; Liu, X.; Wang, Y.; Zhao, B. The Manufacturing Process of Kiwifruit Fruit Powder with High Dietary Fiber and Its Laxative Effect. Molecules 2019, 24, 3813. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Qian, Y.; Zhou, Y.; Wang, R.; Zhao, X. Component analysis of Pu-erh and its anti-constipation effects. Mol. Med. Rep. 2014, 9, 2003–2009. [Google Scholar] [CrossRef]
- Scott, S.M.; Simrén, M.; Farmer, A.D.; Dinning, P.G.; Carrington, E.V.; Benninga, M.A.; Burgell, R.E.; Dimidi, E.; Eikree, A.; Ford, A. Chronic constipation in adults: Contemporary perspectives and clinical challenges. 1: Epidemiology, diagnosis, clinical associations, pathophysiology and investigation. Neurogastroenterol. Motil. 2021, 33, e14050. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef]
- Knowles, C.H.; Farrugia, G. Gastrointestinal neuromuscular pathology in chronic constipation. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 43–57. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Wu, H.; Tang, S.; Zhang, Y.; Yang, B.; Yang, H.; Huang, L. Therapeutic effect of Moringa oleifera leaves on constipation mice based on pharmacodynamics and serum metabonomics. J. Ethnopharmacol. 2022, 282, 114644. [Google Scholar] [CrossRef]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef]
- Yarullina, D.R.; Shafigullin, M.U.; Sakulin, K.A.; Arzamastseva, A.A.; Shaidullov, I.F.; Markelova, M.I.; Grigoryeva, T.V.; Karpukhin, O.Y.; Sitdikova, G.F. Characterization of gut contractility and microbiota in patients with severe chronic constipation. PLoS ONE 2020, 15, e0235985. [Google Scholar] [CrossRef]
- Mudgil, D. The Interaction between Insoluble and Soluble Fiber. 2017. Available online: https://www.researchgate.net/publication/322732961_The_Interaction_Between_Insoluble_and_Soluble_Fiber (accessed on 2 May 2021).
- Chen, T.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Luo, J.; et al. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. Anim. Feed. Sci. Technol. 2019, 260, 114335. [Google Scholar] [CrossRef]
- Su, H.; Chen, J.; Miao, S.; Deng, K.; Liu, J.; Zeng, S.; Zheng, B.; Lu, X. Lotus seed oligosaccharides at various dosages with prebiotic activity regulate gut microbiota and relieve constipation in mice. Food Chem. Toxicol. 2019, 134, 110838. [Google Scholar] [CrossRef]
- Xie, L.; Wang, Y.; Luo, G.; Zhou, W.; Miao, J.; Tang, S.; Jiang, Q.; Guan, Y.; Gao, X. Identification of the multiple bioactive derivatives and their endogenous molecular targets that may mediate the laxative effect of rhubarb in rats. J. Tradit. Chin. Med. Sci. 2020, 7, 210–220. [Google Scholar] [CrossRef]
- Weinheimer, C.J.; Lai, L.; Kelly, D.P.; Kovacs, A. Novel mouse model of left ventricular pressure overload and infarction causing predictable ventricular remodelling and progression to heart failure. Clin. Exp. Pharmacol. Physiol. 2015, 42, 33–40. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.; Qian, Y.; Li, G.; Liu, Z.; Xie, J.; Li, J. Therapeutic Effect of Activated Carbon-Induced Constipation Mice with Lactobacillus fermentum Suo on Treatment. Int. J. Mol. Sci. 2014, 15, 21875–21895. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Zhao, Y.; Yang, X. Bacterial Cellulose Relieves Diphenoxylate-Induced Constipation in Rats. J. Agric. Food Chem. 2018, 66, 4106–4117. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Yan, Q.; Li, Y.; Jiang, Z. Effect of Konjac mannan oligosaccharides on diphenoxylate-induced constipation in mice. J. Funct. Foods 2019, 57, 399–407. [Google Scholar] [CrossRef]
- Guerra, D.D.; Bok, R.; Lorca, R.A.; Hurt, K.J. Protein kinase A facilitates relaxation of mouse ileum via phosphorylation of neuronal nitric oxide synthase. Br. J. Pharmacol. 2020, 177, 2765–2778. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Zhu, J.; Owyang, C. Serotonin released from intestinal enterochromaffin cells mediates luminal non–cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology 2000, 118, 1197–1207. [Google Scholar] [CrossRef]
- Lan, J.; Wang, K.; Chen, G.; Cao, G.; Yang, C. Effects of inulin and isomalto-oligosaccharide on diphenoxylate-induced constipation, gastrointestinal motility-related hormones, short-chain fatty acids, and the intestinal flora in rats. Food Funct. 2020, 11, 9216–9225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, A.; Li, Y.; Cai, M.; Luo, G.; Wu, Y.; Tian, Y.; Xing, L.; Zhang, Q. PICRUSt2 functionally predicts organic compounds degradation and sulfate reduction pathways in an acidogenic bioreactor. Front. Environ. Sci. Eng. 2022, 16, 47. [Google Scholar] [CrossRef]
- Xu, Y.; Shao, M.; Fang, X.; Tang, W.; Zhou, C.; Hu, X.; Zhang, X.; Su, K.-P. Antipsychotic-induced gastrointestinal hypomotility and the alteration in gut microbiota in patients with schizophrenia. Brain Behav. Immun. 2022, 99, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ashafa, A.; Abass, A.; Sunmonu, T.; Ogbe, A. Laxative potential of the ethanolic leaf extract of Aloe vera (L.) Burm. f. in Wistar rats with loperamide-induced constipation. J. Nat. Pharm. 2011, 2, 158. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, B.; Li, T.; Yu, Y.; Men, F.; Zhao, S.; Liu, J.; Zhang, T. Potential targets and the action mechanism of food-derived dipeptides on colitis: Network pharmacology and bioinformatics analysis. Food Funct. 2021, 12, 5989–6000. [Google Scholar] [CrossRef] [PubMed]
- Macho-González, A.; Garcimartín, A.; Redondo, N.; Cofrades, S.; Bastida, S.; Nova, E.; Benedí, J.; Sánchez-Muniz, F.J.; Marcos, A.; López-Oliva, M.E. Carob fruit extract-enriched meat, as preventive and curative treatments, improves gut microbiota and colonic barrier integrity in a late-stage T2DM model. Food Res. Int. 2021, 141, 110124. [Google Scholar] [CrossRef] [PubMed]
- Hurst, N.R.; Kendig, D.M.; Murthy, K.S.; Grider, J.R. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2015, 26, 1586–1596. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Q.; Huang, Y.; Ni, L.; Liu, J.; Jiang, J.; Li, N. Function and clinical implications of short-chain fatty acids in patients with mixed refractory constipation. Color. Dis. 2016, 18, 803–810. [Google Scholar] [CrossRef]
- Wang, J.-K.; Yao, S.-K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid. Based Complement. Altern. Med. 2021, 2021, 5560310. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiu, Z.; Tian, F.; Yu, L.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Pediococcus acidilactici Strains Improve Constipation Symptoms and Regulate Intestinal Flora in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 655258. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, R.; Zhang, X.; Wang, Z.; Wen, J.; Zhou, T. Non-isoflavones Diet Incurred Metabolic Modifications Induced by Constipation in Rats via Targeting Gut Microbiota. Front. Microbiol. 2018, 9, 3002. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Kim, D.-H.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Kim, H.-S.; Seo, K.-H. Modulation of gut microbiota and increase in fecal water content in mice induced by administration of Lactobacillus kefiranofaciens DN1. Food Funct. 2017, 8, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.R.; Kang, S.-Y.; Kim, S.-E.; Lee, S.-J.; Lee, Y.-C.; Sung, M.-K. Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: A randomized placebo-controlled intervention study. World J. Gastroenterol. 2019, 25, 6129–6144. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Pardesi, B.; Roberton, A.M.; Lee, K.C.; Angert, E.R.; Rosendale, D.I.; Boycheva, S.; White, W.L.; Clements, K.D. Distinct microbiota composition and fermentation products indicate functional compartmentalization in the hindgut of a marine herbivorous fish. Mol. Ecol. 2022, 31, 2494–2509. [Google Scholar] [CrossRef]
- Nagano, A.; Ohge, H.; Tanaka, T.; Takahashi, S.; Uemura, K.; Murakami, Y.; Sueda, T. Effects of different types of dietary fibers on fermentation by intestinal Flora. Hiroshima J. Med. Sci. 2018, 67, 1–5. [Google Scholar] [CrossRef]
- Romick-Rosendale, L.E.; Haslam, D.B.; Lane, A.; Denson, L.; Lake, K.; Wilkey, A.; Watanabe, M.; Bauer, S.; Litts, B.; Luebbering, N.; et al. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2018, 24, 2418–2424. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Pang, T.; Leach, S.T.; Katz, T.; Day, A.S.; Ooi, C.Y. Fecal Biomarkers of Intestinal Health and Disease in Children. Front. Pediatr. 2014, 2, 6. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Abdulamir, A.; Fatimah, A.; Anas, O.M.; Shuhaimi, M.; Yazid, A.; Loong, Y. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients Versus Healthy Subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex Interactions Among Diet, Gastrointestinal Transit, and Gut Microbiota in Humanized Mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship Between Microbiota of the Colonic Mucosa vs. Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016, 150, 367–379.e1. [Google Scholar] [CrossRef] [PubMed]



| Group | Day 0 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 |
|---|---|---|---|---|---|---|
| ND | 27.89 ± 2.76 | 29.01 ± 1.51 | 29.51 ± 1.78 | 29.53 ± 1.16 | 30.36 ± 2.48 | 30.56 ± 1.04 |
| Mod | 28.87 ± 2.80 | 28.53 ± 1.57 | 29.02 ± 2.35 | 28.93 ± 1.24 | 28.64 ± 2.69 | 28.57 ± 1.14 |
| HD | 29.41 ± 2.76 | 29.72 ± 1.03 | 30.46 ± 2.74 | 30.53 ± 1.12 | 30.85 ± 1.24 | 31.41 ± 1.79 |
| MD | 29.32 ± 2.34 | 29.55 ± 2.69 | 29.90 ± 1.11 | 30.21 ± 1.23 | 30.39 ± 1.31 | 30.57 ± 2.44 |
| LD | 28.77 ± 2.59 | 29.11 ± 1.26 | 29.33 ± 1.56 | 29.67 ± 1.15 | 29.91 ± 1.10 | 30.08 ± 1.21 |
| Group | Total Length of the Small Intestine (cm) | Ink Propulsion Length (cm) | Ink Propulsion Rate (%) |
|---|---|---|---|
| ND | 47.06 ± 3.50 | 42.33 ± 4.53 | 89.79 ± 2.94 |
| Mod | 49.17 ± 3.94 | 15.98 ± 4.12 ## | 32.15 ± 5.08 ### |
| HD | 49.00 ± 3.26 | 41.20 ± 4.08 ** | 83.99 ± 3.94 *** |
| MD | 47.42 ± 2.84 | 27.26 ± 4.94 * | 57.26 ± 7.76 ** |
| LD | 49.18 ± 1.92 | 25.87 ± 6.55 | 52.42 ± 12.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, A.; Nie, M.; Chen, Z.; Wang, L.; Liu, L.; Wang, F.; Tong, L. Barley Vinegar Relieves Loperamide-Induced Constipation in Mice via the Modulation of the Gut Microbiota and Serum Metabolism. Fermentation 2023, 9, 962. https://doi.org/10.3390/fermentation9110962
Li Y, Wang A, Nie M, Chen Z, Wang L, Liu L, Wang F, Tong L. Barley Vinegar Relieves Loperamide-Induced Constipation in Mice via the Modulation of the Gut Microbiota and Serum Metabolism. Fermentation. 2023; 9(11):962. https://doi.org/10.3390/fermentation9110962
Chicago/Turabian StyleLi, Yan, Aixia Wang, Mengzi Nie, Zhiying Chen, Lili Wang, Liya Liu, Fengzhong Wang, and Litao Tong. 2023. "Barley Vinegar Relieves Loperamide-Induced Constipation in Mice via the Modulation of the Gut Microbiota and Serum Metabolism" Fermentation 9, no. 11: 962. https://doi.org/10.3390/fermentation9110962
APA StyleLi, Y., Wang, A., Nie, M., Chen, Z., Wang, L., Liu, L., Wang, F., & Tong, L. (2023). Barley Vinegar Relieves Loperamide-Induced Constipation in Mice via the Modulation of the Gut Microbiota and Serum Metabolism. Fermentation, 9(11), 962. https://doi.org/10.3390/fermentation9110962








