Metagenomics-Based Analysis of the Effect of Rice Straw Substitution for a Proportion of Whole-Plant Corn Silage on the Rumen Flora Structure and Carbohydrate-Active Enzymes (CAZymes)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collection
2.3. Determination and Methodology of the Indicators of the Rumen Fermentation Parameters
2.4. Experimental Procedures of Metagenomic Sequencing
2.4.1. Sample Testing
2.4.2. Library Construction
2.5. Metagenomic Sequencing
2.5.1. Sequencing Result Pretreatment
2.5.2. Metagenome Assembly
2.5.3. Gene Prediction and Abundance Analysis
2.5.4. Taxonomy Prediction
2.5.5. Common Functional Database Annotations
2.6. Statistical Analysis
3. Results
3.1. Replacing Part of the Whole-Plant Corn Silage with Rice Straw Changed the Rumen Fermentation
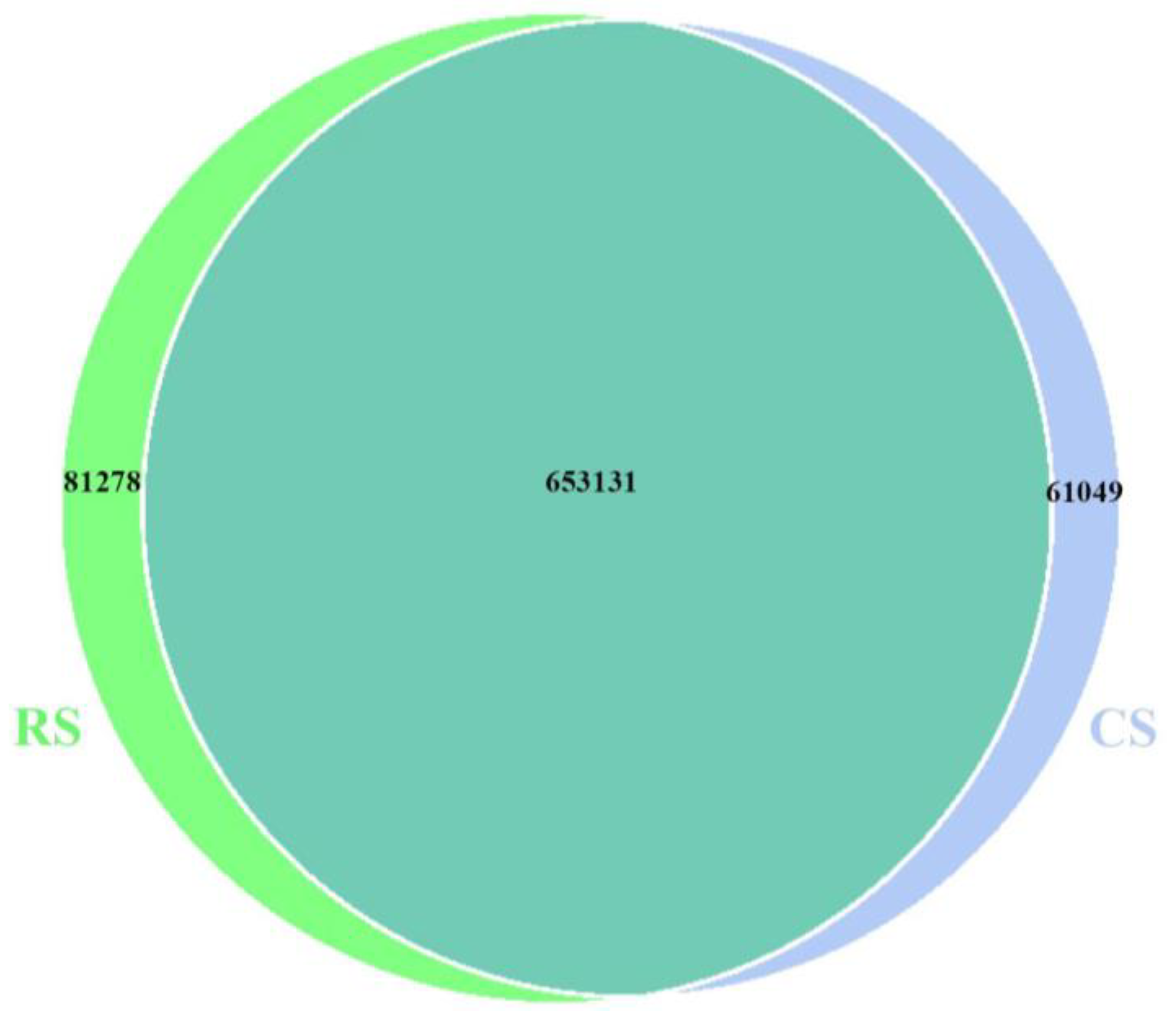
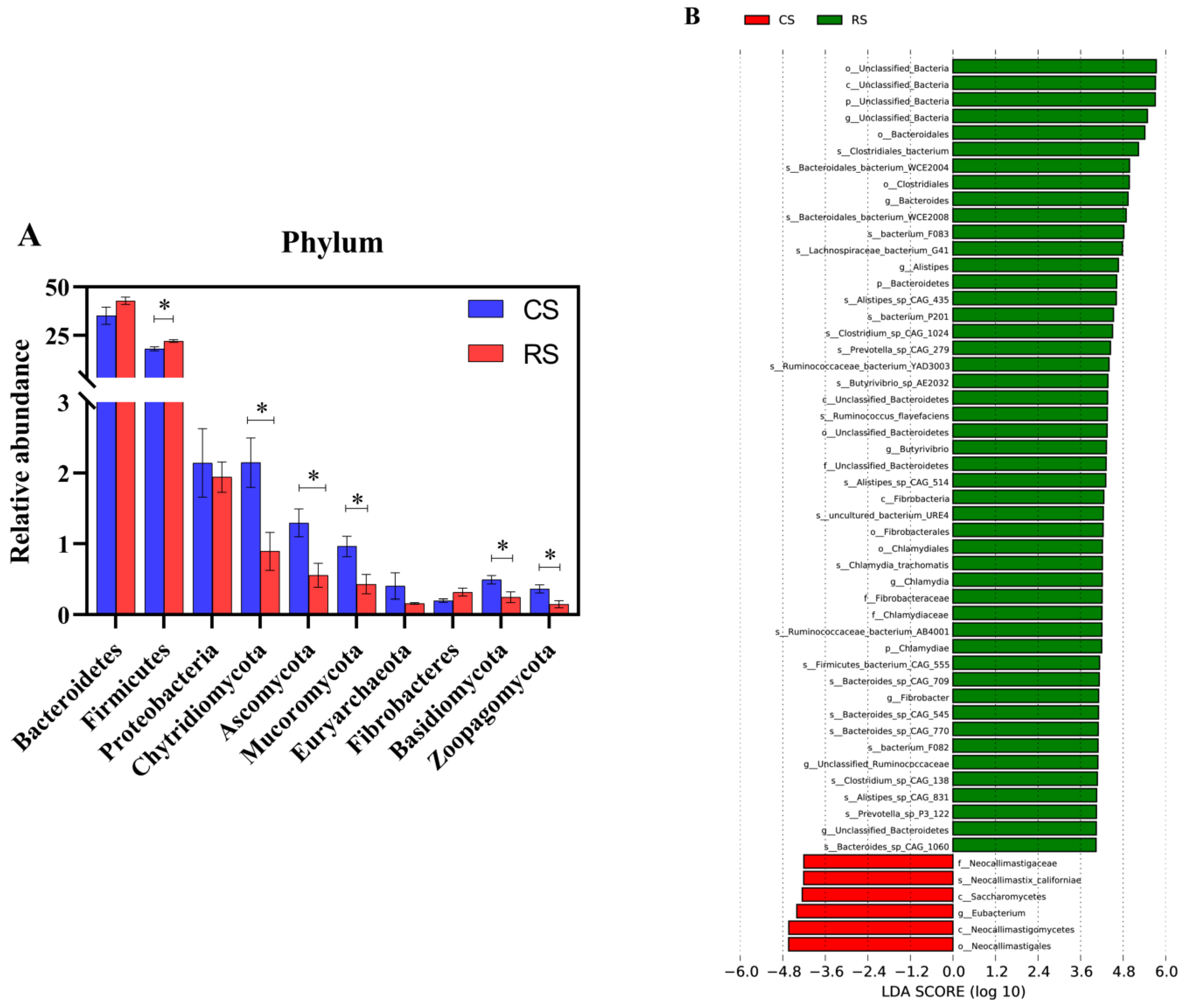
3.2. Replacing Part of the Whole-Plant Corn Silage with Rice Straw Changed the Structure and Composition of the Ruminal Bacteria
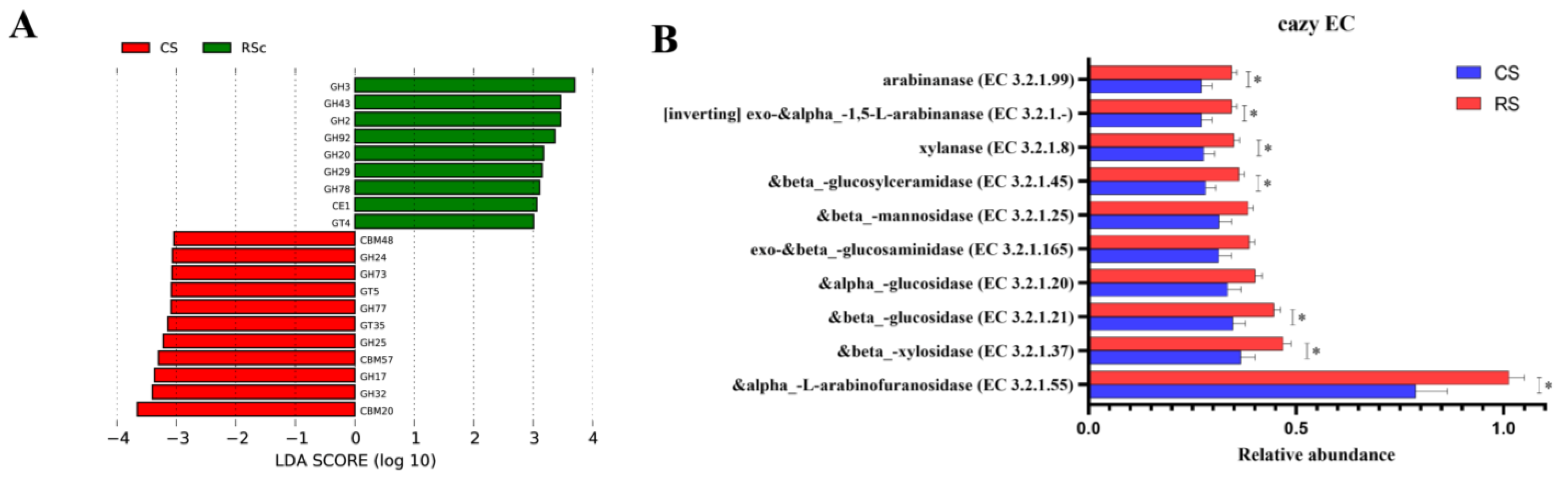
3.3. Functional Analysis of Bacteria and Fungi in the Rumen of the Rice Straw-Substituted Part of the Whole-Plant Corn Silage
3.4. Correlation Analysis of Bacteria and Fungi with CAZymes in the Rumen of the Rice Straw Substituted for Part of the Whole-Plant Corn Silage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akay, H. Grain and Straw Yield of Paddy Cultivars and Feed Quality Traits of Paddy Straw. Gesunde Pflanz. 2022, 74, 549–560. [Google Scholar] [CrossRef]
- Suretno, N.D.; Adriyani, F.Y.; Hevrizen, R. Content and potential of rice straw as a mineral source of zinc in ruminant feed. Iop Conf. Ser. Earth Environ. Sci. 2021, 653, 12022. [Google Scholar] [CrossRef]
- Peripolli, V.; Barcellos, J.O.J.; Prates, Ê.R.; McManus, C.; Silva, L.P.D.; Stella, L.A.; Costa Junior, J.B.G.; Lopes, R.B. Nutritional value of baled rice straw for ruminant feed. Rev. Bras. Zootec. 2016, 45, 392–399. [Google Scholar] [CrossRef]
- Hoerbe, J.B.; Sessim, A.G.; Pereira, G.R.; Brutti, D.D.; Oliveira, T.E.; Barcellos, J.O.J. Cow-calf intensification through the feeding of rice straw. Livest. Sci. 2020, 242, 104296. [Google Scholar] [CrossRef]
- Peripolli, V.; Barcellos, J.O.J.; Prates, Ê.R.; McManus, C.; Stella, L.A.; Camargo, C.M.; Costa Jr, J.B.G.; Bayer, C. Additives on in vitro ruminal fermentation characteristics of rice straw. Rev. Bras. Zootec. 2017, 46, 240–250. [Google Scholar] [CrossRef][Green Version]
- Ma, Y.; Chen, X.; Khan, M.Z.; Xiao, J.; Cao, Z. A Combination of Novel Microecological Agents and Molasses Role in Digestibility and Fermentation of Rice Straw by Facilitating the Ruminal Microbial Colonization. Front. Microbiol. 2022, 13, 948049. [Google Scholar] [CrossRef]
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Muñoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future. Front. Microbiol. 2018, 9, 2161. [Google Scholar] [CrossRef]
- Lobo, R.R.; Faciola, A.P. Ruminal Phages—A Review. Front. Microbiol. 2021, 12, 763416. [Google Scholar] [CrossRef]
- Liang, J.; Nabi, M.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y. Promising biological conversion of lignocellulosic biomass to renewable energy with rumen microorganisms: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 134, 110335. [Google Scholar] [CrossRef]
- Plouhinec, L.; Neugnot, V.; Lafond, M.; Berrin, J. Carbohydrate-active enzymes in animal feed. Biotechnol. Adv. 2023, 65, 108145. [Google Scholar] [CrossRef]
- Nam, N.N.; Do, H.; Loan, T.K.; Lee, N.Y. Metagenomics: An Effective Approach for Exploring Microbial Diversity and Functions. Foods 2023, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Tringe, S.G.; Rubin, E.M. Metagenomics: DNA sequencing of environmental samples. Nat. Rev. Genet. 2005, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Van Gastelen, S.; Antunes-Fernandes, E.C.; Hettinga, K.A.; Klop, G.; Alferink, S.; Hendriks, W.H.; Dijkstra, J. Enteric methane production, rumen volatile fatty acid concentrations, and milk fatty acid composition in lactating Holstein-Friesian cows fed grass silage-or corn silage-based diets. J. Dairy Sci. 2015, 98, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Mende, D.R.; Waller, A.S.; Sunagawa, S.; Jarvelin, A.I.; Chan, M.M.; Arumugam, M.; Raes, J.; Bork, P. Assessment of metagenomic assembly using simulated next generation sequencing data. PLoS ONE 2012, 7, e31386. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Almeida, M.; Juncker, A.S.; Rasmussen, S.; Li, J.; Sunagawa, S.; Plichta, D.R.; Gautier, L.; Pedersen, A.G.; Le Chatelier, E.; et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat. Biotechnol. 2014, 32, 822–828. [Google Scholar] [CrossRef]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Ocean plankton. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, J.; Degen, A.; Liu, H.; Cao, X.; Hao, L.; Shang, Z.; Ran, T.; Long, R. A comparison of average daily gain, apparent digestibilities, energy balance, rumen fermentation parameters, and serum metabolites between yaks (Bos grunniens) and Qaidam cattle (Bos taurus) consuming diets differing in energy level. Anim. Nutr. 2023, 12, 77–86. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C.; Du, M.; Yan, P.; Long, R.; Han, J.; et al. Effects of Dietary Energy Levels on Rumen Fermentation, Microbial Diversity, and Feed Efficiency of Yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lv, F.; Liu, G.; Pang, X.; Han, X.; Wang, X. Effects of starters with different NDF/starch ratio on rumen fermentation parameters and rumen microorganisms in lambs. Front. Vet. Sci. 2023, 10, 1064774. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yin, Y.; Bian, G. Effects of whole maize high-grain diet feeding on colonic fermentation and bacterial community in weaned lambs. Front. Microbiol. 2022, 13, 1018284. [Google Scholar] [CrossRef]
- Xiao, J.X.; Alugongo, G.M.; Chung, R.; Dong, S.Z.; Li, S.L.; Yoon, I.; Wu, Z.H.; Cao, Z.J. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: Ruminal fermentation, gastrointestinal morphology, and microbial community. J. Dairy Sci. 2016, 99, 5401–5412. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Chen, Y.; Liu, J.; Zhang, C.; Irving, B.; Fitzsimmons, C.; Plastow, G.; Guan, L.L. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome 2019, 7, 92. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Pope, P.B.; Denman, S.E.; Jones, M.; Tringe, S.G.; Barry, K.; Malfatti, S.A.; McHardy, A.C.; Cheng, J.F.; Hugenholtz, P.; McSweeney, C.S.; et al. Adaptation to herbivory by the Tammar wallaby includes bacterial and glycoside hydrolase profiles different from other herbivores. Proc. Natl. Acad. Sci. USA 2010, 107, 14793–14798. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.E.; Borneman, W.S. Role of Rumen Fungi in Fiber Degradation. J. Dairy Sci. 1990, 73, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Pitta, D.W.; Pinchak, W.E.; Dowd, S.E.; Osterstock, J.; Gontcharova, V.; Youn, E.; Dorton, K.; Yoon, I.; Min, B.R.; Fulford, J.D.; et al. Rumen Bacterial Diversity Dynamics Associated with Changing from Bermudagrass Hay to Grazed Winter Wheat Diets. Microb. Ecol. 2010, 59, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Solden, L.M.; Hoyt, D.W.; Collins, W.B.; Plank, J.E.; Daly, R.A.; Hildebrand, E.; Beavers, T.J.; Wolfe, R.; Nicora, C.D.; Purvine, S.O.; et al. New roles in hemicellulosic sugar fermentation for the uncultivated Bacteroidetes family BS11. ISME J. 2017, 11, 691–703. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y.; Zubair, M. Effect of substrate load on anaerobic fermentation of rice straw with rumen liquid as inoculum: Hydrolysis and acidogenesis efficiency, enzymatic activities and rumen bacterial community structure. Waste Manag. 2021, 124, 235–243. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Holman, D.B.; Gzyl, K.E. A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol. Ecol. 2019, 95, fiz072. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Xu, H.; Xin, H.; Zhang, Y. Metagenomic Analyses of Microbial and Carbohydrate-Active Enzymes in the Rumen of Holstein Cows Fed Different Forage-to-Concentrate Ratios. Front. Microbiol. 2019, 10, 649. [Google Scholar] [CrossRef]
- Mu, Y.Y.; Qi, W.P.; Zhang, T.; Zhang, J.Y.; Mao, S.Y. Gene function adjustment for carbohydrate metabolism and enrichment of rumen microbiota with antibiotic resistance genes during subacute rumen acidosis induced by a high-grain diet in lactating dairy cows. J. Dairy Sci. 2021, 104, 2087–2105. [Google Scholar] [CrossRef]
- Díaz Carrasco, J.M.; Cabral, C.; Redondo, L.M.; Pin Viso, N.D.; Colombatto, D.; Farber, M.D.; Fernández Miyakawa, M.E. Impact of Chestnut and Quebracho Tannins on Rumen Microbiota of Bovines. Biomed. Res. Int. 2017, 2017, 9610810. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.J.; Gong, J.; Teather, R.M. Group-specific 16S rRNA hybridization probes for determinative and community structure studies of Butyrivibrio fibrisolvens in the rumen. Appl. Environ. Microb. 1997, 63, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.Y.; Huo, W.J.; Zhu, W.Y. Microbiome–metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ. Microbiol. 2016, 18, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.C.; Purvis, H.T.; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; DeSilva, U. Rumen Microbial Population Dynamics during Adaptation to a High-Grain Diet. Appl. Environ. Microb. 2010, 76, 7482–7490. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.P.; Suen, G. The Phylogenomic Diversity of Herbivore-Associated Fibrobacter spp. Is Correlated to Lignocellulose-Degrading Potential. Msphere 2018, 3, 10–1128. [Google Scholar] [CrossRef]
- Azad, E.; Fehr, K.B.; Derakhshani, H.; Forster, R.; Acharya, S.; Khafipour, E.; McGeough, E.; McAllister, T.A. Interrelationships of Fiber-Associated Anaerobic Fungi and Bacterial Communities in the Rumen of Bloated Cattle Grazing Alfalfa. Microorganisms 2020, 8, 1543. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, L.; Chen, X.; Han, X.; Cao, Y.; Yao, J. Metagenomic Analyses of Microbial and Carbohydrate-Active Enzymes in the Rumen of Dairy Goats Fed Different Rumen Degradable Starch. Front. Microbiol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Bohra, V.; Dafale, N.A.; Purohit, H.J. Understanding the alteration in rumen microbiome and CAZymes profile with diet and host through comparative metagenomic approach. Arch. Microbiol. 2019, 201, 1385–1397. [Google Scholar] [CrossRef]
- Nazli, M.H.; Halim, R.A.; Abdullah, A.M.; Hussin, G.; Samsudin, A.A. Potential of feeding beef cattle with whole corn crop silage and rice straw in Malaysia. Trop. Anim. Health Prod. 2018, 50, 1119–1124. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Danu, M.; Ailiesei, G.; Nechita, M.T. An Experimental Study on the Hot Alkali Extraction of Xylan-Based Hemicelluloses from Wheat Straw and Corn Stalks and Optimization Methods. Polymers 2022, 14, 1662. [Google Scholar] [CrossRef]
- Wilkens, C.; Andersen, S.; Dumon, C.; Berrin, J.G.; Svensson, B. GH62 arabinofuranosidases: Structure, function and applications. Biotechnol. Adv. 2017, 35, 792–804. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.; Wang, X.; Wang, B.; Li, H.; Feng, J.; Wu, A. Mutagenesis of UDP-xylose epimerase and xylan arabinosyl-transferase decreases arabinose content and improves saccharification of rice straw. Plant Biotechnol. J. 2021, 19, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, T.; Lim, J.; Gu, F.; Fang, F.; Cheng, L.; Campanella, O.H.; Hamaker, B.R. Acid gelation of soluble laccase-crosslinked corn bran arabinoxylan and possible gel formation mechanism. Food Hydrocoll. 2019, 92, 1–9. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Zhao, S.; Dai, L.; Huang, S.; Liu, X.; Yu, J.; Wang, L. Functional Specificity of Three α-Arabinofuranosidases from Different Glycoside Hydrolase Families in Aspergillus niger An76. J. Agric. Food Chem. 2022, 70, 5039–5048. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Zheng, D.; Xu, Z.; Li, Y.; Shao, S.; Zhang, Y.; Ge, X.; Lu, F. Biochemical characterization of a novel GH43 family β-xylosidase from Bacillus pumilus. Food Chem. 2019, 295, 653–661. [Google Scholar] [CrossRef]
- Terrasan, C.R.F.; Guisan, J.M.; Carmona, E.C. Xylanase and β-xylosidase from Penicillium janczewskii: Purification, characterization and hydrolysis of substrates. Electron. J. Biotechnol. 2016, 23, 54–62. [Google Scholar] [CrossRef]
- Fanchini Terrasan, C.R.; Trobo-Maseda, L.; Moreno-Pérez, S.; Carmona, E.C.; Pessela, B.C.; Guisan, J.M. Co-immobilization and stabilization of xylanase, β-xylosidase and α-l-arabinofuranosidase from Penicillium janczewskii for arabinoxylan hydrolysis. Process Biochem. 2016, 51, 614–623. [Google Scholar] [CrossRef]
- Smaali, I.; Rémond, C.; O Donohue, M.J. Expression in Escherichia coli and characterization of β-xylosidases GH39 and GH-43 from Bacillus halodurans C-125. Appl. Microbiol. Biotechnol. 2006, 73, 582–590. [Google Scholar] [CrossRef]
- Santos, C.R.; Polo, C.C.; Costa, M.C.M.F.; Nascimento, A.F.Z.; Meza, A.N.; Cota, J.; Hoffmam, Z.B.; Honorato, R.V.; Oliveira, P.S.L.; Goldman, G.H.; et al. Mechanistic Strategies for Catalysis Adopted by Evolutionary Distinct Family 43 Arabinanases. J. Biol. Chem. 2014, 289, 7362–7373. [Google Scholar] [CrossRef]
- Inácio, J.M.; de Sá-Nogueira, I. Characterization of abn2 (yxiA), Encoding a Bacillus subtilis GH43 Arabinanase, Abn2, and Its Role in Arabino-Polysaccharide Degradation. J. Bacteriol. 2008, 190, 4272–4280. [Google Scholar] [CrossRef]
- Sorensen, A.; Lubeck, P.S.; Lubeck, M.; Teller, P.J.; Ahring, B.K. β-glucosidases from a new Aspergillus species can substitute commercial β-glucosidases for saccharification of lignocellulosic biomass. Can. J. Microbiol. 2011, 57, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Volkov, P.V.; Rozhkova, A.M.; Zorov, I.N.; Sinitsyn, A.P. Cloning, purification and study of recombinant GH3 family β-glucosidase from Penicillium verruculosum. Biochimie 2020, 168, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Misra, B.N.; Sangwan, N.S. β-Glucosidases from the fungus trichoderma: An efficient cellulase machinery in biotechnological applications. Biomed. Res. Int. 2013, 2013, 203735. [Google Scholar] [CrossRef] [PubMed]



| Item | CS | RS |
|---|---|---|
| NH3-N (mg/dL) | 11.67 ± 0.26 | 11.08 ± 0.15 |
| Acetate (mmol/L) | 72.28 ± 2.18 A | 67.47 + 1.4 B |
| Propionate (mmol/L) | 19.38 ± 0.44 A | 16.32 ± 0.54 B |
| Isobutyrate (mmol/L) | 0.74 ± 0.09 | 0.78 ± 0.12 |
| Butyrate (mmol/L) | 10.86 ± 0.55 A | 7.85 ± 0.55 B |
| Isovalerate (mmol/L) | 1.08 ± 0.05 | 1.01 ± 0.06 |
| Valerate (mmol/L) | 0.97 ± 0.17 | 0.71 ± 0.07 |
| TVFA (mmol/L) | 105.31 ± 2.52 A | 94.14 ± 2.46 B |
| Acetate/propionate | 3.73 ± 0.18 B | 4.13 ± 0.09 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Ye, W.; Cheng, Y.; Ren, W.; Yang, S.; Zhang, L.; Xu, X. Metagenomics-Based Analysis of the Effect of Rice Straw Substitution for a Proportion of Whole-Plant Corn Silage on the Rumen Flora Structure and Carbohydrate-Active Enzymes (CAZymes). Fermentation 2023, 9, 954. https://doi.org/10.3390/fermentation9110954
Ma Y, Ye W, Cheng Y, Ren W, Yang S, Zhang L, Xu X. Metagenomics-Based Analysis of the Effect of Rice Straw Substitution for a Proportion of Whole-Plant Corn Silage on the Rumen Flora Structure and Carbohydrate-Active Enzymes (CAZymes). Fermentation. 2023; 9(11):954. https://doi.org/10.3390/fermentation9110954
Chicago/Turabian StyleMa, Yubin, Wenxing Ye, Yuchen Cheng, Wenyi Ren, Shuangming Yang, Lili Zhang, and Xiaofeng Xu. 2023. "Metagenomics-Based Analysis of the Effect of Rice Straw Substitution for a Proportion of Whole-Plant Corn Silage on the Rumen Flora Structure and Carbohydrate-Active Enzymes (CAZymes)" Fermentation 9, no. 11: 954. https://doi.org/10.3390/fermentation9110954
APA StyleMa, Y., Ye, W., Cheng, Y., Ren, W., Yang, S., Zhang, L., & Xu, X. (2023). Metagenomics-Based Analysis of the Effect of Rice Straw Substitution for a Proportion of Whole-Plant Corn Silage on the Rumen Flora Structure and Carbohydrate-Active Enzymes (CAZymes). Fermentation, 9(11), 954. https://doi.org/10.3390/fermentation9110954




