Using Deep Ocean Water in the Fermentation of Antrodia cinnamomea to Boost Magnesium Ion Bioabsorption and Anti-Inflammatory Effects in the Brain of an Alzheimer’s Disease Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. The Source of DOW
2.3. Plate Culture Method of Antrodia cinnamomea Mycelium
2.4. Seed Culture of Liquid Fermentation for Antrodia cinnamomea Mycelium
2.5. Solid-State Fermentation of Antrodia cinnamomea Mycelium
2.6. Extraction and Analysis Method of Active Components of Antrodia cinnamomea Mycelium
2.7. Magnesium Content Determination Method
2.8. β-1,3-Glucan Analysis Method
2.9. Animal Experiment Grouping and Dose
2.10. Surgical Operation for Establishing Alzheimer’s Disease Model Rats
2.11. Water Maze Test
2.12. Animal Sacrifice and Brain Tissue Processing
2.13. Protein Content Analysis
2.14. ELISA Analysis of Target Protein
2.15. Immunohistochemical Stain of RAGE in Hippocampus
2.16. Statistical Analysis
3. Results
3.1. Growth Variations of A. cinnamomea NTTU 206 Solid-State Fermented Mycelia Cultured in DOW and UPW
3.2. Effects of Adding DOW and MgCl2 on the Functional Components Produced by Fermentation of A. cinnamomea NTTU 206
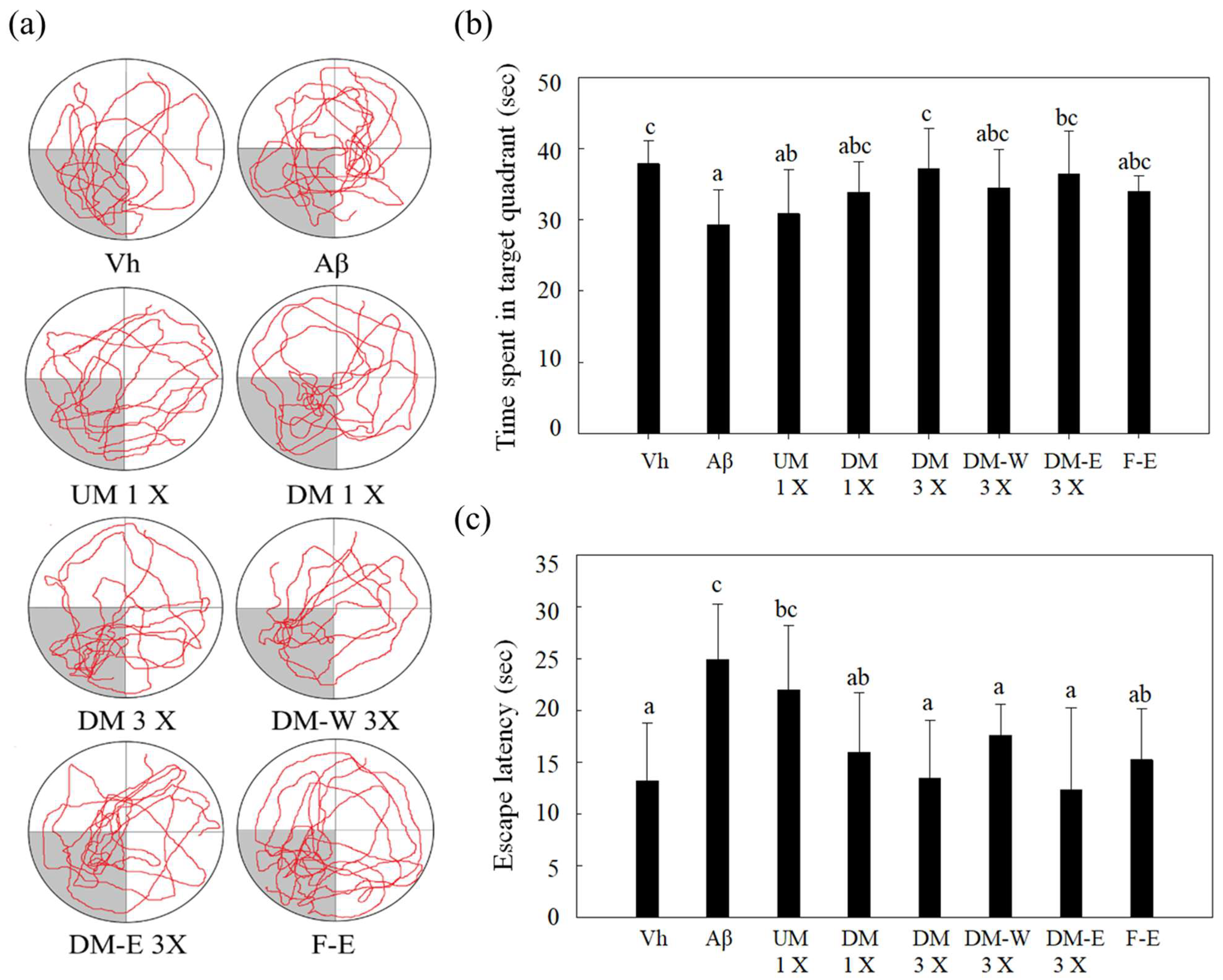
3.3. Effects of A. cinnamomea NTTU 206 Solid-State Fermented Mycelia on Brain-Infused Aβ40 Rats on Memory Test and Spatial Learning Ability
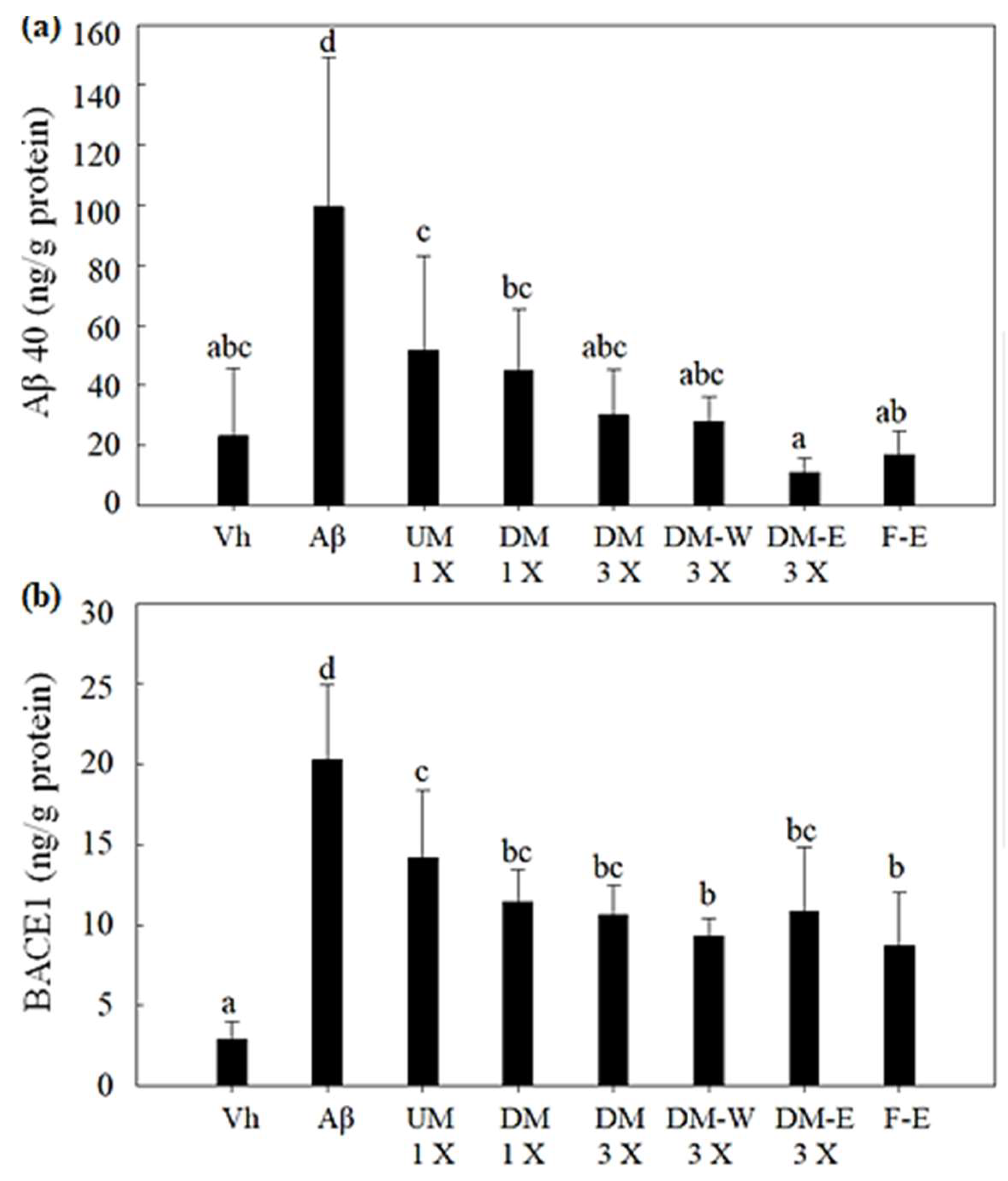
3.4. Effects of A. cinnamomea NTTU 206 Solid-State Fermentation Mycelia on the Expression of Aβ40 and BACE1 Proteins in the Hippocampus of Rats with Brain Infusion of Aβ40
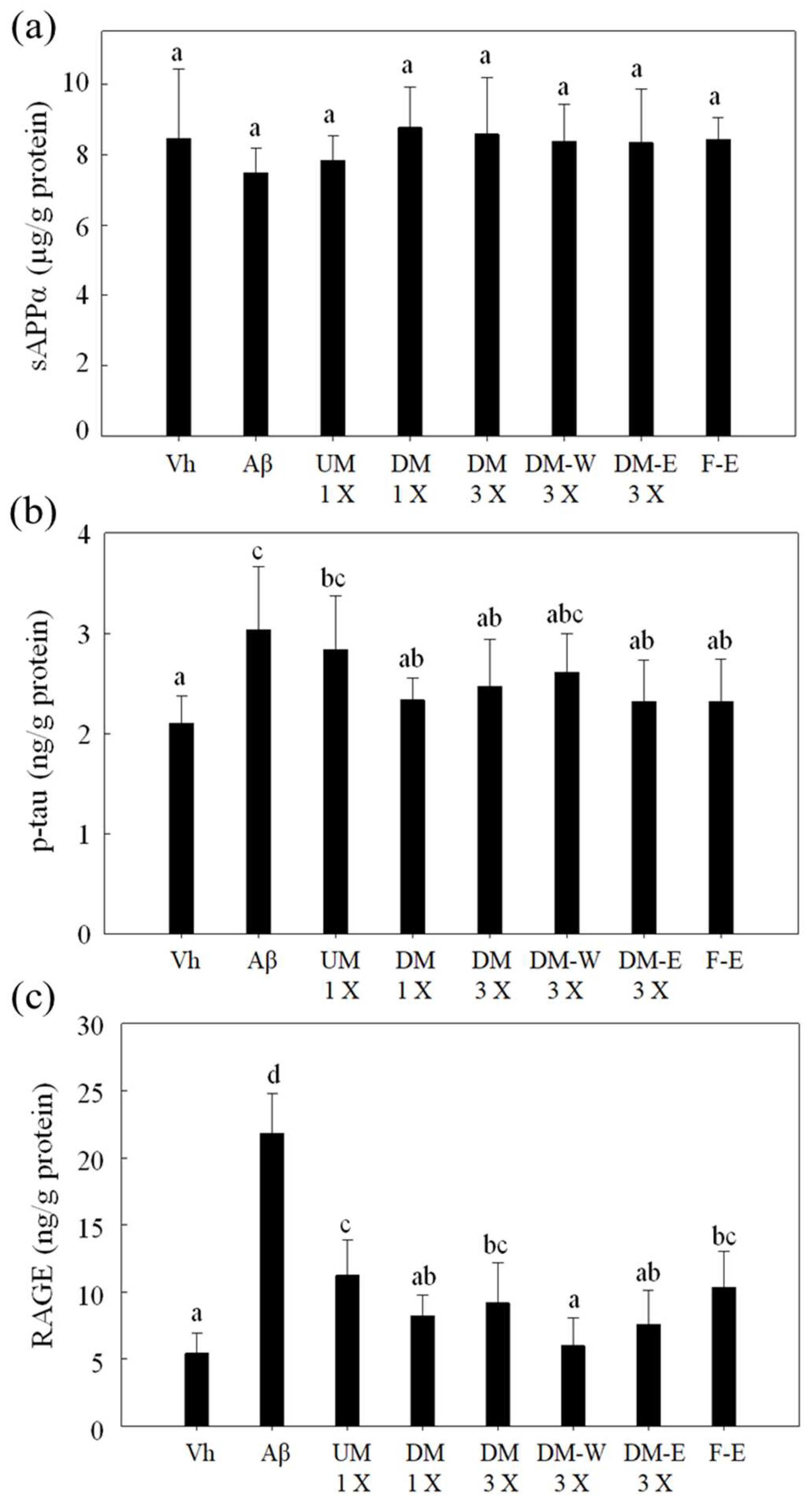
3.5. Effects of A. cinnamomea NTTU 206 Solid-State Fermented Mycelia on the Expression of sAPPα, p-tau, and RAGE Proteins in the Hippocampus of Rats with Brain Infusion of Aβ40
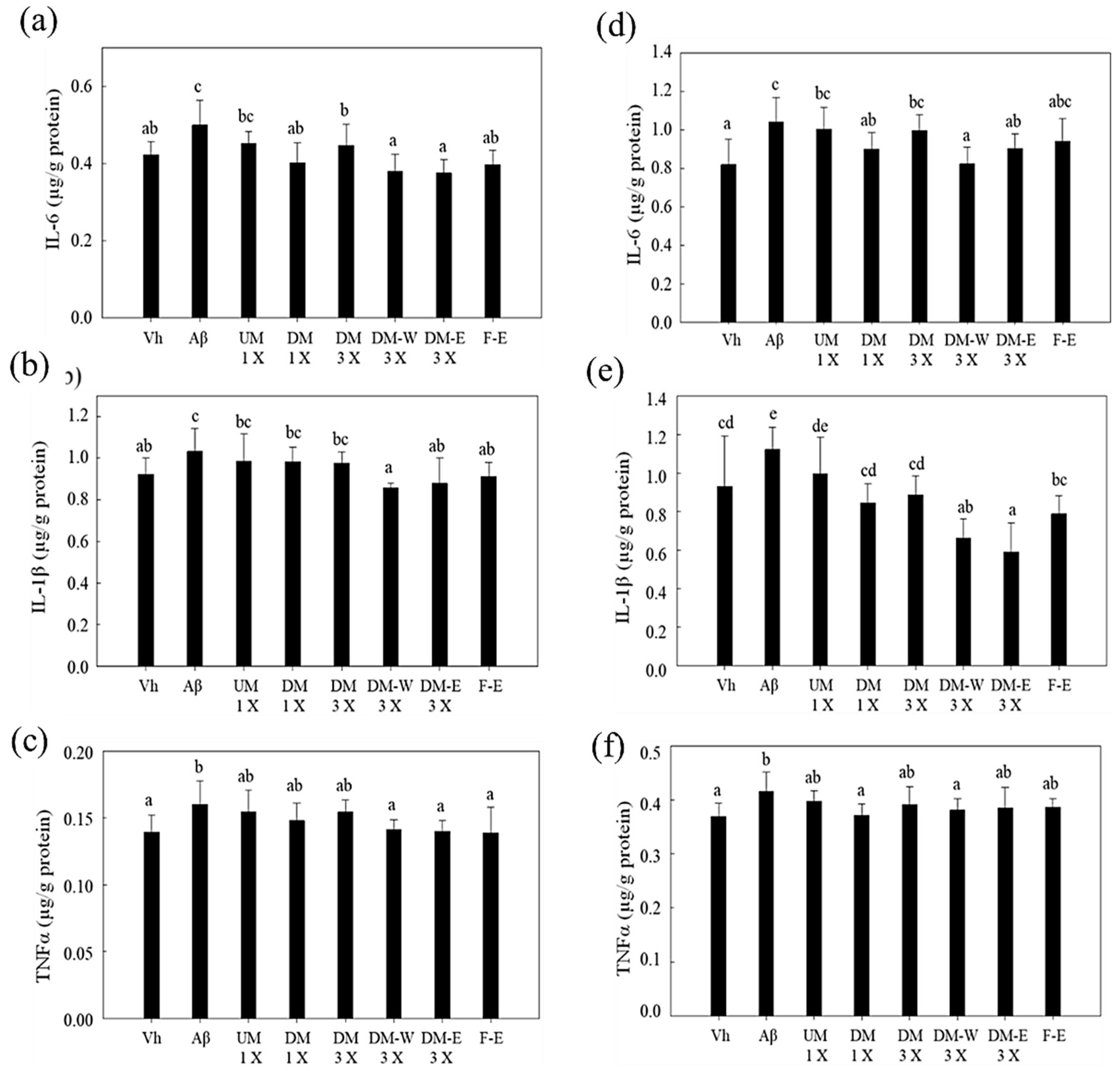
3.6. Effects of A. cinnamomea NTTU 206 Solid-State Fermentation Mycelium on the Expression of IL-6, IL-1β, TNF-α Proteins in the Hippocampus of Rats with Brain Infusion of Aβ40
3.7. Effects of A. cinnamomea NTTU 206 Solid-State Fermentation Mycelia on the Expressions of IL-6, IL-1β, and TNF-α Proteins in the Cerebral Cortex of Rats with Brain Infusion of Aβ40
3.8. Effect of A. cinnamomea NTTU 206 Solid-State Fermented Mycelium on Magnesium Uptake in the Hippocampus of Rats with Brain Infusion of Aβ40
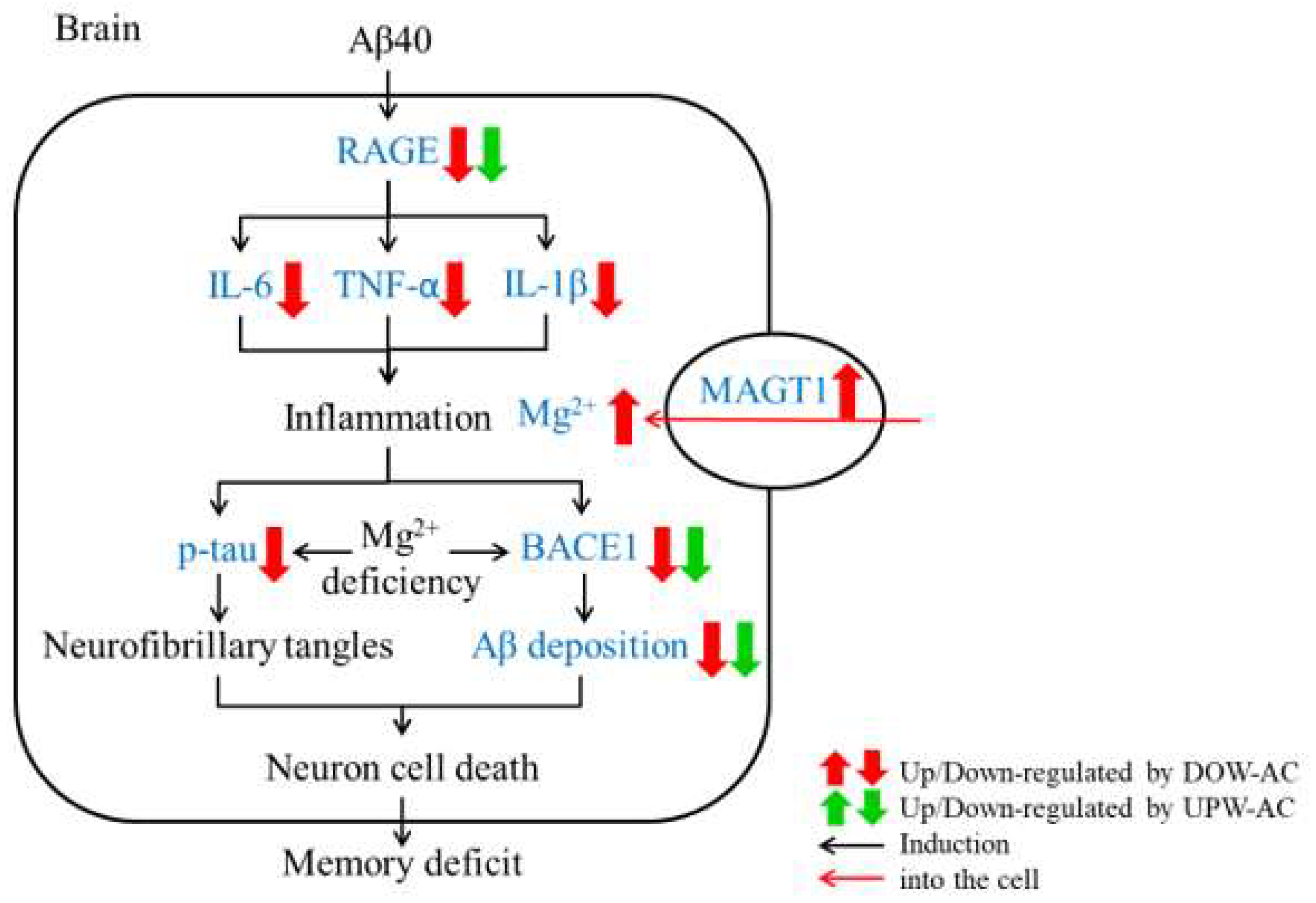
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Simic, G. Pathological tau proteins in argyrophilic grain disease. Lancet Neurol. 2002, 1, 276. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.C.; Esiri, M.M.; Hiorns, R.W.; Wilcock, G.K.; Powell, T.P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1985, 82, 4531–4534. [Google Scholar] [CrossRef] [PubMed]
- Uylings, H.B.; de Brabander, J.M. Neuronal changes in normal human aging and Alzheimer’s disease. Brain Cogn. 2002, 49, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Koo, E.H. Biology and pathophysiology of the amyloid precursor protein. Mol. Neurodegener. 2011, 6, 27. [Google Scholar] [CrossRef]
- Lee, D.C.; Rizer, J.; Hunt, J.B.; Selenica, M.L.; Gordon, M.N.; Morgan, D. Review: Experimental manipulations of microglia in mouse models of Alzheimer’s pathology: Activation reduces amyloid but hastens tau pathology. Neuropathol. Appl. Neurobiol. 2013, 39, 69–85. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, S.; Lee, D.Y.; Lee, C. Increasing anti-Abeta-induced neurotoxicity ability of Antrodia camphorata-fermented product with deep ocean water supplementary. J. Sci. Food Agric. 2016, 96, 4690–4701. [Google Scholar] [CrossRef]
- Wang, L.C.; Kuo, I.U.; Tsai, T.Y.; Lee, C.L. Antrodia camphorata-fermented product cultured in deep ocean water has more liver protection against thioacetamide-induced fibrosis. Appl. Microbiol. Biotechnol. 2013, 97, 9955–9967. [Google Scholar] [CrossRef]
- Li, H.X.; Wang, J.J.; Lu, C.L.; Gao, Y.J.; Gao, L.; Yang, Z.Q. Review of Bioactivity, Isolation, and Identification of Active Compounds from Antrodia cinnamomea. Bioengineering 2022, 9, 494. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, S.E.; Wang, J.J.; Tsai, T.Y.; Lin, C.H.; Pan, T.M.; Lee, C.L. In vitro and in vivo comparisons of the effects of the fruiting body and mycelium of Antrodia camphorata against amyloid beta-protein-induced neurotoxicity and memory impairment. Appl. Microbiol. Biotechnol. 2012, 94, 1505–1519. [Google Scholar] [CrossRef]
- Cao, Y.N.; Yue, S.S.; Wang, A.Y.; Xu, L.; Hu, Y.T.; Qiao, X.; Wu, T.Y.; Ye, M.; Wu, Y.C.; Qi, R. Antrodia cinnamomea and its compound dehydroeburicoic acid attenuate nonalcoholic fatty liver disease by upregulating ALDH2 activity. J. Ethnopharmacol. 2022, 292, 115146. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.K.; Lee, M.H.; Chao, C.H.; Hsu, Y.C. Physiochemical changes and mechanisms of anti-inflammation effect of sulfated polysaccharides from ammonium sulfate feeding of Antrodia cinnamomea. Int. J. Biol. Macromol. 2020, 148, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, A.; Qu, Y.; Wang, Z.; Zhang, Y.; Liu, Y.; Wang, N.; Teng, L.; Wang, D. Ameliorative effects of Antrodia cinnamomea polysaccharides against cyclophosphamide-induced immunosuppression related to Nrf2/HO-1 signaling in BALB/c mice. Int. J. Biol. Macromol. 2018, 116, 8–15. [Google Scholar] [CrossRef]
- Wang, H.C.; Chu, F.H.; Chien, S.C.; Liao, J.W.; Hsieh, H.W.; Li, W.H.; Lin, C.C.; Shaw, J.F.; Kuo, Y.H.; Wang, S.Y. Establishment of the metabolite profile for an Antrodia cinnamomea health food product and investigation of its chemoprevention activity. J. Agric. Food Chem. 2013, 61, 8556–8564. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lee, C.L. Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients 2021, 13, 1732. [Google Scholar] [CrossRef]
- Sheu, M.J.; Chou, P.Y.; Lin, W.H.; Pan, C.H.; Chien, Y.C.; Chung, Y.L.; Liu, F.C.; Wu, C.H. Deep sea water modulates blood pressure and exhibits hypolipidemic effects via the AMPK-ACC pathway: An in vivo study. Mar. Drugs 2013, 11, 2183–2202. [Google Scholar] [CrossRef]
- Chang, W.T.; Lu, T.Y.; Cheng, M.C.; Lu, H.C.; Wu, M.F.; Hsu, C.L. Deep Sea Water Improves Abnormalities in Lipid Metabolism through Lipolysis and Fatty Acid Oxidation in High-Fat Diet-Induced Obese Rats. Mar. Drugs 2017, 15, 386. [Google Scholar] [CrossRef]
- Chang, C.Y.; Yang, P.X.; Yu, T.L.; Lee, C.L. Cordyceps cicadae NTTU 868 Mycelia Fermented with Deep Ocean Water Minerals Prevents D-Galactose-Induced Memory Deficits by Inhibiting Oxidative Inflammatory Factors and Aging-Related Risk Factors. Nutrients 2023, 15, 1968. [Google Scholar] [CrossRef]
- Geethangili, M.; Tzeng, Y.M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid. Based Complement. Alternat. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef]
- Jiang, H.; Hampel, H.; Prvulovic, D.; Wallin, A.; Blennow, K.; Li, R.; Shen, Y. Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 69. [Google Scholar] [CrossRef]
- Lin, E.S.; Chen, Y.H. Factors affecting mycelial biomass and exopolysaccharide production in submerged cultivation of Antrodia cinnamomea using complex media. Bioresour. Technol. 2007, 98, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Shedletzky, E.; Unger, C.; Delmer, D.P. A microtiter-based fluorescence assay for (1,3)-beta-glucan synthases. Anal. Biochem. 1997, 249, 88–93. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: The New Coronal Set; Elsevier Academic: London, UK, 2005. [Google Scholar]
- Yamaguchi, Y.; Miyashita, H.; Tsunekawa, H.; Mouri, A.; Kim, H.C.; Saito, K.; Matsuno, T.; Kawashima, S.; Nabeshima, T. Effects of a novel cognitive enhancer, spiro[imidazo-[1,2-a]pyridine-3,2-indan]-2(3H)-one (ZSET1446), on learning impairments induced by amyloid-beta1-40 in the rat. J. Pharmacol. Exp. Ther. 2006, 317, 1079–1087. [Google Scholar] [CrossRef]
- Lee, C.L.; Kuo, T.F.; Wang, J.J.; Pan, T.M. Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid beta-infused rat by repressing amyloid beta accumulation. J. Neurosci. Res. 2007, 85, 3171–3182. [Google Scholar] [CrossRef]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Cheung, B.S.; Hyman, B.T.; Irizarry, M.C. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002, 59, 1381–1389. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Arancio, O.; Zhang, H.P.; Chen, X.; Lin, C.; Trinchese, F.; Puzzo, D.; Liu, S.; Hegde, A.; Yan, S.F.; Stern, A.; et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004, 23, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Tarkowski, E.; Issa, R.; Sjogren, M.; Wallin, A.; Blennow, K.; Tarkowski, A.; Kumar, P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol. Aging 2002, 23, 237–243. [Google Scholar] [CrossRef]
- Blum-Degen, D.; Muller, T.; Kuhn, W.; Gerlach, M.; Przuntek, H.; Riederer, P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 1995, 202, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Mrak, R.E.; Griffin, W.S. Potential inflammatory biomarkers in Alzheimer’s disease. J. Alzheimers Dis. 2005, 8, 369–375. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Lee, C.L. Cordyceps cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain. Fermentation 2021, 7, 39. [Google Scholar]
- Zhu, D.; Su, Y.; Fu, B.; Xu, H. Magnesium Reduces Blood-Brain Barrier Permeability and Regulates Amyloid-beta Transcytosis. Mol. Neurobiol. 2018, 55, 7118–7131. [Google Scholar] [CrossRef]
- Lee, C.L. The advantages of deep ocean water for the development of functional fermentation food. Appl. Microbiol. Biotechnol. 2015, 99, 2523–2531. [Google Scholar] [CrossRef]
- Matsumoto, A.; Itoh, K.; Seki, T.; Motozaki, K.; Matsuyama, S. Human brain carboxypeptidase B, which cleaves beta-amyloid peptides in vitro, is expressed in the endoplasmic reticulum of neurons. Eur. J. Neurosci. 2001, 13, 1653–1657. [Google Scholar] [CrossRef]
- Eckman, E.A.; Watson, M.; Marlow, L.; Sambamurti, K.; Eckman, C.B. Alzheimer’s disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J. Biol. Chem. 2003, 278, 2081–2084. [Google Scholar] [CrossRef] [PubMed]
- Ayyalasomayajula, N.; Suresh, C. Mechanistic comparison of current pharmacological treatments and novel phytochemicals to target amyloid peptides in Alzheimer’s and neurodegenerative diseases. Nutr. Neurosci. 2018, 21, 682–694. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Iwatsubo, T.; Wolfe, M.S. Presenilins and gamma-secretase: Structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006304. [Google Scholar] [CrossRef]
- Golde, T.E.; Estus, S.; Younkin, L.H.; Selkoe, D.J.; Younkin, S.G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science 1992, 255, 728–730. [Google Scholar] [CrossRef]
- Vetrivel, K.S.; Meckler, X.; Chen, Y.; Nguyen, P.D.; Seidah, N.G.; Vassar, R.; Wong, P.C.; Fukata, M.; Kounnas, M.Z.; Thinakaran, G. Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J. Biol. Chem. 2009, 284, 3793–3803. [Google Scholar] [CrossRef] [PubMed]
- Pant, M.K.; Amin, N.D.; Amin, N.; Pant, H.C. Phosphorylation Activity in the Alzheimer’s Disease and Normal Brain is Modulated by Microtubule-Associated Protein, Tau in Vitro. J. Alzheimers Dis. 1999, 1, 169–182. [Google Scholar] [CrossRef]
- Meraz-Rios, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernandez, J.; Campos-Pena, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Wu, Z.; Zlokovic, B.V. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke 2004, 35, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.C.; Tung, Y.T.; Kuo, Y.H.; Liao, J.W.; Tsai, H.C.; Chong, K.Y.; Chen, H.L.; Chen, C.M. Anti-inflammatory effects of Antrodia camphorata, a herbal medicine, in a mouse skin ischemia model. J. Ethnopharmacol. 2015, 159, 113–121. [Google Scholar] [CrossRef]
- Chang, C.H.; Hsu, C.C.; Lee, A.S.; Wang, S.W.; Lin, K.T.; Chang, W.L.; Peng, H.C.; Huang, W.C.; Chung, C.H. 4-Acetylantroquinonol B inhibits lipopolysaccharide-induced cytokine release and alleviates sepsis through of MAPK and NFkappaB suppression. BMC Complement. Altern. Med. 2018, 18, 108. [Google Scholar] [CrossRef]
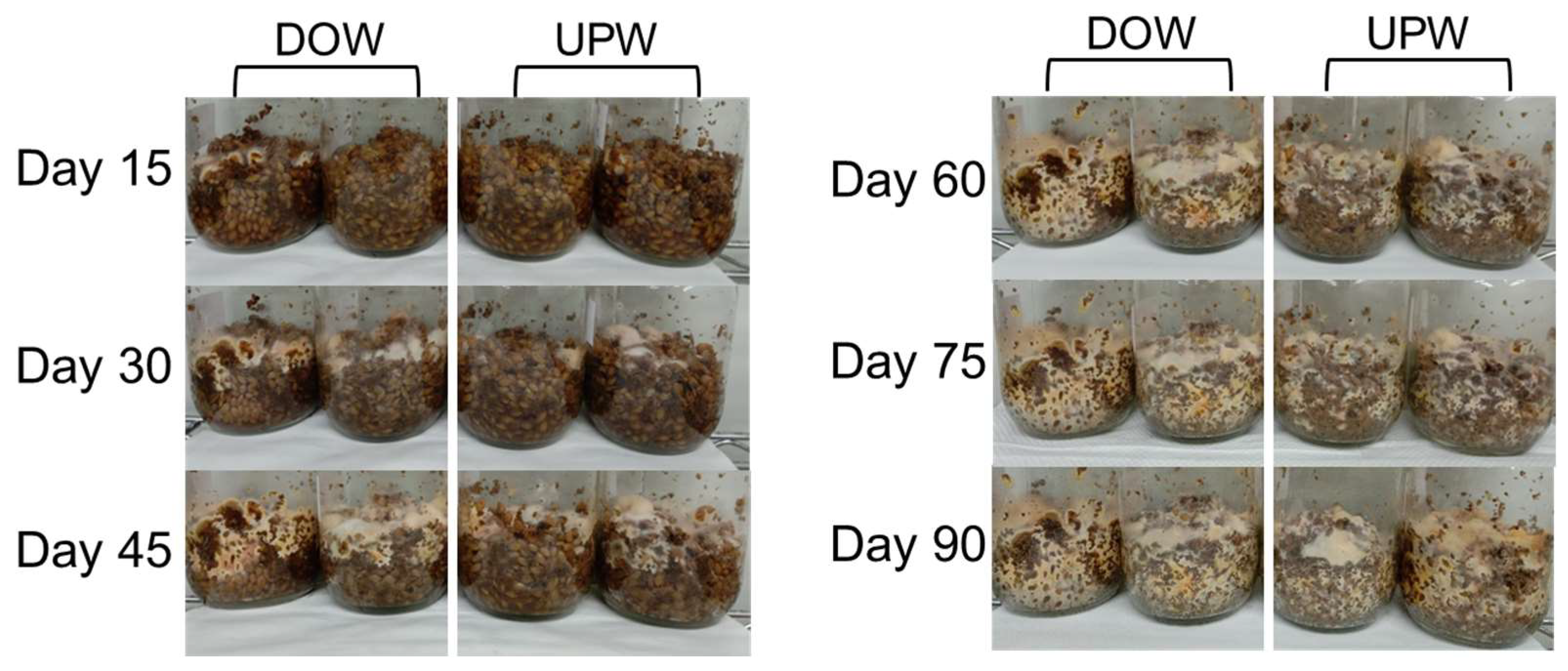




| Culture Water | 4-AAQB (mg/Plate) | DSA (mg/Plate) | DEA (mg/Plate) |
|---|---|---|---|
| UPW | 0.27 ± 0.03 b | 48.38 ± 0.81 a | 44.17 ± 2.14 a |
| DOW | 0.16 ± 0.03 a | 82.57 ± 4.99 c | 98.96 ± 13.36 c |
| MgCl2 | 0.36 ± 0.06 c | 68.55 ± 3.93 b | 68.20 ± 8.20 b |
| Culture Water | 4-AAQB (mg/kg) | DSA (mg/kg) | DEA (mg/kg) | β-1,3-Glucan@@@(mg/kg) |
|---|---|---|---|---|
| UPW | 621 ± 54 a | 3294 ± 1525 a | 1231 ± 624 a | 5495 ± 1215 a |
| DOW | 719 ± 114 a | 7184 ± 1071 b | 2874 ± 631 b | 5874 ± 501 a |
| MgCl2 | 642 ± 24 a | 5260 ± 150 ab | 2137 ± 213 ab | 5388 ± 517 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.-Y.; Tzeng, D.-Y.; Lee, C.-L. Using Deep Ocean Water in the Fermentation of Antrodia cinnamomea to Boost Magnesium Ion Bioabsorption and Anti-Inflammatory Effects in the Brain of an Alzheimer’s Disease Rat Model. Fermentation 2023, 9, 893. https://doi.org/10.3390/fermentation9100893
Xu T-Y, Tzeng D-Y, Lee C-L. Using Deep Ocean Water in the Fermentation of Antrodia cinnamomea to Boost Magnesium Ion Bioabsorption and Anti-Inflammatory Effects in the Brain of an Alzheimer’s Disease Rat Model. Fermentation. 2023; 9(10):893. https://doi.org/10.3390/fermentation9100893
Chicago/Turabian StyleXu, Ting-Yu, De-Yu Tzeng, and Chun-Lin Lee. 2023. "Using Deep Ocean Water in the Fermentation of Antrodia cinnamomea to Boost Magnesium Ion Bioabsorption and Anti-Inflammatory Effects in the Brain of an Alzheimer’s Disease Rat Model" Fermentation 9, no. 10: 893. https://doi.org/10.3390/fermentation9100893
APA StyleXu, T.-Y., Tzeng, D.-Y., & Lee, C.-L. (2023). Using Deep Ocean Water in the Fermentation of Antrodia cinnamomea to Boost Magnesium Ion Bioabsorption and Anti-Inflammatory Effects in the Brain of an Alzheimer’s Disease Rat Model. Fermentation, 9(10), 893. https://doi.org/10.3390/fermentation9100893







