Beetroot Stalk Extract as a Functional Colorant for Stirred Yogurt Beverages: Effect on Nutritional Value and Stability during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Beetroot Stalk Water Extract (BSE)
2.3. Biological Characterization of BSE
2.3.1. Determination of Total Phenolics
2.3.2. Determination of Total Flavonoids
2.3.3. HPLC Profile
2.3.4. Betalain Content
2.3.5. Antioxidant Activity
DPPH• Scavenging Activity
ABTS• Scavenging Activity
2.3.6. Antimicrobial Effect of BSE
2.4. Stability of Betalains at pH 4–5
2.5. Preparation of Raspberry Flavor and Stirred Yogurt
2.6. Sensory Evaluation
2.7. Physical Properties of Yogurt
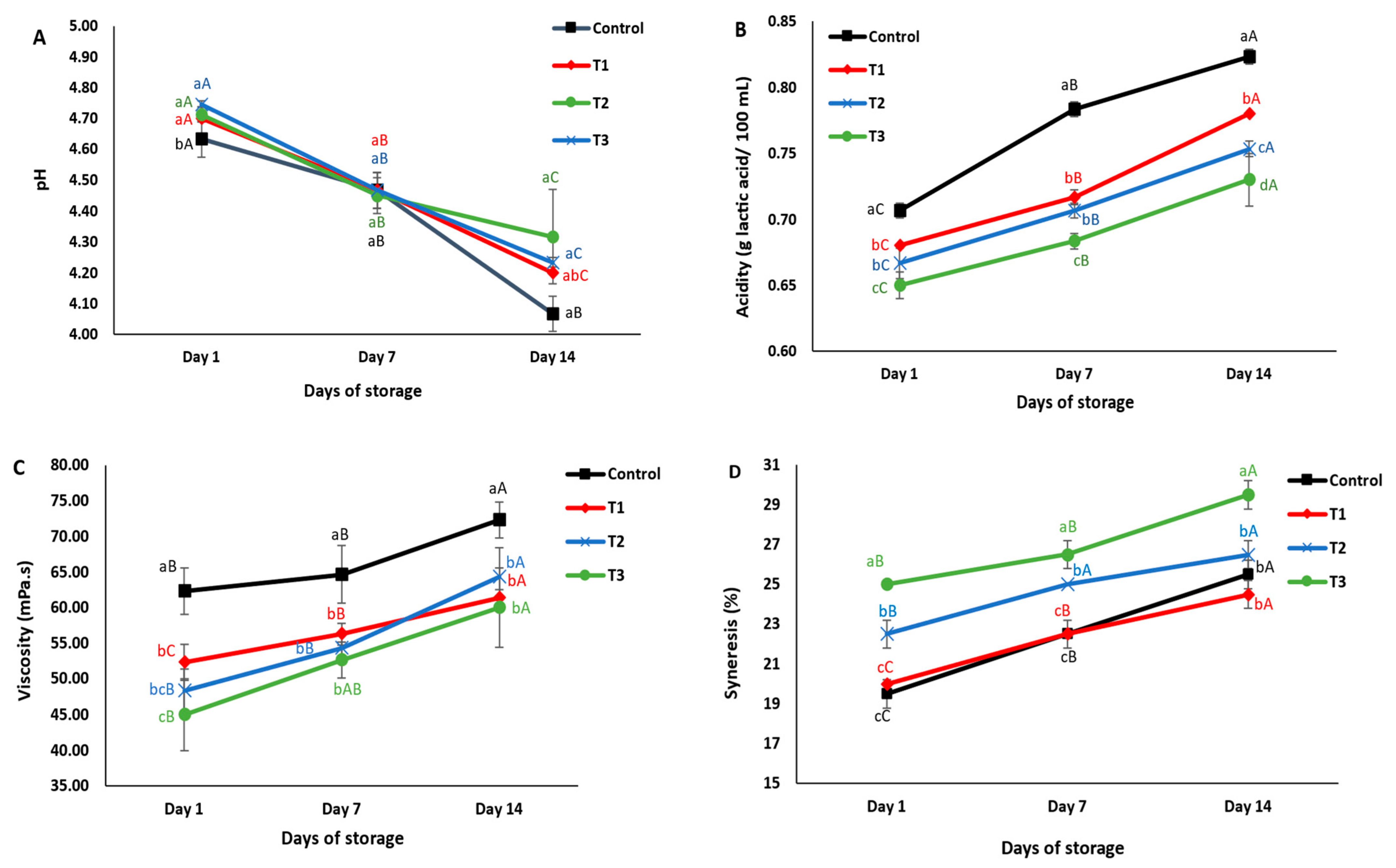
2.7.1. pH and Acidity
2.7.2. Viscosity
2.7.3. Syneresis
2.7.4. Color
2.8. Bioactive Components, Betalains, and Antioxidant Activity of Fortified Yogurt
2.9. Viability of Lactic Acid Bacteria (LAB)
2.10. Shelf Life and Microbial Load of Yogurt
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Beetroot Stalk Extract (BSE)
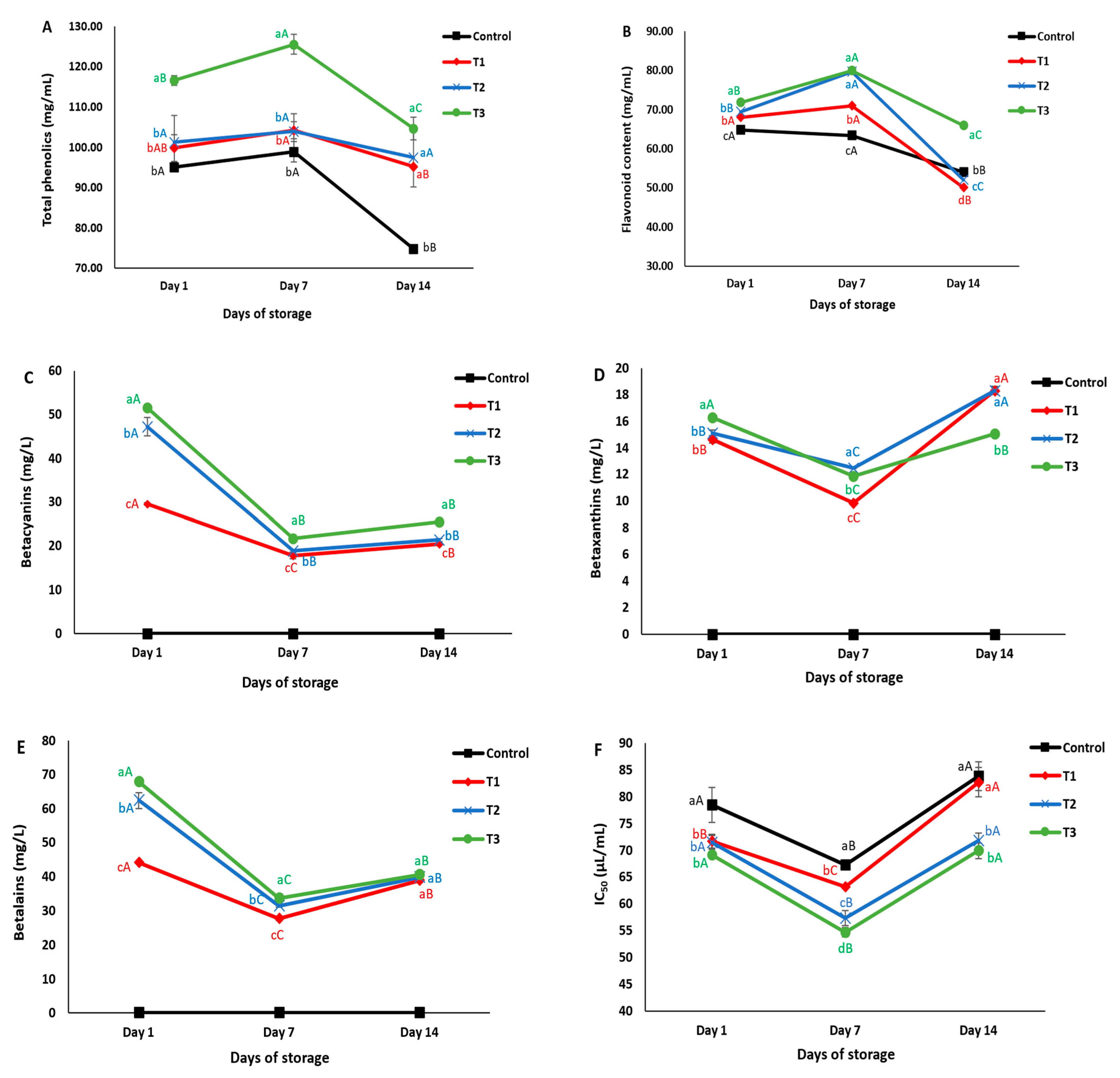
3.1.1. Phenolic and Flavonoid Content
3.1.2. Phenolics Profile of BSE
3.1.3. Betalain Content
3.1.4. Antioxidant Activity
3.1.5. Antimicrobial Activity
3.2. Stability of Betalains at pH 4–5
3.3. Sensory Evaluation
3.4. Physicochemical Properties
3.4.1. pH and Acidity
3.4.2. Viscosity
3.4.3. Syneresis
3.4.4. Color
3.5. Bioactive Components of Yogurt and Their Stability during Storage
3.5.1. Total Phenolic and Flavonoid Content
3.5.2. Betalain Content
3.5.3. Antioxidant Activity
3.6. LAB Viability
3.7. Microbial Load of Yogurt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mousavi, M.; Heshmati, A.; Garmakhany, A.D.; Vahidinia, A.; Taheri, M. Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed-enriched stirred probiotic yogurt by using response surface methodology. LWT 2019, 102, 80–88. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Effect of encapsulated beet extracts (Beta vulgaris) added to yogurt on the physicochemical characteristics and antioxidant activity. Molecules 2021, 26, 4768. [Google Scholar] [CrossRef] [PubMed]
- AdebayoTayo, B.; Akpeji, S. Probiotic Viability, Physicochemical and Sensory Properties of Probiotic Pineapple Juice. Fermentation 2016, 2, 20. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. The Effect of Refrigerated Storage on Anti-Diabetic and Antioxidant Potency of Probiotic Yogurt Treated with Some Medicinal Plants. Fermentation 2023, 9, 427. [Google Scholar] [CrossRef]
- Aleman, R.S.; Marcia, J.; Page, R.; Kazemzadeh Pournaki, S.; Martín-Vertedor, D.; Manrique-Fernández, V.; Montero-Fernández, I.; Aryana, K. Effects of Yogurt with Carao (Cassia grandis) on Intestinal Barrier Dysfunction, α-glycosidase Activity, Lipase Activity, Hypoglycemic Effect, and Antioxidant Activity. Fermentation 2023, 9, 566. [Google Scholar] [CrossRef]
- Stuivenberg, G.A.; Chmiel, J.A.; Akouris, P.P.; White, J.; Wilcox, H.; Seney, S.; Burton, J.P.; Reid, G. Supplementing Yogurt with Probiotic Bifidobacteria to Counter Chronic Kidney Disease. Fermentation 2023, 9, 391. [Google Scholar] [CrossRef]
- Echegaray, N.; Guzel, N.; Kumar, M.; Guzel, M.; Hassoun, A.; Lorenzo, J.M. Recent advancements in natural colorants and their application as coloring in food and in intelligent food packaging. Food Chem. 2023, 404, 134453. [Google Scholar] [CrossRef] [PubMed]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Yuan, Y.; Tian, Y.; Gao, S.; Zhang, X.; Gao, X.; He, J. Effects of environmental factors and fermentation on red raspberry anthocyanins stability. LWT 2023, 173, 114252. [Google Scholar] [CrossRef]
- Soutelino, M.E.M.; Silva, D.B.d.; Rocha, R.d.S.; de Oliveira, B.C.R.; Esmerino, E.A.; Cruz, A.G.d.; Mársico, E.T.; Silva, A.C.d.O. Yogurt added with beetroot extract: Physicochemical parameters, biological activities and sensory evaluation by CATA method. Int. J. Food Sci. Tech. 2023, 58, 3303–3309. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.-S. The effect of pH treatment and refrigerated storage on natural colourant preparations (betacyanins) from red pitahaya and their potential application in yoghurt. LWT 2017, 80, 437–445. [Google Scholar] [CrossRef]
- Schneider-Teixeira, A.; Molina-García, A.D.; Alvarez, I.; Dello Staffolo, M.; Deladino, L. Application of betacyanins pigments from Alternanthera brasiliana as yogurt colorant. LWT 2022, 159, 113237. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products; Yield and Production Quantity of Crops. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 September 2023).
- Abdo, E.M.; Shaltout, O.E.-S.; Ali, S.; Mansour, H.M. A Functional Orange Juice Fortified with Beetroot By-Products Attenuates Hyperlipidemia and Obesity Induced by A High-Fat Diet. Antioxidants 2022, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Ben Haj Koubaier, H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and phenolic compositions, antioxidant activity of Tunisian red beet (Beta vulgaris L. conditiva) roots and stems extracts. Int. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef]
- Abdo, E.; El-Sohaimy, S.; Shaltout, O.; Abdalla, A.; Zeitoun, A. Nutritional Evaluation of Beetroots (Beta vulgaris L.) and Its Potential Application in a Functional Beverage. Plants 2020, 9, 1752. [Google Scholar] [CrossRef]
- Lasta, H.F.B.; Lentz, L.; Rodrigues, L.G.G.; Mezzomo, N.; Vitali, L.; Ferreira, S.R.S. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- De Oliveira, S.P.A.; do Nascimento, H.M.A.; Rodrigues, N.P.A.; Sampaio, K.B.; Lima, M.d.S.; da Conceição, M.L.; de Souza, E.l. Different parts from the whole red beet (Beta vulgaris L.) valorization with stimulatory effects on probiotic lactobacilli and protection against gastrointestinal conditions. Food Biosci. 2023, 52, 102439. [Google Scholar] [CrossRef]
- Dos Santos, C.D.; Ismail, M.; Cassini, A.S.; Marczak, L.D.F.; Tessaro, I.C.; Farid, M. Effect of thermal and high pressure processing on stability of betalain extracted from red beet stalks. J. Food Sci. Technol. 2018, 55, 568–577. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Multivariate statistical analysis and optimization of ultrasound-assisted extraction of natural pigments from waste red beet stalks. J. Food Sci. Technol. 2016, 53, 792–799. [Google Scholar] [CrossRef]
- Abdo, E.M.; Allam, M.G.; Gomaa, M.A.E.; Shaltout, O.E.; Mansour, H.M.M. Valorization of whey proteins and beetroot peels to develop a functional beverage high in proteins and antioxidants. Front. Nutr. 2022, 9, 984891. [Google Scholar] [CrossRef]
- Vasconcellos, J.; Conte-Junior, C.; Silva, D.; Pierucci, A.P.; Paschoalin, V.; Alvares, T.S. Comparison of total antioxidant potential, and total phenolic, nitrate, sugar, and organic acid contents in beetroot juice, chips, powder, and cooked beetroot. Food Sci. Biotechnol. 2016, 25, 79–84. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Calinoiu, L.F.; Dulf, F.V.; Stefanescu, B.E.; Crisan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebovic, T.N.C.; Čanadanović-Bruneta, J.M.C.; Ćetković, G.S.C.; Čanadanović, V.M.C.; Djilasa, S.M.; Šaponjac, V.T.T.S. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Mansour, H.M.M.; El-Sohaimy, S.A.; Zeitoun, A.M.; Abdo, E.M. Effect of Natural Antioxidants from Fruit Leaves on the Oxidative Stability of Soybean Oil during Accelerated Storage. Antioxidants 2022, 11, 1691. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Porto, M.R.A.; Okina, V.S.; Pimentel, T.C.; Prudencio, S.H. Physicochemical stability, antioxidant activity, and acceptance of beet and orange mixed juice during refrigerated storage. Beverages 2017, 3, 36. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Rocha, F.; Marques, C.S.; de Sousa, L.S.; Minim, V.P.R.; Pires, A.C.d.S.; Minim, L.A.; Stringheta, P.C.; Jones, O.G.; Vidigal, M.C.T.R. Betalains nanodispersions: Effects on betalains stability and on rheological properties of Greek yogurt. Food Res. Int. 2022, 159, 111583. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; Soliman, T.N.; Darwish, A.M.G. Evaluation of date palm pollen (Phoenix dactylifera L.) encapsulation, impact on the nutritional and functional properties of fortified yoghurt. PLoS ONE 2019, 14, e0222789. [Google Scholar] [CrossRef]
- Naklong, K.; Therdtatha, P.; Sumonsiri, N.; Leksawasdi, N.; Techapun, C.; Rachtanapun, P.; Taesuwan, S.; Nunta, R.; Khemacheewakul, J. Microencapsulation of Bifidobacterium breve to Enhance Microbial Cell Viability in Green Soybean Yogurt. Fermentation 2023, 9, 296. [Google Scholar] [CrossRef]
- Darwish, A.M.G.; Soliman, T.N.; Elhendy, H.A.; El-Kholy, W.M. Nano-encapsulated Iron and Folic Acid-Fortified Functional Yogurt Enhance Anemia in Albino Rats. Front. Nutr. 2021, 8, 654624. [Google Scholar] [CrossRef]
- Caldas-Cueva, J.P.; Morales, P.; Ludeña, F.; Betalleluz-Pallardel, I.; Chirinos, R.; Noratto, G.; Campos, D. Stability of betacyanin pigments and antioxidants in ayrampo (Opuntia soehrensii britton and rose) seed extracts and as a yogurt natural colorant. J. Food Process. Preserv. 2016, 40, 541–549. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.-J. Microencapsulated saffron floral waste extracts as functional ingredients for antioxidant fortification of yogurt: Stability during the storage. LWT 2023, 184, 114976. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Juárez-Moya, J.; Santellano-Estrada, E.; Chávez-Martínez, A. Stirred yogurt added with beetroot extracts as an antioxidant source: Rheological, sensory, and physicochemical characteristics. J. Food Process. Preserv. 2021, 45, e15628. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Sawicki, T.; Bartoszek, A. The comparison of betalain composition and chosen biological activities for differently pigmented prickly pear (Opuntia ficus-indica) and beetroot (Beta vulgaris) varieties. Int. J. Food Sci. Nutr. 2019, 70, 442–452. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Alam, M.A. Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT-Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Savatović, S.S.; Ćetković, G.S.; Vulić, J.J.; Djilas, S.M.; Markov, S.L.; Cvetković, D.D. Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J. Food Sci. 2011, 29, 575–585. [Google Scholar] [CrossRef]
- John, S.; Monica, J.; Priyadarshini, S.; Sivaraj, C.; Arumugam, P. Antioxidant and antibacterial activities of Beta vulgaris L. peel extracts. Int. J. Pharma Res. Health Sci. 2017, 5, 1974–1979. [Google Scholar]
- Martin JG, P.; Porto, E.; Corrêa, C.B.; Alencar, S.M.; Gloria, E.M.; Cabral IS, R.; Aquino, L.M. Antimicrobial potential and chemical composition of agro-industrial wastes. J. Nat. Prod. 2012, 5, 27–36. [Google Scholar]
- Spence, C.; Levitan, C.A. Explaining Crossmodal Correspondences Between Colours and Tastes. i-Perception 2021, 12, 20416695211018223. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, A.; Weerasingha, V.; Jayarathna, S.; Priyashantha, H. Quality parameters of natural phenolics and its impact on physicochemical, microbiological, and sensory quality attributes of probiotic stirred yogurt during the storage. Food Chem. X 2022, 14, 100332. [Google Scholar] [CrossRef]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage. LWT 2019, 102, 230–236. [Google Scholar] [CrossRef]
- Asiimwe, A.; Kigozi, J.B.; Muyonga, J. Physicochemical Properties, Sensory Acceptance and Storage Stability of Yogurt Flavored with Refractance Window Dried Passion Fruit Powder. Asian Food Sci. J. 2021, 20, 38–49. [Google Scholar] [CrossRef]
- De Campo, C.; Queiroz Assis, R.; Marques da Silva, M.; Haas Costa, T.M.; Paese, K.; Stanisçuaski Guterres, S.; de Oliveira Rios, A.; Hickmann Flôres, S. Incorporation of zeaxanthin nanoparticles in yogurt: Influence on physicochemical properties, carotenoid stability and sensory analysis. Food Chem. 2019, 301, 125230. [Google Scholar] [CrossRef]
- Ghasempour, Z.; Javanmard, N.; Langroodi, A.M.; Alizadeh-Sani, M.; Ehsani, A.; Kia, E.M. Development of probiotic yogurt containing red beet extract and basil seed gum; techno-functional, microbial and sensorial characterization. Biocatal. Agric. Biotechnol. 2020, 29, 101785. [Google Scholar] [CrossRef]
- Kulaitienė, J.; Vaitkevičienė, N.; Levickienė, D. Studies on Proximate Composition, Mineral and Total Phenolic Content of Yogurt Bites Enriched with Different Plant Raw Material. Fermentation 2021, 7, 301. [Google Scholar] [CrossRef]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]


| Whole Milk | Raspberry Concentrate | BSE * | |
|---|---|---|---|
| Control | 90 | 10 | 0 |
| T1 | 89 | 10 | 1 |
| T2 | 88 | 10 | 2 |
| T3 | 85 | 10 | 5 |
| Bioactive Components | |
| Total phenolics (mg/g) | 139.87 ± 0.45 |
| Total flavonoids (mg/g) | 31.17 ± 2.24 |
| Betacyanins (mg/L) | 254.38 ± 13.61 |
| Betaxanthins (mg/L) | 202.45 ± 4.99 |
| Betalains (mg/L) | 456.82 ± 18.60 |
| Phenolics profile (µL/mL) | |
| Chlorogenic acid | 36.52 |
| Ferulic acid | 17.57 |
| Gallic acid | 8.43 |
| Syringic acid | 4.46 |
| Methyl gallate | 0.66 |
| Caffeic acid | 0.43 |
| Coumaric acid | 0.12 |
| Catechin | 8.89 |
| Rutin | 8.65 |
| Antioxidant activity (µL/mL) | |
| DPPH• IC50 | 207.33 ± 4.19 |
| ABTS• IC50 | 64.69 ± 0.71 |
| Antimicrobial activity (mm) | |
| Staphylococcus aureus NCTC 10788 | 13.10 ± 0.14 |
| Escherichia coli BA 12296 | 14.70 ± 0.42 |
| Salmonella senfteneberg ATCC 8400 | 19.80 ± 0.42 |
| Day 1 | Day 7 | Day 14 | |
|---|---|---|---|
| pH 4 | |||
| Betacyanins (mg/L) | 247.5 ± 12.96 a | 147.13 ± 7.13 b | 152.17 ± 11.67 b |
| Betaxanthins (mg/L) | 199.88 ± 8.62 a | 103.63 ± 0.45 c,* | 122.88 ± 0.45 b |
| Betalains (mg/L) | 447.38 ± 21.58 a | 250.75 ± 6.68 b,* | 275.05 ± 12.12 b |
| pH 5 | |||
| Betacyanins (mg/L) | 214.5 ± 1.3 a | 157.21 ± 0.65 b | 138.88 ± 5.83 c |
| Betaxanthins (mg/L) | 191.54 ± 2.27 a | 128.01 ± 1.36 b,* | 108.12 ± 6.81 c |
| Betalains (mg/L) | 406.04 ± 0.97 a | 285.22 ± 2.01 b,* | 247.00 ± 0.97 c |
| Control | T1 | T2 | T3 | |
|---|---|---|---|---|
| LAB (107) | ||||
| Day 1 | 4.73 ± 0.25 aC | 0.90 ± 0.21 bC | 0.72 ± 0.11 bC | 0.70 ± 0.2 bC |
| Day 7 | 73.67 ± 3.21 aA | 55.33 ± 1.53 bA | 36.00 ± 3.46 cA | 25.17 ± 0.29 dA |
| Day 14 | 37.00 ± 1.00 aB | 3.13 ± 0.17 bB | 2.60 ± 0.17 bB | 3.13 ± 0.15 bB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, E.M.; Mansour, H.M.M.; Darwish, A.M.G.; El-Sohaimy, S.A.; Gomaa, M.A.E.; Shaltout, O.E.; Allam, M.G. Beetroot Stalk Extract as a Functional Colorant for Stirred Yogurt Beverages: Effect on Nutritional Value and Stability during Storage. Fermentation 2023, 9, 878. https://doi.org/10.3390/fermentation9100878
Abdo EM, Mansour HMM, Darwish AMG, El-Sohaimy SA, Gomaa MAE, Shaltout OE, Allam MG. Beetroot Stalk Extract as a Functional Colorant for Stirred Yogurt Beverages: Effect on Nutritional Value and Stability during Storage. Fermentation. 2023; 9(10):878. https://doi.org/10.3390/fermentation9100878
Chicago/Turabian StyleAbdo, Eman M., Hanem M. M. Mansour, Amira M. Galal Darwish, Sobhy Ahmed El-Sohaimy, Mohamed A. E. Gomaa, Omayma E. Shaltout, and Marwa G. Allam. 2023. "Beetroot Stalk Extract as a Functional Colorant for Stirred Yogurt Beverages: Effect on Nutritional Value and Stability during Storage" Fermentation 9, no. 10: 878. https://doi.org/10.3390/fermentation9100878
APA StyleAbdo, E. M., Mansour, H. M. M., Darwish, A. M. G., El-Sohaimy, S. A., Gomaa, M. A. E., Shaltout, O. E., & Allam, M. G. (2023). Beetroot Stalk Extract as a Functional Colorant for Stirred Yogurt Beverages: Effect on Nutritional Value and Stability during Storage. Fermentation, 9(10), 878. https://doi.org/10.3390/fermentation9100878











