Enhancing Pullulan Production in Aureobasidium pullulans through UV Mutagenesis Breeding and High-Throughput Screening System
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Construction of Plasmid
2.3. Expression and Purification of Pullulan Screening Marker
2.4. Induced Expression of Fluorescent Detection Proteins and Optimization of the System
2.5. Construction of Mutant Strain Library through UV Mutagenesis
2.6. Flow Cytometry Screening of Mutant Strains
2.7. Cultivation in Microplates and Fermentation after Screening
2.8. Determination of the Yield and Viscosity of Strain Production “Pullulan”
2.9. Determination of the Molecular Weight of Strain Production “Pullulan”
3. Results
3.1. Expression of Screening Marker and Optimization Results of Detection Conditions
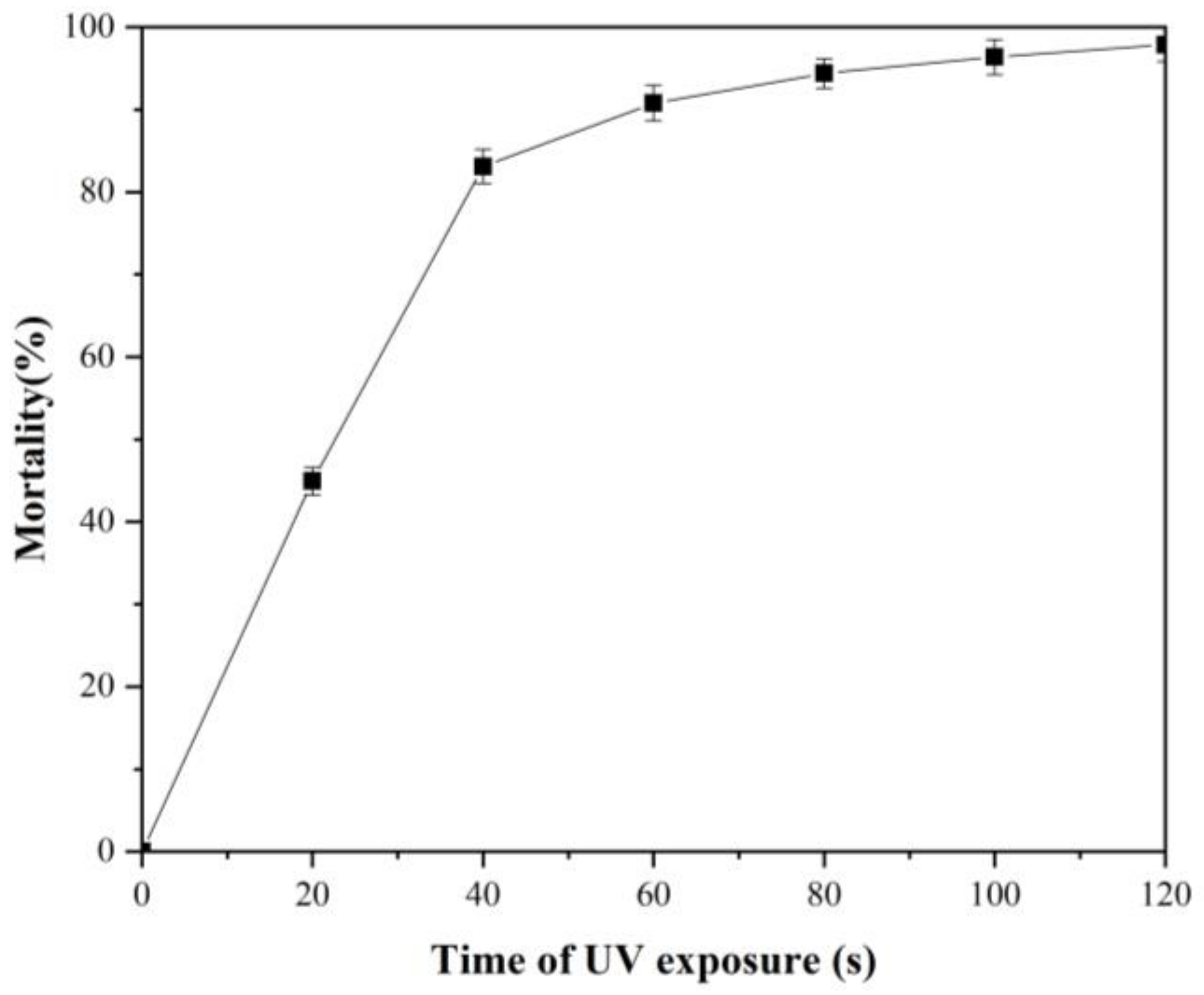
3.2. Result of Mortality of A. pullulans Irradiated by UV
3.3. Flow Cytometry Analysis and Sorting of Aureobasidium pullulans
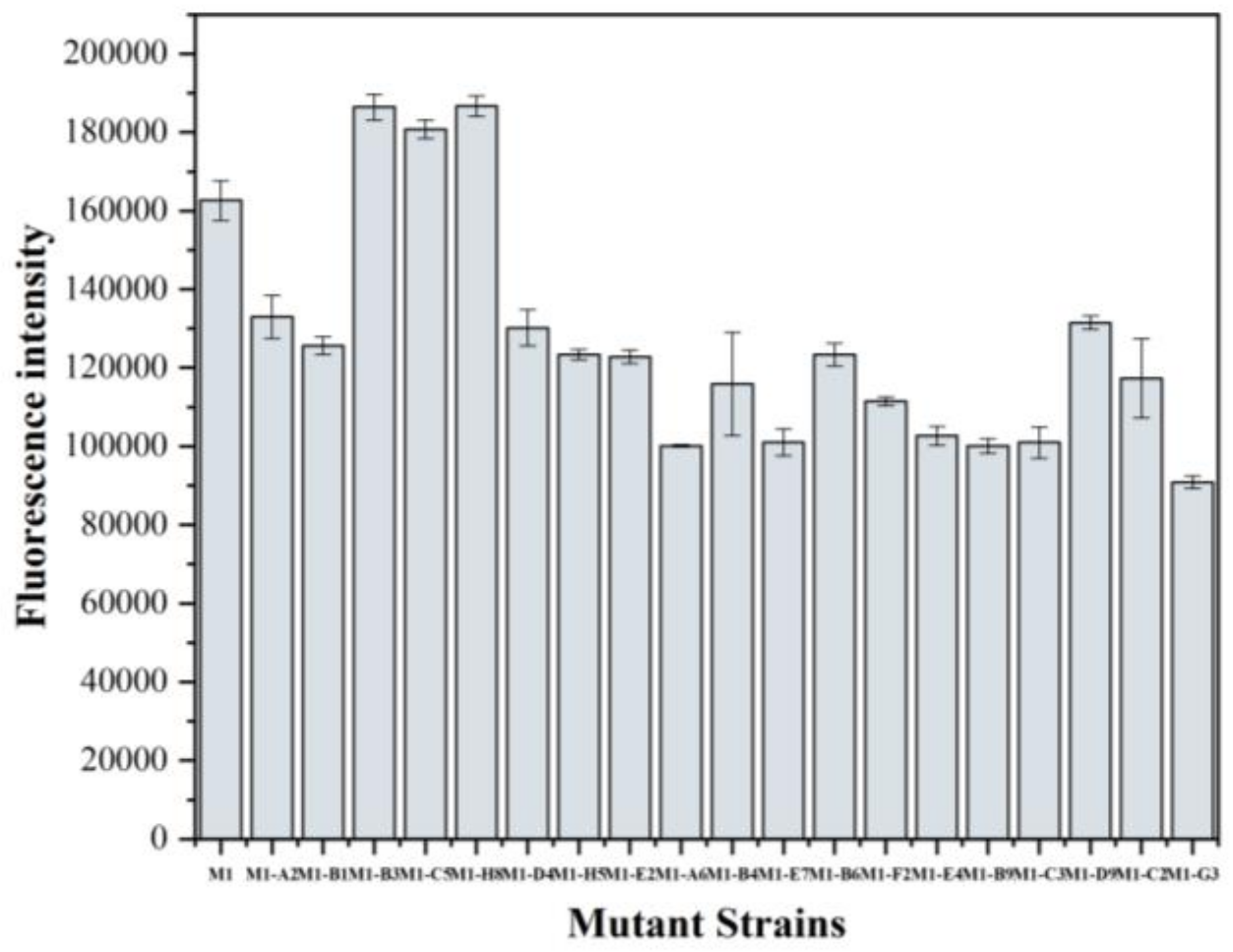
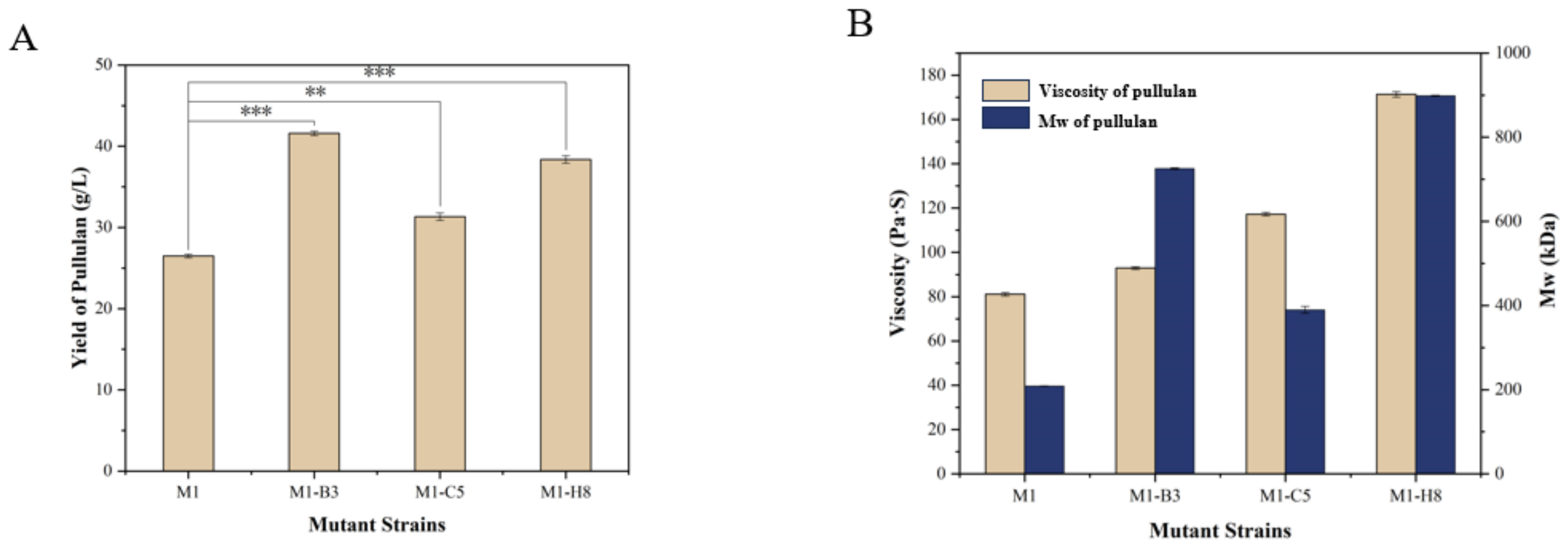
3.4. Screening of the Mutant Strains and Determination of the Product
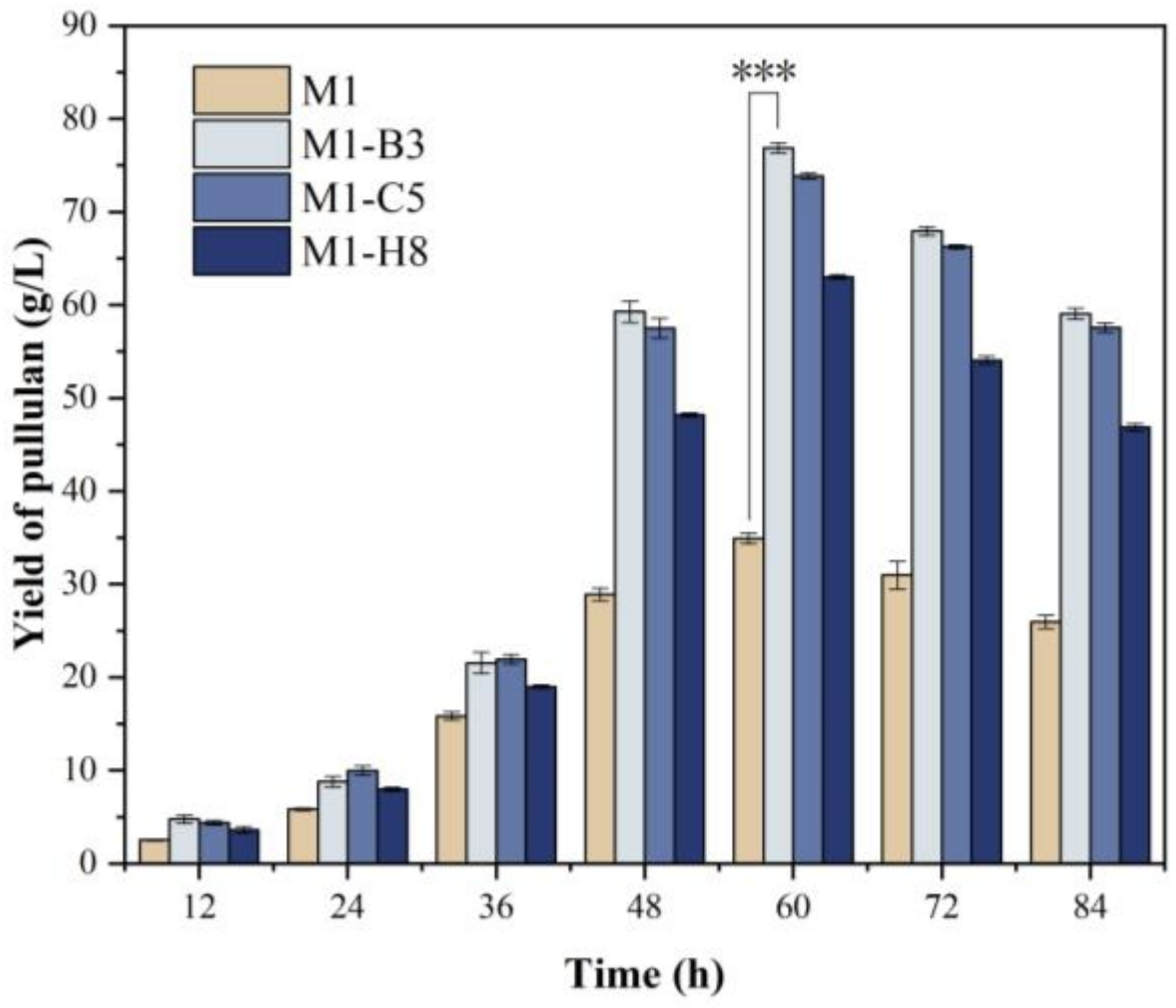
3.5. Fermentation of Mutant Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chi, Z.; Wang, F.; Chi, Z.; Yue, L.; Liu, G.; Zhang, T. Bioproducts from Aureobasidium pullulans, a biotechnologically important yeast. Appl. Microbiol. Biotechnol. 2009, 82, 793–804. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Hassan, M.; Kennedy, J.F. Pullulan in biomedical research and development—A review. Int. J. Biol. Macromol. 2021, 166, 694–706. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef]
- Rekha, M.; Sharma, C. Pullulan as a promising biomaterial for biomedical applications: A perspective. Trends Biomater Artif Organs 2007, 20, 116–121. [Google Scholar]
- Prasongsuk, S.; Lotrakul, P.; Ali, I.; Bankeeree, W.; Punnapayak, H. The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol. 2017, 63, 129–140. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Lu, Y.; Ren, Z.-Y.; Chi, Z.; Liu, G.-L.; Chi, Z.-M. CreA is directly involved in pullulan biosynthesis and regulation of Aureobasidium melanogenum P16. Curr. Genet. 2016, 63, 471–485. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Zhang, L.; Diao, M.; Ling, P.; Wang, F. Correlation between the synthesis of pullulan and melanin in Aureobasidium pullulans. Int. J. Biol. Macromol. 2021, 177, 252–260. [Google Scholar] [CrossRef]
- Wu, S.; Chen, H.; Jin, Z.; Tong, Q. Effect of two-stage temperature on pullulan production by Aureobasidium pullulans. World J. Microbiol. Biotechnol. 2009, 26, 737–741. [Google Scholar] [CrossRef]
- Sheng, L.; Zhu, G.; Tong, Q. Effect of uracil on pullulan production by Aureobasidium pullulans CGMCC1234. Carbohydr. Polym. 2014, 101, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, A.; Mody, K.; Jha, B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Kerppola, T.K. Bimolecular Fluorescence Complementation: Visualization of Molecular Interactions in Living Cells. Methods Cell Biol. 2008, 85, 431–470. [Google Scholar] [CrossRef]
- Van Bueren, A.L.; Boraston, A.B. The Structural Basis of α-Glucan Recognition by a Family 41 Carbohydrate-binding Module from Thermotoga maritima. J. Mol. Biol. 2007, 365, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Shiota, K.; Yoshikawa, J.; Ogawa, S.; Aoyagi, H. Simple method for analyzing the purity of protease-containing samples by acid-treatment SDS-PAGE. J. Biosci. Bioeng. 2019, 128, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Spriestersbach, A.; Kubicek, J.; Schäfer, F.; Block, H.; Maertens, B. Purification of His-Tagged Proteins. In Methods in Enzymology; Academic Press: New York, NY, USA, 2015; pp. 1–15. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Su, Y.; Zhu, Y.; Zhang, G.; Zhao, H.; Liu, H.; Yang, Y.; Nian, R.; Zhang, H.; et al. Pullulan production from synthetic medium by a new mutant of Aureobasidium pullulans. Prep. Biochem. Biotechnol. 2017, 47, 963–969. [Google Scholar] [CrossRef]
- Hernandez, V.A.; Evans, P.D. Melanization of the wood-staining fungus Aureobasidium pullulans in response to UV radiation. Wood Fiber Sci. 2015, 47, 120–124. [Google Scholar]
- Hu, D.; Tang, K.; Miao, Z. Preparation of catalitaxol-deficient defective strains by using ultraviolet mutagenesis. Chin. J. Biotechnol. 2017, 33, 838–848. [Google Scholar]
- Ong, S.-C.; Kao, C.-Y.; Chiu, S.-Y.; Tsai, M.-T.; Lin, C.-S. Characterization of the thermal-tolerant mutants of Chlorella sp. with high growth rate and application in outdoor photobioreactor cultivation. Bioresour. Technol. 2010, 101, 2880–2883. [Google Scholar] [CrossRef]
- Bürling, K.; Hunsche, M.; Noga, G.; Pfeifer, L.; Damerow, L. UV-induced fluorescence spectra and lifetime determination for detection of leaf rust (Puccinia triticina) in susceptible and resistant wheat (Triticum aestivum) cultivars. Funct. Plant Biol. 2011, 38, 337–345. [Google Scholar] [CrossRef]
- Akagi, S.; Nakajima, C.; Tanaka, Y.; Kurihara, Y. Flow cytometry-based method for rapid and high-throughput screening of hybridoma cells secreting monoclonal antibody. J. Biosci. Bioeng. 2018, 125, 464–469. [Google Scholar] [CrossRef]
- Veal, D.; Deere, D.; Ferrari, B.; Piper, J.; Attfield, P. Fluorescence staining and flow cytometry for monitoring microbial cells. J. Immunol. Methods 2000, 243, 191–210. [Google Scholar] [CrossRef]
- Hu, H.; Takada, A.; Takahashi, Y. Intrinsic viscosity of Pullulan in ionic liquid solutions studied by rheometry. J. Rheol. 2014, 42, 191–196. [Google Scholar] [CrossRef]
- An, C.; Ma, S.-J.; Xue, W.-J.; Liu, C.; Ding, H. Comparative study of different molecular weight pullulan productions by Aureobasidium pullulans. Biotech 2019, 9, 156. [Google Scholar]
- Kerppola, T.K. Bimolecular Fluorescence Complementation (BiFC) Analysis as a Probe of Protein Interactions in Living Cells. Annu. Rev. Biophys. 2008, 37, 465–487. [Google Scholar] [CrossRef]
- Miller, K.E.; Kim, Y.; Huh, W.-K.; Park, H.-O. Bimolecular Fluorescence Complementation (BiFC) Analysis: Advances and Recent Applications for Genome-Wide Interaction Studies. J. Mol. Biol. 2015, 427, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2003, 62, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E. UV Signature Mutations. Photochem. Photobiol. 2014, 91, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-X.; Chen, X.-J.; Chen, W.-R.; Li, M.-S.; Fang, Y.; Li, D.-S.; Ren, Y.-Z.; Liu, D.-Q. Enhanced production of pullulan in Aureobasidium pullulans by a new process of genome shuffling. Process Biochem. 2011, 46, 792–795. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Wei, G.; Dong, Y. Media optimization for elevated molecular weight and mass production of pigment-free pullulan. Carbohydr. Polym. 2012, 89, 928–934. [Google Scholar] [CrossRef]
- Müller, S.; Nebe-von-Caron, G. Functional single-cell analyses: Flow cytometry and cell sorting of microbial populations and communities. FEMS Microbiol. Rev. 2010, 34, 554–587. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Y.; Peng, Z.; Du, P.; Du, X.; Zuo, G.; Wang, C.; Li, P.; Wang, J.; Wang, R. Exploration of microbiome diversity of stacked fermented grains by flow cytometry and cell sorting. Front. Microbiol. 2023, 14, 1160552. [Google Scholar] [CrossRef]
- Zigon, E.S.; Purseglove, S.M.; Toxavidis, V.; Rice, W.; Tigges, J.; Chan, L.L.Y. A rapid single cell sorting verification method using plate-based image cytometry. Cytom. Part A 2018, 93, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, G.-L.; Chi, Z.; Hu, Z.; Chi, Z.-M. Genetics of trehalose biosynthesis in desert-derived Aureobasidium melanogenum and role of trehalose in the adaptation of the yeast to extreme environments. Curr. Genet. 2017, 64, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Kachhawa, D.K.; Bhattacharjee, P.; Singhal, R.S. Studies on downstream processing of pullulan. Carbohydr. Polym. 2003, 52, 25–28. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Feng, Z.; Zeng, Q.; Zeng, J.; Liu, H.; Deng, P.; Li, S.; Li, N.; Wang, J. Enhancing Pullulan Production in Aureobasidium pullulans through UV Mutagenesis Breeding and High-Throughput Screening System. Fermentation 2024, 10, 103. https://doi.org/10.3390/fermentation10020103
Zhang S, Feng Z, Zeng Q, Zeng J, Liu H, Deng P, Li S, Li N, Wang J. Enhancing Pullulan Production in Aureobasidium pullulans through UV Mutagenesis Breeding and High-Throughput Screening System. Fermentation. 2024; 10(2):103. https://doi.org/10.3390/fermentation10020103
Chicago/Turabian StyleZhang, Shuyue, Zhe Feng, Qingming Zeng, Junhao Zeng, Huijing Liu, Pan Deng, Shangyu Li, Nan Li, and Junqing Wang. 2024. "Enhancing Pullulan Production in Aureobasidium pullulans through UV Mutagenesis Breeding and High-Throughput Screening System" Fermentation 10, no. 2: 103. https://doi.org/10.3390/fermentation10020103
APA StyleZhang, S., Feng, Z., Zeng, Q., Zeng, J., Liu, H., Deng, P., Li, S., Li, N., & Wang, J. (2024). Enhancing Pullulan Production in Aureobasidium pullulans through UV Mutagenesis Breeding and High-Throughput Screening System. Fermentation, 10(2), 103. https://doi.org/10.3390/fermentation10020103




