Abstract
Antrodia cinnamomea (AC), a rare fungus endemic to Taiwan, contains high levels of various secondary metabolites, notably triterpenoids, having useful medicinal and pharmacological properties. Techniques for increasing the production of AC triterpenoids (ACT) for medicinal purposes are a high research priority. We measured and compared the biomass and ACT content of AC mycelia under various liquid fermentation culture conditions. Relative gene expression levels of ten enzymes involved in the mevalonate (MVA) pathway and “subsequent group modification pathway” were determined, and correlation analysis was performed to evaluate the roles of these enzyme genes in ACT synthesis. Two representative genes encoding the enzymes lanosterol synthase (AcLSS) and sterol C-24 reductase (AcERG4), whose activity is closely associated with ACT content, were selected for homologous expression. AcLSS and AcERG4 were separately linked to plasmid pCT74, and transformed into prepared AC protoplasts to obtain two recombinant strains, termed RpLSS and RpERG4, by polyethylene glycol (PEG)-CaCl2-mediated protoplast transformation. Upregulated expression levels of AcLSS and AcERG4 (1.78- and 1.41-fold, respectively) were associated with significantly higher (1.82- and 1.37-fold, respectively) ACT content in the recombinant strains in comparison with the wild-type. Our findings provide a theoretical and practical basis for the enhancement of ACT production using homologous expression techniques.
1. Introduction
Antrodia cinnamomea (AC) (synonyms: Antrodia camphorata, Taiwanofungus camphoratus) is a rare fungus (class: Agaricomycetes), which is parasitic on the hollow, decaying inner trunk wall of Cinnamomum kanehirae (“small-flowered camphor tree”, family Lauraceae; endemic to Taiwan) [1]. AC displays a wide range of medicinal and pharmacological activities, including anti-inflammatory [2], antioxidative [3,4], hepatoprotective [3], neuroprotective [5], and antitumor [6,7,8,9] activities, resulting from a high content of active secondary metabolites such as terpenoids [10], polysaccharides [11], and maleic and succinic acid derivatives [12]. Triterpenoids, a class of terpenoids, are present in many medicinal fungi and often display strong physiological activities. AC triterpenoids (ACT) have greater variety and stronger activities than triterpenoids from Ganoderma lucidum and other widely used medicinal fungi [13]. Over 100 ACT, mostly lanostane- and ergostane-type, have been isolated to date [10,14].
Excessive logging of C. kanehirae during the past two decades has greatly reduced availability of natural AC fruiting bodies [15]. Under “forest farming” techniques, AC grows extremely slowly. In contrast, liquid fermentation (inoculation of AC into liquid medium) is more rapid [16], and the addition of inducers may increase ACT content [17]. Still, production of ACT remains insufficient to meet medicinal market demand in Southeast Asian countries.
Detailed studies of ACT biosynthesis at the molecular level are essential for improving production techniques. Partial sequencing and annotation of the AC genome and transcriptome since 2014 have helped elucidate the main biosynthetic pathways for triterpenoid compounds, and functions of related enzymes [18]. Transcriptome prediction results showed indicate that ACT can be synthesized via the mevalonate (MVA) pathway through formation of terpene skeletons, the subsequent formation of lanosterol skeletons through the intermediate product squalene and, finally, the generation of various triterpenoids through a series of downstream group modifications. The initial enzyme driving this process is acetyl-CoA C-acetyltransferase (AACT), whose activity was shown to increase total triterpenoid content in G. lucidum [19]. Hydroxymethylglutaryl-CoA reductase (HMGR) is a rate-limiting enzyme in isoprenoid biosynthesis. HMGR overexpression enhanced squalene production in hairy roots of Salvia miltiorrhiza (“red sage”; family Lamiaceae) [20]. Mevalonate pyrophosphate decarboxylase (MVD) catalyzes the decarboxylation activity, and triterpenoid accumulation in G. lucidum was increased by MVD overexpression [21]. Farnesyl pyrophosphate (FPP) is converted into a 2,3-epoxysqualene skeleton through activity of squalene synthase (SQS) and squalene epoxidase (SE), which enhance levels of terpenoids as direct precursors [22]. Lanosterol synthase (LSS), a member of the oxidosqualene cyclase superfamily, catalyzes formation of triterpenoid product lanosterol, the precursor for subsequent generation of other triterpenoids [23]. Lanosterol is modified by a series of ergosterol synthase (ERG) genes to form ergosterol [24]. Few studies have focused on detailed functions of genes involved in ACT synthesis, or associated strategies for increasing triterpenoid content.
Identification and characterization of ACT synthesis-related genes will be facilitated by development of efficient genetic transformation systems for knockout of target genes or insertion of new genes, as a platform for molecular genetic manipulation. Various genetic transformation systems for filamentous fungi [25] and mushrooms [26] have been described during recent decades. Approaches used for fungi include PEG-CaCl-mediated protoplast transformation [27], Agrobacterium tumefaciens-mediated transformation [28], electroporation [29], the gene gun method [30], and restriction enzyme-mediated integration [31]. Few studies have addressed genetic transformation systems for AC. L-Z Ai’s group used PEG-CaCl-mediated protoplast transformation to construct AC strains overexpressing 4-hydroxybenzoate polyprenyltransferase biosynthesis-related genes (ubiA, CoQ2), and observed a 2-fold increase in production of antroquinonol (AQ), a bioactive component with strong anticancer effects [32]. Analogously, it may be possible to enhance triterpenoid yield through manipulation of the AC genome.
We describe here correlations of ACT content with expression levels of several key enzyme genes, and construction of recombinant AC strains with high triterpenoid production based on homologous expression of selected genes. Our findings provide a useful platform for future large-scale development and application of ACT.
2. Materials and Methods
2.1. Strains and Reagents
AC strain FJ-01 was obtained from the Microbial Genetic Stock Center of Huazhong Agricultural University (Wuhan, China), maintained on potato dextrose agar (PDA) slants at 4 °C, and activated in PDA plate medium for 25 day at 28 °C. Escherichia coli strain DH5 (Tsingke Biotechnology Co.; Beijing, China) was used as cloning host. Plasmid shuttle vector pCT74 from Miaoling Biology (Wuhan, China) was maintained in strain DH5. Yeast mold (YM) medium was from Hopebio to Technology Co. (Qingdao, China). Casein peptone and yeast extract were from Oxoid (Basingstoke, UK). Agar was from neoFroxx GmbH (Einhausen, Germany). Oleanolic acid standard (96% purity) and ampicillin were from Yuanye Bio-Technology Co. (Shanghai, China). Hygromycin B was from Biosharp (Beijing, China). A 2× GS Taq PCR mix and Uniclone One Step Seamless Cloning Kit were from Genesand Biotech Co. (Beijing, China). M5 DL2000 plus DNA Marker were from Mei5 Biotechnology Co. (Beijing, China). HiScript II QRT SuperMix for qPCR, AceQ qPCR SYBR Green Master Mix, and HiScript II 1st Strand cDNA Synthesis Kit were from Vazyme Biotech Co. (Nanjing, China). Psp5II and alkaline phosphatase were from Thermo. HighPure Maxi Plasmid kit was from Tiangen Biotech Co. (Beijing, China). AxyPrep PCR Clean-up kit was from Corning Life Sciences Co. (Wujiang, China). I-5TM 2× High-Fidelity Master Mix was from Tsingke Biotechnology Co. (Beijing, China). Oleic acid was from Aladdin Holding Group Co. (Shanghai, China). Other reagents and chemicals, all of analytical grade, were from Sinopharm Chemical Reagent Co. (Shanghai, China).
2.2. Determination of Mycelial Biomass and ACT Content under Various Culture Conditions
Four hyphae pieces (each 1 cm) were taken from plates and incubated (7 day, 28 °C, 160 rpm) in seed culture medium (corn flour 47.8 g/L, YM medium 31.9 g/L, pH 5.5) [7]. Seeds were then inoculated into fermentation medium (10% v/v) formulated with various combinations of carbon source (glucose (GL) or fructose (FR) 40 g/L), nitrogen source (beef powder [BP] or yeast extract [YE] 10 g/L with MgSO and KHPO 0.5 g/L), and inducer (chitooligosaccharides (COS) 1 g/L or oleic acid (OA) 6% v/v), and fermented for periods of 4, 8, 12, and 16 day. Mycelia were collected by vacuum filtration, dried at 50 °C, dry weight was measured, and mycelial biomass (Mb; g/L) was calculated as:
where Md is the mycelial dry weight and Vb is the volume of fermentation broth.
Mycelial powder was extracted with 80% ethanol, filtered under reduced pressure, and ACT content was measured by vanillin-glacial acetic acid method using oleanolic acid standard curve [33]. A solution of citric acid standard (mass fraction 0.02%) in methanoic acid was prepared, divided into 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 mL portions followed by methanoic acid evaporation, added to 5% vanillin-acetic acid solution (0.3 mL) and perchloric acid (1 mL), heated at 70 °C for 25 min in a sealed container, cooled rapidly in ice water, added to acetic acid to volume 10 mL, and absorbance at wavelength 550 nm was measured by spectrophotometry. A standard curve was plotted (x-axis: mass of citric acid; y-axis: absorbance) and a regression equation was obtained:
ACT content (ACTc, mg/g), defined as amount of triterpenoids produced per g mycelia, was calculated as:
where c is the triterpenoid concentration of filtrate calculated by Equation (2), and Vf is the volume of flitrate.
2.3. Correlation Analysis of ACT Content and Enzyme Expression Levels in Synthetic Pathway
Expression levels of key enzyme genes involved in ACT synthetic pathway under various culture conditions were analyzed by quantitative PCR (qPCR) using cDNA library as template and GAPDH gene as internal reference. qPCR primers (sequences shown in Table 1) for AACT, HMGR, MVD, IDI, FPS, SQS, SE, LSS, ERG27, ERG4, and GAPDH genes were designed using Primer-BLAST program (https://www.ncbi.nlm.nih.gov/tools/primer-blast/, accessed on 30 September 2021). The qPCR program was 95 °C for 5 min; 40 cycles of 95 °C for 10 s and 60 °C for 30 s; 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. Correlation analysis of ACT content and enzyme expression levels was performed using IBM SPSS Statistics 20 software (https://www.ibm.com/support/pages/node/723799, accessed on 30 October 2021).

Table 1.
Primers for PCR and qPCR.
2.4. Construction of Plasmids pCT74-AcLSS and pCT74-AcERG4
For construction of these plasmids, AcLSS and AcERG4 sequences were cloned using primer pairs IAcLSS-F/R and IAcERG4-F/R (Table 1) from the genome template, and linked into the pCT74 vector (Figure 3A) linearized by Psp5II (Thermo Scientific; Waltham, MA, USA) through seamless cloning (Genesand Biotech, Beijing). pCT74 is a GFP expression vector commonly used in filamentous fungi since 2001, based on pBlue -SGFP-TYG-nos-KS. The transformant was recognized by EGFP promoted by pToxA and hygromycinB resistance. During the construction of the homologous expression plasmid, the gene was inserted between the ToxA promoter and EGFP. The plasmid was validated by Psp5II digestion.
2.5. Preparation of AC Protoplasts
AC mycelia were activated in 200 mL liquid fermentation medium (LFM; 25 g glucose, 5 g peptone, 3 g maltose, 3 g yeast extract, 1 g KHPO, 1 g MgSO·7HO, 1 g VB/L, pH 5.5) for 7 day. The resulting AC mycelial pellet was collected by filtration, ground into a slurry, washed 2× with sterile water and mannitol solution (0.6 mol/L), and centrifuged (7000× g, 10 min). The resulting sludge was resuspended in lysis buffer (10 mg/mL lysing enzyme + 5 mg/mL snail enzyme + 5 mg/mL cellulase), and reacted 6 h at 30 °C, 80 rpm. Lysate was filtered with defatted cotton, centrifuged (2500× g, 20 min), washed 2× with mannitol solution as above, resuspended in 2 mL STC buffer (1.2 mol/L sorbitol, 10 mM/L Tris-HCl, 50 mM CaCl, pH 7.5), distributed into 10 tubes, and stored at −80 °C. The protoplast number was determined using a hemocytometer. Protoplasts (100 L) were spread on regeneration medium TB3 (casein acid hydrolysate 3 g/L, yeast extract 3 g/L, sucrose 200 g/L) and incubated 7 day at 28 °C. The regeneration rate (Rr; %) was calculated as:
where Nrc is the number of regenerated colonies and Ncp is the number of coated protoplasts.
2.6. Homologous Expression of AcLSS and AcERG4
Plasmid solution ( 100 ng/L; 50 L) was mixed gently in a tube with protoplasts ( 6 × 10 /mL; 200 L), mannitol solution (0.6 mol/L; 100 L), and polyethylene glycol (PEG)-CaCl solution (40% PEG 4000; 1 mL), placed on ice for 1 h, added with 500 L PEG-CaCl solution, and kept for 30 min at room temperature with stirring (5 min intervals). A total of 1 mL of STC buffer, 5 mL of LFM, and 10 L of Amp solution (100 mg/mL) were added during the recovery process, and the resulting mixture was incubated for 48 h at 28 °C, 85 rpm. The mixture was then spread onto LFM solid plates containing 30 g/mL hygromycin B, and incubated for 7 day at 28 °C. Transformation efficiency was calculated based on the amount of plasmid added during transformation and the number of transformants grown on hygromycin B-resistant plates. Hygromycin B resistance gene fragment was detected by PCR using primer pair hygR-F/hygR-R (Table 1). Expression of enhanced green fluorescent protein (EGFP), a reporter gene, was measured by laser confocal microscopy with excitation wavelength 488 nm and reception range 500–550 nm. Transformants were amplified as seeds and inoculated into a fermentation medium for 12 day, with oleic acid as inducer. ACT content and expression of key genes were evaluated as described in Section 2.3.
2.7. Statistical Analysis
Experiments were performed in triplicate. Data were expressed as mean ± SD, and differences between means were analyzed using the SPSS Statistics program. Differences were considered significant for p < 0.05, and highly significant for p < 0.01.
3. Results
3.1. Changes in Mycelial Biomass and ACT Content under Various Fermentation Conditions
Liquid fermentation culture of AC was performed for 16 day using alternative carbon sources (GL, FR), nitrogen sources (BP, YE), and inducers (COS, OA). The experimental groups showed clear differences in mycelial biomass (Figure 1A) and ACT content (Figure 1B), and both these parameters increased during early and middle fermentation stages. For the OA group, maximal levels were observed on day 12 for mycelial biomass (11.03 g/L) and ACT content (36.39 mg/g). These findings demonstrate that mycelial growth and secondary metabolite synthesis varied depending on culture conditions.
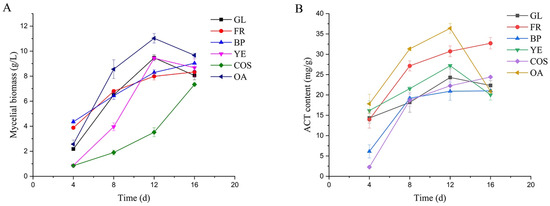
Figure 1.
Effects of culture conditions on mycelial growth and production of Antrodia cinnamomea triterpenoids: (A) Mycelial biomass. (B) ACT content. GL: glucose as carbon source; FR: fructose as carbon source; BP: beef powder as nitrogen source; YE: yeast extract as nitrogen source; COS: chitooligosaccharides as inducer; OA: oleic acid as inducer.
3.2. Expression Levels of Key Genes in ACT Synthetic Pathways
Ten representative key enzymes (AACT, HMGR, MVD, IDI, FPS, SQS, SE, LSS, ERG27, ERG4) involved in the MVA pathway and “subsequent group modification pathway” were selected on the basis of previous reports and KEGG database analysis. Relative expression levels of genes encoding these enzymes were measured by qPCR under various culture conditions. Levels were upregulated during the initial stage and subsequently downregulated (Figure 2). During days 4–8, ACT content showed highest rate of increase, corresponding to high levels of related enzyme genes in triterpenoid synthetic pathway. During days 8–12, ACT content increase rate declined, as did enzyme gene levels. Presumably, secondary metabolites began to accumulate and ACT synthesis entered plateau phase during this period.
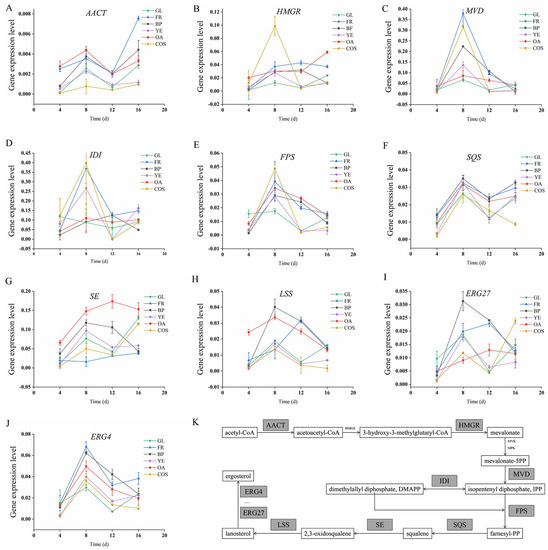
Figure 2.
Relative expression levels of key enzyme genes (A–J) in the ACT synthetic pathway (K) under various culture conditions. AACT: acetyl-CoA C-acetyltransferase; HMGR: hydroxymethylglutaryl-CoA reductase; MVD: mevalonate pyrophosphate decarboxylase; IDI: isopentenyl-diphosphate delta-isomerase; FPS: farnesyl diphosphate synthase; SQS: squalene synthase; SE: squalene epoxidase; LSS: lanosterol synthase; ERG27: sterol 3-keto reductase; ERG4: sterol C-24 reductase. Culture condition notations as in Figure 1.
During days 12–16, enzyme gene levels varied depending on enzyme function. The level of the AACT gene, which acts at the beginning of MVA pathway to transfer acetyl-CoA for secondary metabolic production, continued to increase. Levels remained fairly constant for MVD, IDI, and FPS, the genes responsible for formation of triterpenoid precursor squalene. Levels of LSS, ERG27, and ERG4, which encode key enzymes involved in downstream group modification, were strongly affected by culture conditions.
3.3. Correlation Analysis of ACT Content and Synthesis Gene Expression
Relationships between ACT content and enzyme gene expression levels were evaluated by correlation analysis (Table 2). ACT content was positively correlated with levels of key genes in the MVA pathway and subsequent group modification pathway. Among these, levels of LSS and SQS showed significant (p < 0.01) positive correlation (respective correlation coefficients 0.541 and 0.523) with ACT content. Thus, effect on ACT content was slightly stronger for LSS level than for SQS level. Levels of AACT, HMGR, FPS, SE, and ERG4 were also correlated with ACT content, with somewhat lower significance (p < 0.05). In view of these findings, we selected the LSS gene as a target for homologous expression.

Table 2.
Pearson correlation coefficients among relative expression levels of enzymes and ACT content.
Expression levels of the various enzymes also showed positive correlations with each other. FPS and ERG4 levels had the highest correlation coefficient (0.899). ERG4 also had strong correlations with levels of other genes. We concluded that levels of upstream key enzyme genes in ACT synthesis had regulatory effects on levels of downstream key enzyme genes. Related enzyme genes in MVA pathway may be regulated through a common molecular mechanism.
3.4. Construction of Plasmid for Homologous Transformation
For homologous overexpression of key genes involved in ACT synthesis, two recombinant plasmids harboring AcLSS (2205 bp) and AcERG4 (1437 bp) gene sequences (respectively termed pCT74-AcLSS and pCT74-AcERG4) were constructed based on vector pCT74. The plasmids were constructed by homologous recombination, and verified by enzyme digestion. They displayed three bands as a result of differing conformations (Figure 3B). The accuracy of recombination was verified based on relative differences in fragment length of inserted genes and sequencing results. Linearization of recombinant plasmids with PspII endonuclease yielded a linear empty carrier (5790 bp in lanes 1, 2, and 3) and target gene fragments (2205 bp in lane 1 and 1437 bp in lane 2) (Figure 3C), consistent with estimated positions.
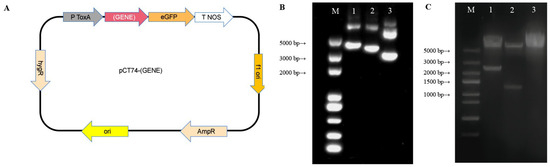
Figure 3.
Gel electrophoresis of recombinant plasmids pCT74-AcLSS, pCT74-AcERG4, and pCT74, and their enzyme-digested products: (A) Scheme of pCT74. (B) Plasmids. M: DL5000 marker; lane 1: pCT74-AcLSS; lane 2: pCT74-AcERG4; lane 3: pCT74. (C) Products from endonuclease Psp5II digestion of plasmids. Lanes as in Panel (B).
3.5. Protoplast Preparation and PEG-CaCl-Mediated Transformation
Protoplasts were generated at concentration 6.45 × 10 cell/mL under optimized enzymatic hydrolysis conditions, and plasmids were then transformed into wild-type protoplasts by PEG-CaCl-mediated transformation. Transformants were grown for three generations on LFM solid plates containing 30 g/mL hygromycin B. Positive transformants were verified by PCR amplification of hygR gene integrated into genomes (Figure 4A). Length of hygR gene was 1026 bp, and successful transformation of plasmids into genome was confirmed by gel image using wild-type (lane 4) as control. Target gene construction was amplified from cDNA, and lengths of AcLSS and AcERG4 were determined, respectively, as 2205 and 1437 bp by matching positions in Figure 4B. The wild-type of AC in lane 4 showed that the primers designed according to the cDNA sequence could not amplify the sequence in the genome, only annealed to the sequence of CDS in the vector.
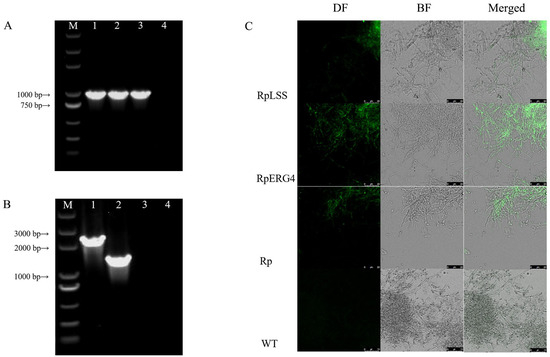
Figure 4.
Screening validation of positive transformants: (A) Amplification of hygromycin B resistant gene from AC transformant genomes. M: DL5000; 1: pCT74-AcLSS transformant; 2: pCT74-AcERG4 transformant; 3: pCT74 transformant; 4: wild-type. (B) Amplification of AcLSS and AcERG4 genes from AC transformant genomes. Lanes as in Panel A. (C) Confocal microscopic imaging of EGFP in mycelia. RpLSS: pCT74-AcLSS recombinant; RpERG4: pCT74-AcERG4 recombinant; Rp: pCT74 recombinant; WT: wild-type; DF: dark field (EGFP); BF: bright field.
Transformation efficiencies for pCT74-AcLSS and pCT74-AcERG4 were, respectively, 14.44 and 10.17 transformants/g. Reporter gene EGFP expression was evaluated by confocal microscopy (Figure 4C). Green fluorescence in the fluorescence field was observed for pCT74-AcLSS, pCT74-AcERG4, and pCT74, overlapping with positions of mycelia in the bright field. No fluorescence was observed for wild-type mycelia. These findings demonstrate successful expression of reporter genes in the recombinant plasmids, and indicate that the transformants can be regarded as recombinant strains (respectively termed RpLSS, RpERG4, and Rp) that harbor plasmids pCT74-AcLSS, pCT74-AcERG4, and pCT74.
3.6. Enhanced Target Gene Expression Levels and ACT Content in Recombinant Strains
Recombinant strains as above were incubated in seed culture medium for 7 day, and then fermented under conditions used for OA group (see Section 3.1). Their ACT contents were determined by vanillin-perchloric acid method. Biomass did not differ significantly among the three strains; however, ACT content was significantly (p < 0.01) 1.82 times higher for RpLSS (55.54 mg/g) and 1.37 times for RpERG4 (41.76 mg/g) than for Rp (Figure 5A,B).
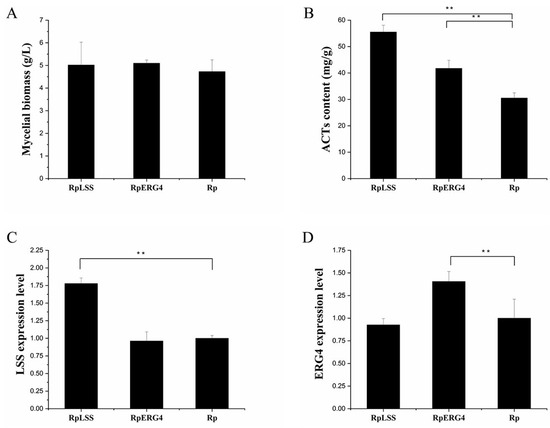
Figure 5.
Liquid fermentation studies of AC recombinant strains RpLSS, RpERG4, and Rp. (A) Mycelial biomass. (B) ACT content. (C) Relative expression levels of AcLSS genes. (D) Relative expression levels of AcERG4 genes. ** p < 0.01.
RNA was extracted for qPCR analysis of relative target gene expression levels in RpLSS and RpERG4, with Rp as negative control (Figure 5C,D). Levels of AcLSS in RpLSS and AcERG4 in RpERG4 were, respectively, 1.78 and 1.41 times higher than corresponding levels in Rp. Our findings, taken together, indicate that target gene expression was enhanced by recombinant plasmids, and that ACT synthesis was strongly promoted through homologous expression of key enzyme genes involved in triterpenoid synthetic pathway.
4. Discussion
Antrodia cinnamomea (AC) is a rare medicinal fungus containing abundant secondary metabolites, and its extracts have been applied therapeutically for a variety of diseases and disorders. Many studies during the past decade have described isolation, identification, and synthetic analysis of its active metabolites [34,35,36]. Triterpenoids, a group of structurally diverse metabolites, are of particular interest. Detailed studies of ACT synthetic pathway-related enzymes have been useful for optimizing fermentation conditions and increasing ACT content [37,38]. Characterization of triterpenoids from the medicinal fungus G. lucidum, and cloning and homologous expression of key synthesis-related genes, have led to significant increases in G. lucidum triterpenoid content [39]. In the case of AC, a few ACT synthesis-related genes (e.g., 2,3-oxidosqualene cyclase gene) have been cloned [40]; however, most studies have focused on effects of culture conditions on gene expression levels [17,41].
W.H. Li’s group, in 2014, performed whole-genome sequencing and gene annotation of AC, and predicted major ACT biosynthetic pathways [18]. Using KEGG database information on triterpenoid synthetic pathways, they annotated 20 terpenoid skeleton synthesis-related enzyme genes, nine sesquiterpene and triterpene synthesis-related enzyme genes, seven terpenoid quinone synthesis-related enzyme genes, and 119 cytochrome P450 genes in the genome, and described a putative triterpenoid ACT synthetic pathway. ACT synthesis-related genes have been assigned to two pathways (MVA pathway and “subsequent group modification pathway”) based on analysis of triterpenoid synthetic pathways in other species. Synthesis from acetyl to CoA of squalene, an intermediate compound at the beginning of MVA pathway, is catalyzed by a series of enzymes (HMGR, MVD, IDI, FPS, and SQS; see Introduction). Formation of lanosterol, the essential substance for ACT synthesis, is catalyzed by SE and LSS enzymes. In the subsequent group modification pathway, ERG group enzymes (ERG27, ERG4) are activated to convert lanosterol into ergosterol, leading to formation of ergostane-type, lanostane-type, and other ACT types. As in the review published in 2021 [10], over 100 AC triterpenoids were classified into ergostane-type and lanostane-type according to the chemical skeletons. Combining the experimental results from the proposed biosynthesis pathways and the research progress on ACT, we investigated the relationship between ACT content and key genes involved in the biosynthetic pathway to enhance the metabolic process. Several methods have been used for cloning of cDNA and CDS (protein-coding) sequences of these triterpenoid synthesis-related enzyme genes. Z.X. Lin’s group used RACE PCR technique to obtain full-length cDNA sequences of MVD and SE genes in AC [42]. F.H. Chu’s group isolated a 2,3-oxidosqualene cyclase gene (AcOSC) and showed that the encoded enzyme promoted production of cytotoxic triterpenoids [40].
Our 2021 study demonstrated strong effects of artificial culture conditions on secondary metabolic processes in the parasitic chaga fungus (Inonotus obliquus) [43]. In the present study, we examined effects in ACT synthesis of various combinations of fermentation parameters (carbon source, nitrogen source, inducer) on expression levels of 10 key enzyme genes involved in MVA pathway and subsequent group modification pathway. Such effects varied depending on the gene. ACT content was significantly correlated with SQS and LSS expression, suggesting that increased LSS expression might enhance ACT synthesis. ERG4 evidently plays a more important role than does ERG27 in the final stage of ACT synthesis, in view of their correlation coefficients (Section 3.3), indicating that ERG4 is an important target for increasing ACT production.
Mycelial structures of large fungi are much more complex than structures of yeast or other unicellular organisms, and were characterized later. Genetic transformation of AC was accomplished for the first time in 2009. H.S. Tsay’s group used Agrobacterium-mediated transformation to introduce a T-DNA vector carrying cauliflower mosaic virus (CaMV) 35S promoter and hygromycin phosphotransferase (hph II) gene into AC mycelia, thereby constructing an activation marker system [44]. F.H. Chu’s group introduced CDS sequence of AcOSC into pCAMBIA1301 using CaMV 35S heterologous promoter and AC endoglucanase homologous promoter, to construct expression plasmids pCAM-AcOSC and pCAM-1112p/AcOSC [40]. Agrobacterium-mediated transformation of AC mycelia resulted in high expression of OSC. Z.X. Lin’s group used pCX62 and pCT74 as expression plasmids to construct a PEG-CaCl-mediated genetic transformation system for AC protoplasts, and achieved expression of EGFP [42]. L-Z Ai’s group optimized the above transformation system by replacing the promoter of pCT74 vector with the GPD promoter of pAN7-1 vector, and introduced antroquinonol synthesis-related genes ubiA and CoQ2 to enhance antroquinonol synthesis through homologous overexpression [32]. Here, we used PEG-CaCl-mediated transformation to construct homologous expression recombinant strains RpLSS and RpERG4 harboring pCT74-AcLSS and pCT74-AcERG4 plasmids. Fermentation culture studies revealed significantly increased ACT content in mycelia of these recombinant strains, providing a useful basis for improved techniques to increase ACT production in the future.
5. Conclusions
Antrodia cinnamomea triterpenoid (ACT) production was significantly increased by upregulating expression of MVA pathway and “subsequent group modification pathway” genes. Correlation coefficients between ACT content and relative expression levels of key enzyme genes in ACT synthetic pathway were determined. AcLSS and AcERG4 were identified as target genes for construction of homologous recombinant expression strains RpLSS and RpERG4. Upregulation of these two genes significantly increased ACT content in fermentation culture studies. Our findings provide a theoretical and practical basis for enhancement of ACT production using homologous expression techniques.
Author Contributions
S.Z.: Original draft. M.F.: Investigation. J.H.: Formal analysis. Y.L.: Software. Y.M.: Writing—review and editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 42271056) and the Program of Scientific and Technological Talents Service to Business of Hubei Province (Grant No. 2023DJC096).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships that could potentially influence the studies or findings described in this paper.
References
- Chang, T.; Chou, W. Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan. Mycol. Res. 1995, 99, 756–758. [Google Scholar] [CrossRef]
- Chen, J.J.; Lin, W.J.; Liao, C.H.; Shieh, P.C. Anti-inflammatory benzenoids from Antrodia camphorata. J. Nat. Prod. 2007, 70, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, G.; Shen, M.Y.; Lin, K.H.; Lan, M.H.; Wu, L.Y.; Chou, D.S.; Lin, C.H.; Su, C.H.; Sheu, J.R. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J. Agr. Food Chem. 2003, 51, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Y.; Yen, G.C. Antioxidant properties of Antrodia camphorata in submerged culture. J. Agr. Food Chem. 2002, 50, 3322–3327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Shiao, Y.J.; Lin, R.D.; Shao, Y.Y.; Lai, M.N.; Lin, C.C.; Ng, L.T.; Kuo, Y.H. Neuroprotective Diterpenes from the Fruiting Body of Antrodia camphorata. J. Nat. Prod. 2006, 69, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, Y.C.; Kuo, P.L.; Ng, L.T.; Kuo, Y.H.; Lin, C.C. Apoptotic effects of extract from Antrodia camphorata fruiting bodies in human hepatocellular carcinoma cell lines. Cancer Lett. 2005, 221, 77–89. [Google Scholar] [CrossRef]
- Li, Y.; Ge, J.; Li, Y.; Zheng, S.; Liu, Y.; Liang, Y.; Mei, Y. Isolation, Purification, and Antitumor Activity of a Novel Active Protein from Antrodia cinnamomea Liquid Fermentation Mycelia. Fermentation 2023, 9, 185. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Wang, Z.; Zang, W.; Rao, P.; Liang, Y.; Mei, Y. Alpha-terpineol affects synthesis and antitumor activity of triterpenoids from Antrodia cinnamomea mycelia in solid-state culture. Food Funct. 2018, 9, 6517–6525. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, D.; Zang, W.; Zhu, H.; Wu, P.; Mei, Y.; Liang, Y. A polysaccharide from Antrodia cinnamomea mycelia exerts antitumor activity through blocking of TOP1/TDP1-mediated DNA repair pathway. Int. J. Biol. Macromol. 2018, 120, 1551–1560. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef]
- Yang, C.M.; Zhou, Y.J.; Wang, R.J.; Hu, M.L. Anti-angiogenic effects and mechanisms of polysaccharides from Antrodia cinnamomea with different molecular weights. J. Ethnopharmacol. 2009, 123, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hirakawa, A.; Gao, J.J.; Kakuda, H.; Shiro, M.; Komatsu, Y.; Sheu, C.c.; Hattori, M. Five New Maleic and Succinic Acid Derivatives from the Mycelium of Antrodia camphorata and Their Cytotoxic Effects on LLC Tumor Cell Line. J. Nat. Prod. 2004, 67, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid. Based Complement. Altern. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.C.; Wu, T.Y.; Chang, F.R.; Lin, W.Y.; Hsu, Y.M.; Cheng, F.T.; Lu, C.Y.; Yen, M.H.; Tsui, Y.T.; Chen, H.L.; et al. Chemical profiling of the cytotoxic triterpenoid-concentrating fraction and characterization of ergostane stereo-isomer ingredients from Antrodia camphorata. J. Pharm. Biomed. Anal. 2012, 58, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Kuo, D.C.; Lin, C.C.; Ho, K.C.; Lin, T.P.; Hwang, S.Y. Historical spatial range expansion and a very recent bottleneck of Cinnamomum kanehiraeHay.(Lauraceae) in Taiwan inferred from nuclear genes. BMC Evol. Biol. 2010, 10, 124. [Google Scholar] [CrossRef]
- Ho, Y.C.; Lin, M.T.; Duan, K.J.; Chen, Y.S. The hepatoprotective activity against ethanol-induced cytotoxicity by aqueous extract of Antrodia cinnamomea. J. Chin. Inst. Chem. Eng. 2008, 39, 441–447. [Google Scholar] [CrossRef]
- Meng, L.; Luo, B.; Yang, Y.; Faruque, M.O.; Zhang, J.; Li, X.; Hu, X. Addition of vegetable oil to improve triterpenoids production in liquid fermentation of medicinal fungus Antrodia cinnamomea. J. Fungi 2021, 7, 926. [Google Scholar] [CrossRef]
- Lu, M.Y.J.; Fan, W.L.; Wang, W.F.; Chen, T.; Tang, Y.C.; Chu, F.H.; Chang, T.T.; Wang, S.Y.; Li, M.y.; Chen, Y.H.; et al. Genomic and transcriptomic analyses of the medicinal fungus Antrodia cinnamomea for its metabolite biosynthesis and sexual development. Proc. Natl. Acad. Sci. USA 2014, 111, E4743–E4752. [Google Scholar] [CrossRef]
- Fang, X.; Shi, L.; Ren, A.; Jiang, A.L.; Wu, F.L.; Zhao, M.W. The cloning, characterization and functional analysis of a gene encoding an acetyl-CoA acetyltransferase involved in triterpene biosynthesis in Ganoderma lucidum. Mycoscience 2013, 54, 100–105. [Google Scholar] [CrossRef]
- Dai, Z.; Cui, G.; Zhou, S.F.; Zhang, X.; Huang, L. Cloning and characterization of a novel 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Salvia miltiorrhiza involved in diterpenoid tanshinone accumulation. J. Plant Physiol. 2011, 168, 148–157. [Google Scholar] [CrossRef]
- Shi, L.; Qin, L.; Xu, Y.; Ren, A.; Fang, X.; Mu, D.; Tan, Q.; Zhao, M. Molecular cloning, characterization, and function analysis of a mevalonate pyrophosphate decarboxylase gene from Ganoderma lucidum. Mol. Biol. Rep. 2012, 39, 6149–6159. [Google Scholar] [CrossRef]
- Asadollahi, M.A.; Maury, J.; Schalk, M.; Clark, A.; Nielsen, J. Enhancement of farnesyl diphosphate pool as direct precursor of sesquiterpenes through metabolic engineering of the mevalonate pathway in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2010, 106, 86–96. [Google Scholar]
- Shang, C.H.; Shi, L.; Ren, A.; Qin, L.; Zhao, M.W. Molecular cloning, characterization, and differential expression of a lanosterol synthase gene from Ganoderma lucidum. Biosci. Biotech. Biochem. 2010, 74, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Kristan, K.; Rižner, T.L. Steroid-transforming enzymes in fungi. J. Steroid Biochem. 2012, 129, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.Z.; Zhu, Y.L.; Huang, P.W.; Yang, Q.; Dai, C.C. Strategies for gene disruption and expression in filamentous fungi. Appl. Microbiol. Biotechnol. 2019, 103, 6041–6059. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lou, H.; Hu, J.; Liu, Z.; Chen, Q. Macrofungi: A review of cultivation strategies, bioactivity, and application of mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Shen, M.; Liu, J.; Li, Y.; Xu, S.; Chen, L.; Shi, G.; Ding, Z. Establishment of an efficient polyethylene glycol (peg)-mediated transformation system in Pleurotus eryngii var. ferulae using comprehensive optimization and multiple endogenous promoters. J. Fungi 2022, 8, 186. [Google Scholar] [CrossRef]
- Liu, R.; Kim, W.; Paguirigan, J.A.; Jeong, M.H.; Hur, J.S. Establishment of Agrobacterium tumefaciens-mediated transformation of Cladonia macilenta, a model lichen-forming fungus. J. Fungi 2021, 7, 252. [Google Scholar] [CrossRef]
- Dong, Z.; Gao, N.; Deng, B.; Huang, X.; Hu, C.; Chen, P.; Wu, Q.; Lu, C.; Pan, M. Stable transformation of fluorescent proteins into Nosema bombycis by electroporation. Parasites Vectors 2022, 15, 141. [Google Scholar] [CrossRef]
- Schmoll, M.; Zeilinger, S. Resistance marker-and gene gun-mediated transformation of Trichoderma reesei. In Methods in Molecular Biology; Humana: New York, NY, USA, 2021; pp. 55–62. [Google Scholar]
- Attri, C.; Swati; Kulshrestha, S. Restriction enzyme-mediated insertional mutagenesis: An efficient method of Rosellinia necatrix transformation. Arch. Microbiol. 2018, 200, 189–194. [Google Scholar] [CrossRef]
- Liu, X.; Xia, Y.; Zhang, Y.; Liang, L.; Xiong, Z.; Wang, G.; Song, X.; Ai, L. Enhancement of antroquinonol production via the overexpression of 4-hydroxybenzoate polyprenyltransferase biosynthesis-related genes in Antrodia cinnamomea. Phytochemistry 2021, 184, 112677. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, X.; Tong, J.; Liu, P.; Lin, Z. ITS sequence analysis of Antrodia cinnamomea and total triterpenoid detection of liquid fermentation mycelium. Southwest China J. Agric. Sci. 2015, 28, 2649–2654. [Google Scholar]
- Li, B.; Kuang, Y.; He, J.B.; Tang, R.; Xu, L.L.; Leung, C.H.; Ma, D.L.; Qiao, X.; Ye, M. Antcamphorols A–K, cytotoxic and ROS scavenging triterpenoids from Antrodia camphorata. J. Nat. Prod. 2019, 83, 45–54. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, X.; Qiao, X.; Ji, S.; Liu, K.; Yeh, C.t.; Tzeng, Y.m.; Guo, D.; Ye, M. Antcamphins A–L, ergostanoids from Antrodia camphorata. J. Nat. Prod. 2014, 77, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; An, R.; Huang, Y.; Ji, S.; Li, L.; Tzeng, Y.m.; Guo, D.a.; Ye, M. Separation of 25R/S-ergostane triterpenoids in the medicinal mushroom Antrodia camphorata using analytical supercritical-fluid chromatography. J. Chromatogr. A 2014, 1358, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chen, C.Y.; Chien, S.C.; Hsiao, W.W.; Chu, F.H.; Li, W.H.; Lin, C.C.; Shaw, J.F.; Wang, S.Y. Metabolite profiles for Antrodia cinnamomea fruiting bodies harvested at different culture ages and from different wood substrates. J. Agr. Food Chem. 2011, 59, 7626–7635. [Google Scholar] [CrossRef]
- Luo, Z.S.; Lu, Z.M.; Hu, M.M.; Li, X.Y.; Xu, G.Q.; Gong, J.S.; Shi, J.S.; Xu, Z.H. Characterization of Cinnamomum kanehirae Extract-Stimulated Triterpenoids Synthesis in Submerged Fermentation of Antrodia camphorata via Untargeted Metabolomics. J. Agr. Food Chem. 2023, 71, 9175–9186. [Google Scholar] [CrossRef]
- You, H.; Sun, B.; Li, N.; Xu, J.W. Efficient expression of heterologous genes by the introduction of the endogenous glyceraldehyde-3-phosphate dehydrogenase gene intron 1 in Ganoderma lucidum. Microb. Cell Fact. 2021, 20, 164. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lee, Y.R.; Tsao, N.W.; Wang, S.Y.; Shaw, J.F.; Chu, F.H. Characterization of the 2, 3-oxidosqualene cyclase gene from Antrodia cinnamomea and enhancement of cytotoxic triterpenoid compound production. J. Nat. Prod. 2015, 78, 1556–1562. [Google Scholar] [CrossRef]
- Liu, H.; Xing, H.; Jin, Y.; Liu, J.; Tzeng, Y.M.; Deng, L.; Wang, F. Application of multiple strategies to improve the production of the potential cancer drug 4-acetylantroquinonol B (4-aaqb) by the rare fungus Antrodia cinnamomea. Appl. Biochem. Biotechnol. 2022, 194, 2720–2730. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.J.; Shao, E.S.; Lin, Z.X. Molecular cloning and expression analysis of mevalonate pyrophosphate decarboxylase in Antrodia cinnamomea. MATEC Web Conf. 2016, 64, 03001. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Fang, M.; Li, T.; Liang, Y.; Mei, Y. Carbon source affects synthesis, structures, and activities of mycelial polysaccharides from medicinal fungus Inonotus obliquus. J. Microbiol. Biotechnol. 2021, 31, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.C.F.; Su, Y.H.; Kanagarajan, S.; Agrawal, D.C.; Tsay, H.S. Development of an activation tagging system for the basidiomycetous medicinal fungus Antrodia cinnamomea. Mycol. Res. 2009, 113, 290–297. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).