3.2. Proximate Chemical Composition
The proximate chemical composition of PMC is shown in
Table 3. For all cheese samples, acidity significantly (
p < 0.05) increased as the storage increased. This may be due to the starters’ activity in breaking down the lactose into lactic acid in the cheese curd [
29,
30]. Furthermore, a decrease in pH could be due to fatty acids that form in varying amounts as the products of the probiotic bacteria pathway as described by Fooks et al. [
31]. The acidity (%) of PMC (control) was the lowest compared to those of other samples, and this value increased from 0.81% to 1.13% through the 28 days of storage at 5 ± 1 °C. However, the acidity (%) of PMC5 (supplemented with 1.5% WPC) was higher (
p < 0.05) than that of the other samples and increased from 0.95% to 1.34% during the storage time. It has been observed that the samples with WPC added (PMC4 and PMC5) recorded higher values of acidity than the samples with SMP added (PMC2 and PMC3). This could be because WPC is a growth-promoting substance that stimulates Bifidobacteria activity in breaking lactose into lactic acid [
32]. It has been reported that the addition of WPC in mozzarella cheese manufacturing led to higher acidity values [
33].
There was a significant (
p < 0.05) increase in TS values in all cheeses. This may be attributed to the shrinkage of the curd as a result of the formation of lactic acid, which helps to remove whey from the cheese mass [
34]. Data obtained showed that the samples with WPC added (PMC4 and PMC5) showed higher (
p < 0.05) TS values than those of other treatments and ranged from 55.88% for PMC4 at 0 days to 58.40% for PMC5 at 28 days of storage. The high TS values in these samples could be associated with their higher acidity levels, making cheese expel more whey [
35]. It has been reported that the addition of WPC to buffalo’s milk in manufacturing mozzarella cheese led to a slight increase in TS [
30].
The fat content of the PMC samples positively correlated with the TS values, meaning that the samples of PMC that gained higher TS contained higher fat content. Similar results were obtained Metzger et al. [
24]
A highly significant (p < 0.05) increase in TP% values was observed as storage time increased in all PMC samples. This could be due to moisture loss as a result of the acidity progress during storage. Although protein can break down into a soluble form in cheese by Bifidobacterium starter activity, soluble protein is still calculated as TP. Therefore, the addition of SMP or WPC in cheese led to a significant (p < 0.05) increase in TP values. However, the samples with WPC added (PMC4 and PMC5) had higher TP values than the samples with SMP added and ranged from 18.76% for PMC4 at 0 days to 22.78% for PMC5 after 28 days of storage. This was due to the high protein content in WPC (87.5%) compared with that of SMP, which contains 37.1%.
The soluble nitrogen (SN) values (
Table 3) differed significantly (
p < 0.05) between PMC samples. PMC1 (control) scored the lowest value of SN percentage and ranged from 0.24% when fresh to 0.29% after 28 days of storage. WPC and SMP addition led to a significant (
p < 0.05) increase in total protein percentage values, which allowed the starter culture to break down more protein to SN; hence, the SN percentage values significantly (
p < 0.05) increased [
36]. The samples with WPC added (PMC3 and PMC4) recorded the highest values of SN percentage (ranged from 0.27% for PMC4 when fresh to 0.32% for PMC5 after 4 weeks of storage) compared to those of the samples with SMP added (PMC2 and PMC3). It has been reported that WPC is a growth-promoting substance that encourages
Bifidobacterium in the breakdown of protein into SN [
33,
34]. The results also showed a significant increase (
p < 0.05) in the SN values of PMC throughout the 28 days, which may be due to the activities of the starter or microflora that reached the cheese during storage and/or proteolysis with a proteolytic enzyme. It has been reported that the SN of white, soft cheeses made with probiotics shows an increase at the end of the storage period [
35,
36,
37].
The ash values of the PMC samples indicated that a significant (
p < 0.05) increase occurred in ash content as storage time progressed. This increase was a response to the progressive moisture loss that occurred during storage [
38]. PMC1 (control) recorded the lowest value of ash compared to the other treatments and ranged from 3.46 to 4.42 during the 28-day storage period. Adding SMP and WPC significantly (
p < 0.05) increased the total ash content in cheese samples. However, the samples with WPC added had lower ash content values than those of the samples with SMP added, which is considered very normal due to the high ash content in SMP (6.61%) compared to WPC, which contains 2.30%.
The calcium values showed a significant reduction in the calcium content of PMC samples when SMP or WPC was added, especially PMC5 (ranging from 0.75 when fresh to 0.87 at the end of shelf life). This may be due to the high acidity levels, which were formed by the addition of WPC to cheese milk during storage. Increasing the acidity of milk causes an increase in non-micellar calcium. In cheese production, the micellar calcium of milk is retained in the cheese, while the non-micellar calcium is lost in the serum. Therefore, as a result of the increased acidity of milk, a high level of non-micellar calcium caused the observed decrease in the calcium content of the cheese [
39].
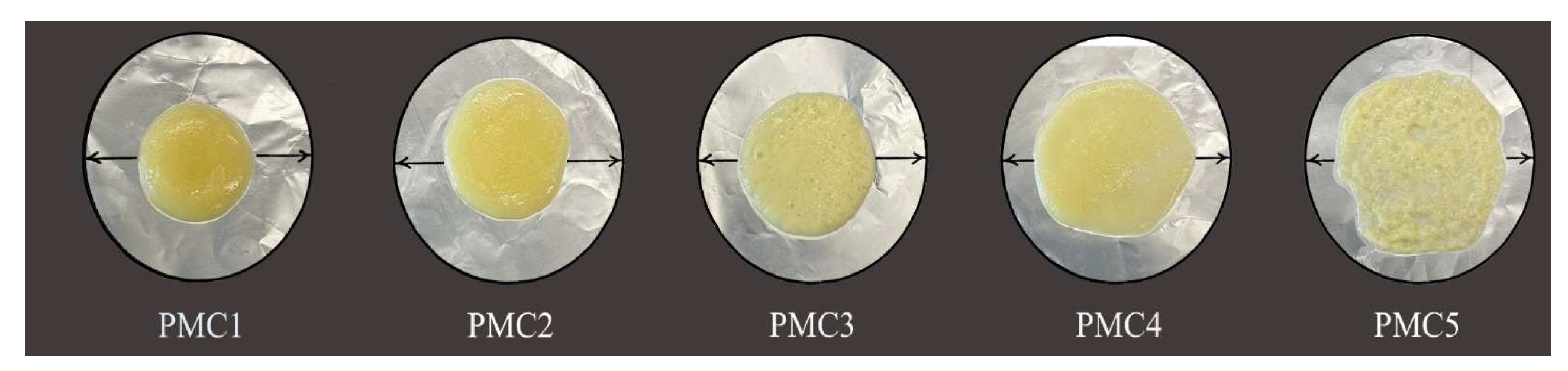
3.4. Schreiber Melt Test
Meltability is defined as the ability of cheese particles to flow together and form a continuous melted mass [
42]. Mozzarella cheese is used for pizza; therefore, as meltability increases, mozzarella cheese quality improves [
43].
Table 5 and
Figure 1 depict that as storage time progressed, the meltability of the PMC samples also increased. This could be due to proteolysis and the degradation of the milk protein matrix [
44]. PMC1 (control) recorded the lowest values of mutability compared to those of the other samples and ranged from 219.80 mm when fresh to 560.00 mm at 28 days of the storage period; this could be due to its low acidity levels in this treatment compared to those of the other samples [
45,
46]. Both SMP and WPC addition had a significant (
p < 0.05) impact on increasing the meltability of cheese. However, the samples with WPC added (PMC4 and PMC5) had higher meltability values than the samples with SMP added (PMC2 and PMC3). This could be due to the fact that WPC stimulated starter culture in breaking down more protein and raising the acidity levels, which led to an increase in soluble calcium, which is partly removed. All of these factors had a crucial role in increasing the meltability of the cheese during the test [
45,
46,
47,
48]. It has been reported that the addition of WPC has a positive effect on the meltability rates of mozzarella cheese [
49].
3.6. Sensory Characteristics
The sensory properties of PMC are shown in
Table 6. The color and appearance of all cheeses did not markedly change (
p > 0.05) by adding SMP or WPC. This difference may be because they used rennet casein in their study, but we used whole cow milk. As storage time progressed, the color points significantly (
p < 0.05) decreased in all cheese samples. This could be due to more protein breakdown by starter activity, which could negatively affect the cheese appearance [
51].
In all PMC samples, the progressive storage time significantly (
p < 0.05) improved the cheese texture. This could be because the hard casein matrix turned to a soft texture because of the slow proteolysis under the action of the endogenous enzymes, rennet enzymes, and the added starters proteolytic enzymes [
27]. The samples with WPC added (PMC4 and PMC5) had the highest points for body and texture, compared to the samples with SMP added (ranged from 24.00/35.00 point for PMC2 when fresh to 33.00/35.00 for PMC3 at the end of storage). This could be due to the low moisture content in these samples. Additionally, WPC can stimulate the growth of Bifidobacteria, which separates proteolytic enzymes during storage [
52,
53,
54,
55,
56,
57].
The flavor of all cheeses was improved over 28 d of storage at 5 ± 1 °C. This could be due to the metabolic activities of starter cultures that may produce flavor compounds like those in fermented milk. Also, rennet and indigenous enzymes are vital to producing flavor in PMC during cheese ripening [
53]. The trained panelists gave higher scores to the samples with WPC added compared to the samples with SMP added. This could be due to the role of WPC in the stimulation of the growth of Bifidobacteria, which led to more proteolysis enzymes that had an important effect on flavor [
54,
55].
The obtained data depicted that the highest scoring points (94/100) were for PMC5 for 4-week-old cheese, followed by PMC4 (91/100), then PMC3 (83/100), and lastly PMC2 (77/100).
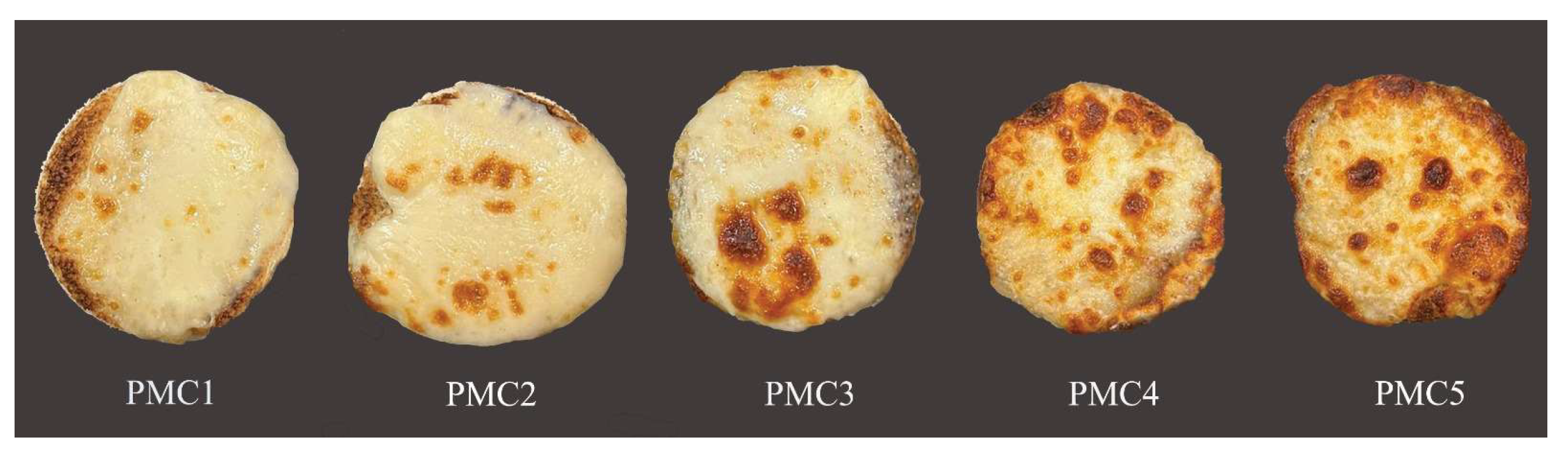
3.7. Color Characteristics of Baked Pizza
The color parameters are important because they have a major impact on the visual acceptability of consumers [
56]. The color characteristics of the PMC samples after baking on a pizza are presented in
Table 7 and
Figure 2. The obtained data showed that the lightness (L*) value of PMC1 (control) was higher (
p < 0.05) than that of the other samples. The addition of SMP and WPC led to a significant (
p < 0.05) decrease in the lightness value (more browning) and an increase in redness (+a*) and yellowness (+b*). The samples with WPC added (PMC4 and PMC5) recorded the lowest values of lightness (54.12 and 51.23, respectively) and the highest values of redness (17.49 and 18.78, respectively) and yellowness (35.33 and 38.21, respectively), compared to the samples with SMP added. The browning of PMC is caused by the Maillard reaction and is closely related to its baking. Also, browning and blistering are important quality characteristics for a pizza’s baking performance [
57,
58,
59,
60,
61]. Browning index (BI) results (
Table 7) were calculated from all color parameters of PMC. The data revealed that mature, 28-day-old samples with WPC added (PMC4 and PMC5) had greater BI (browning index) values (120.86 and 146.48, respectively) than those of the samples with SMP added (PMC2 and PMC3), which recorded BI values (65.79 and 70.03, respectively). This indicated a higher concentration of non-enzymatic browning in mature cheeses made by WPC addition, which could be due to the greater proteolytic levels seen in the samples with WPC added. Higher proteolysis may lead to a greater concentration of accessible amino groups that participate in the Maillard browning reaction [
56]. Additionally, the sugar consumed by starter culture can impact color in mozzarella [
22]. A similar observation stated that brown color in mozzarella cheese increased as a pizza baked by adding WPC during mozzarella cheese manufacturing [
62].
3.8. Bifidobacterium Count
The colonies of
Bifidobacteria bifidum (BB) of PMC during 28 days of storage are illustrated in
Table 8. The
Bifidobacteria bifidum (BB) count significantly (
p < 0.05) increased up to 14 days, followed by a decrease at the end of the storage period. The gradual decrease in BB counts was attributed to high acidity and the forming of lactic and acetic acids, which reduced the viability of BB counts [
58,
59]. Similar observations stated that the numbers of
Bifidobacterium decreased slowly after the first 2 weeks, followed by a sharper decrease towards the end of the ripening period of soft cheese [
61]. PMC1 (control) recorded the lowest number of BB compared to those of other samples during 28 days of storage and decreased from 6.45 cfu/g when fresh to 5.42 cfu/g at the end of storage. The addition of WPC had the positive effect of increasing the numbers of BB. This positive effect occurred by adding SMP but with a lower rate than WPC addition. For instance, PMC5, which contained 1.5% WPC, recorded the highest number of BB compared to PMC3, which contained 1.5% SMP; this could be due to the effect of whey protein in stimulating the growth of BB [
62]. After 28 days of storage, it was observed that the numbers of BB were >6 log cfu/g in the samples with WPC added (PMC4 and PMC5) compared to the samples with SMP added (PMC2 and PMC3), which recorded 5.78 and 5.94, respectively, during the same storage period. It has been reported that for
Bifidobacterium to exert their beneficial effects on the host, they must be able to survive and reach the GI tract in sufficient numbers, at least 10
6 cfu/g [
63].