3.1. Optimization of Alkaline Hydrogen Peroxide Pretreatment of Wheat Straw
The degradation of holocellulose occurs along with delignification, which indicates that holocellulose recovery and lignin removal are not suitable responses during pretreatment. The main aim of pretreatment is to enhance the enzymatic digestibility of substrates for higher yields of fermentable sugar during the following enzymatic hydrolysis. Therefore, glucose and xylose yields during enzymatic hydrolysis of the pretreated WS were more suitable responses for the pretreatment optimization. It was reported that a mild temperature (<100 °C) can achieve significant delignification and high glucan enrichment during AHP pretreatment [
16,
17]. In addition, too high of a temperature can result in more carboxylic acids, such as acetic acid, because acetyl groups are removed from hemicellulose during pretreatment. The produced acids can markedly change the pH in a reaction mixture from pH 11.9 to pH 5.63 [
10]. During AHP pretreatment, pH 11.5 is always adopted, as hydrogen peroxide will dissociate to produce the hydroperoxyl anion (HOO
−), which is responsible for delignification [
10]. Therefore, a mild temperature (<100 °C) and pH 11.5 were prioritized in this work.
According to the analysis of the PBD (
Table 1), the pretreatment temperature, hydrogen peroxide concentration and pretreatment time had significant effects on the sugar production. The temperature and time also have significant effects during the pretreatment of corn stover [
18] and wheat straw [
19]. The temperature had a positive effect on the sugar production, as a higher temperature could enhance the reaction rate constant. A high temperature can also improve the pretreatment efficiency of oil palm trunks [
20] and corn stover [
21,
22], respectively. In addition, the hydrogen peroxide concentration also had a positive effect on sugar production, as a higher concentration of it could improve the delignification degree. The other factors, including the solid loading and agitation speed, had insignificant effects on the sugar yields. The optimization of the significant factors was carried out in subsequent experiments by adopting a lower agitation speed (100 rpm) and higher solid loading (200 g/L).
The results indicate that both the glucose and xylose yields reached a plateau while the pretreatment temperature, hydrogen peroxide concentration and pretreatment time were 49.0 °C, 70 g/L and 7.5 h, respectively. Too low levels of the three factors can result in lower delignification and too high levels can degrade more holocellulose, along with produce higher delignification, which is also not conducive to fermentable sugar yields. The statistical analysis of the BBD (
Table 2) indicated that the linear terms, namely,
x1,
x2 and
x3, and the square terms, namely,
x12,
x22 and
x32, had significant effects on the glucose and xylose yields. The interaction terms, namely,
x1x2,
x1x3 and
x2x3, had insignificant effects on the glucose yield, whereas only
x1x3 had a significant effect on the xylose yield.
F values of the models (
F = 86.69,
F = 94.83) and lack of fit (
F = 6.23,
F = 7.83), along with
p-values of the models (
p = 0.000,
p = 0.000) and lack of fit (
p = 0.141,
p = 0.115), indicate that the obtained experimental data for glucose and xylose yields were a good fit with the models, respectively. The values of
R2 (99.4%, 99.4%) and adjusted
R2 (98.2%, 98.4%) also indicate the accuracy of the models.
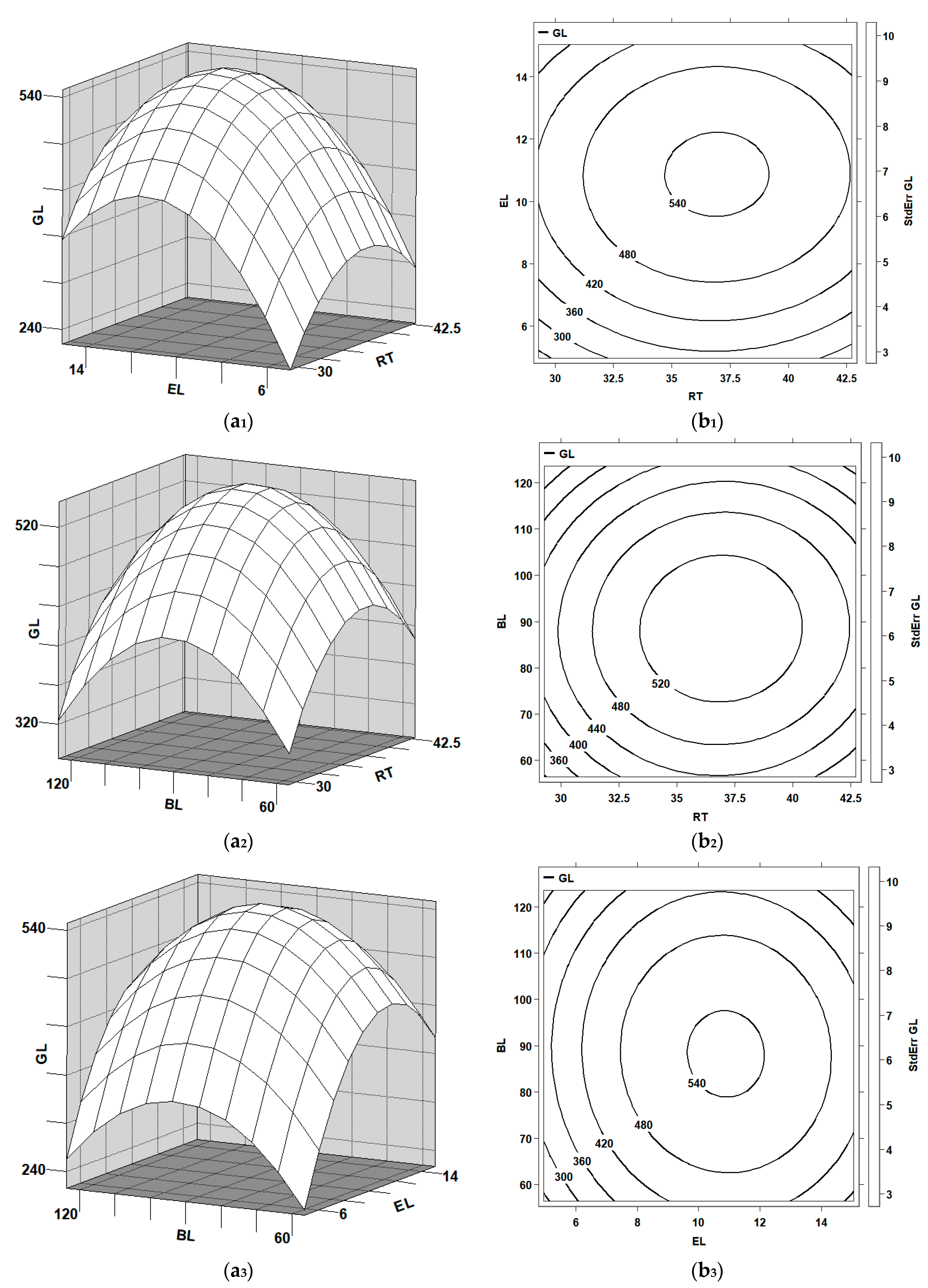
Three-dimensional response surface plots and the corresponding contour plots were used to demonstrate the combined effects of each variable’s pair on yields of glucose and xylose, as shown in
Figure 1 and
Figure 2, respectively. As shown in
Figure 1(a
1,b
1) and
Figure 2(a
1,b
1), while the time was fixed at its middle level (7.5 h), the optimal regions of the temperature and hydrogen peroxide concentration for the yields of glucose and xylose were 48 °C–50 °C and 70 g/L–75 g/L, respectively. As shown in
Figure 1(a
2,b
2), while the hydrogen peroxide concentration was set to be its middle level (70 g/L), the optimal regions of the temperature and time for the glucose yield were 48 °C–50 °C and 7.4 h–7.6 h, respectively. Under the same conditions in
Figure 2(a
2,b
2), the optimal regions of the temperature and time for the xylose yield were 48 °C–50 °C and 7.6 h–7.8 h, respectively. As shown in
Figure 1(a
3,b
3), while the temperature was fixed at its middle level (49 °C), the optimal regions of the hydrogen peroxide concentration and time for the glucose yield were 70 g/L–75 g/L and 7.4 h–7.6 h, respectively. Under the same conditions in
Figure 2(a
3,b
3), the optimal regions of the hydrogen peroxide concentration and time for the xylose yield were 70 g/L–75 g/L and 7.6 h–7.8 h, respectively.
According to canonical analysis, the maximal glucose (233.7 mg/gds) could be obtained while WS was pretreated using 71 g/L hydrogen peroxide solution (x2 = 0.084) at 49.5 °C (x1 = 0.085) for 7.5 h (x3 = 0.056). The maximal xylose (91.9 mg/gds) could be obtained while WS was pretreated using 72 g/L hydrogen peroxide solution (x2 = 0.14405) at 49.6 °C (x1 = 0.11218) for 7.7 h (x3 = 0.25303).
To validate the predicted conditions, the WS was pretreated using 71 g/L hydrogen peroxide solution at 50 °C for 7.6 h. After the enzymatic hydrolysis, the yields of glucose (234.2 mg/gds) and xylose (92.5 mg/gds) (average of three replicates) were obtained, which were close to the predicted values from the models.
In addition, the contents of cellulose, hemicellulose and lignin in the raw WS (dry weight 20.00 g) were 43.52%, 22.41% and 9.80%, respectively. After pretreatment, the contents of cellulose, hemicellulose and lignin in the pretreated WS (dry weight 16.04 g) were 52.92%, 23.56% and 3.05%, respectively. After the calculation, the pretreatment resulted in 80.2% solid recovery, 97.5% cellulose recovery, 84.3% hemicellulose recovery and 75.0% lignin removal, respectively. Comparisons of the above parameters in this work with those in other previous reports are shown in
Table 3.
It was obvious that cellulose recovery (97.5%) and hemicellulose recovery (84.3%) in this work were the most competitive among the different reports. Though the lignin removal of other studies, namely, 95.2% [
23] and 78.7% [
24], were higher than that (75.0%) in this work, the cellulose recovery (92.59%, 83.1%) and hemicellulose recovery (31.39%, 45.4%) in the two previous reports were lower than those (97.5%, 84.3%) in this work. This indicates that higher delignification could result in more degradation of holocellulose. Compared with the lignin removal in other studies, including 56.5% [
25], 62.1% [
26], 67.6% [
27] and 71.4% [
28], the lignin removal (75.0%) in this work was more competitive, which could facilitate the enzymatic digestibility of holocellulose. In conclusion, the optimized pretreatment conditions in this work could retain holocellulose in a large amount, along with simultaneously producing a relatively considerable lignin removal.
Relative to the pretreatment conditions in different reports in
Table 3, the solid loading (200 g/L) adopted in this work was the most competitive, which could enhance pretreatment efficiency and reduce the requirement of reaction vessels. Of course, direct comparisons of temperature and time were not available, as higher temperature always corresponded with shorter time and lower temperature corresponded with longer time, respectively. Even so, the pretreatment temperature (50 °C) in this work was similar to that (50 °C) described by Yu et al. (2020) [
24]. Meanwhile, the time (7.6 h) in this work was shorter than that (24 h) in the work described by Yu et al. (2020) [
24], which indicates that the optimized pretreatment conditions in this work could enhance the pretreatment efficiency and reduce the energy cost for pretreatment. Although the time (7.6 h) in this work was longer than that (6.0 h) described by Zhao et al. (2017) [
27], the higher solid loading (200 g/L) and lower temperature (50 °C) in this work were more competitive. Furthermore, compared with pretreatment conditions described by Li et al. (2019) [
23], Yu et al. (2020) [
24], Chen et al. (2018) [
25] and Yuan et al. (2018) [
26], the uncomplicated experimental operations in this work could reduce manual operations and facilitate large-scale application.
3.2. Optimization of Enzymatic Hydrolysis of the Pretreated Wheat Straw
Tween-80 can protect the activation of cellulases, which may be decreased by lignin; swell the fiber; increase the surface area; and improve the adsorption of enzyme to cellulose [
29]. Therefore, it was adopted during the enzymatic hydrolysis in this work. The statistical analysis of PBD in
Table 4 indicated that the reaction time, enzyme loading and biomass loading had significant effects on glucose and xylose yields. Gupta and Parkhey (2014) [
30] and Pandey and Negi (2015) [
31] reported similar results for the enzymatic hydrolysis of fallen pine foliage and rice straw, respectively. The reaction time plays an important role during enzymatic hydrolysis. Insufficient reaction time can result in low sugar yields, whereas sugar yields always decrease in the later stage of enzymatic hydrolysis due to the recrystallization of cellulose and attachment of enzymes on the amorphous regions of cellulose. Enzyme loading always has an important effect on sugar yields. Too low of an enzyme loading is unfavorable to sugar yields, whereas too high of an enzyme loading is also adverse to sugar yields by increasing the rate of transglycosylation reactions, along with hydrodynamic instability, improper mixing and suspension of slurry [
32]. Biomass loading also has an important role during enzymatic hydrolysis. Too low of a biomass loading can enhance the requirement of reaction vessels. Too high of a biomass loading can result in poor stirring, enzymatic feedback inhibition by end-products and a decrease in the synergistic action of cellulases. In this work, the Tween-80 concentration had an insignificant effect on the sugar yields, which was similar to the results reported by Jin et al. (2016) [
33]. In addition, the reaction temperature had a negative effect and the reaction pH had a positive effect on sugar yields. Meanwhile, the reaction temperature had a significant on sugar yields during the enzymatic hydrolysis of sugarcane bagasse [
34]. The reaction pH had a significant effect on the enzymatic hydrolysis of cotton stalk [
35]. Different effects of the same factors on sugar yields were perhaps related to differences in substrates and cellulase properties. Based on the above results, enzymatic hydrolysis was carried out at 50 °C and pH 4.8 with a supplement of Tween-80 (0.3%,
w/
v) in the following steps.
The results of the steepest ascent method indicate that the glucose and xylose yields reached a plateau while the reaction time, enzyme loading and biomass loading were 36.0 h, 10.0 FPU/g
ds and 90 g/L, respectively. The analysis results of the CCD are shown in
Table 5. It indicates that the linear terms, namely,
X1,
X2 and
X3, and square terms, namely,
X12,
X22 and
X32, had significant effects on the glucose and xylose yields. The interaction terms, namely,
X1X2,
X1X3 and
X2X3, had insignificant effects on the glucose yield. Meanwhile, only
X1X2 had a significant effect on the xylose yield. High
F values of the models (199.12, 203.66) and lack of fit (3.40, 3.25), along with
p-values of the models (
p = 0.000,
p = 0.000) and lack of fit (
p = 0.103,
p = 0.111), indicate that the obtained data were a good fit with the models. High values of
R2 (99.4%, 99.5%) and adjusted
R2 (98.9%, 99.0%) also demonstrated the accuracy of the models.
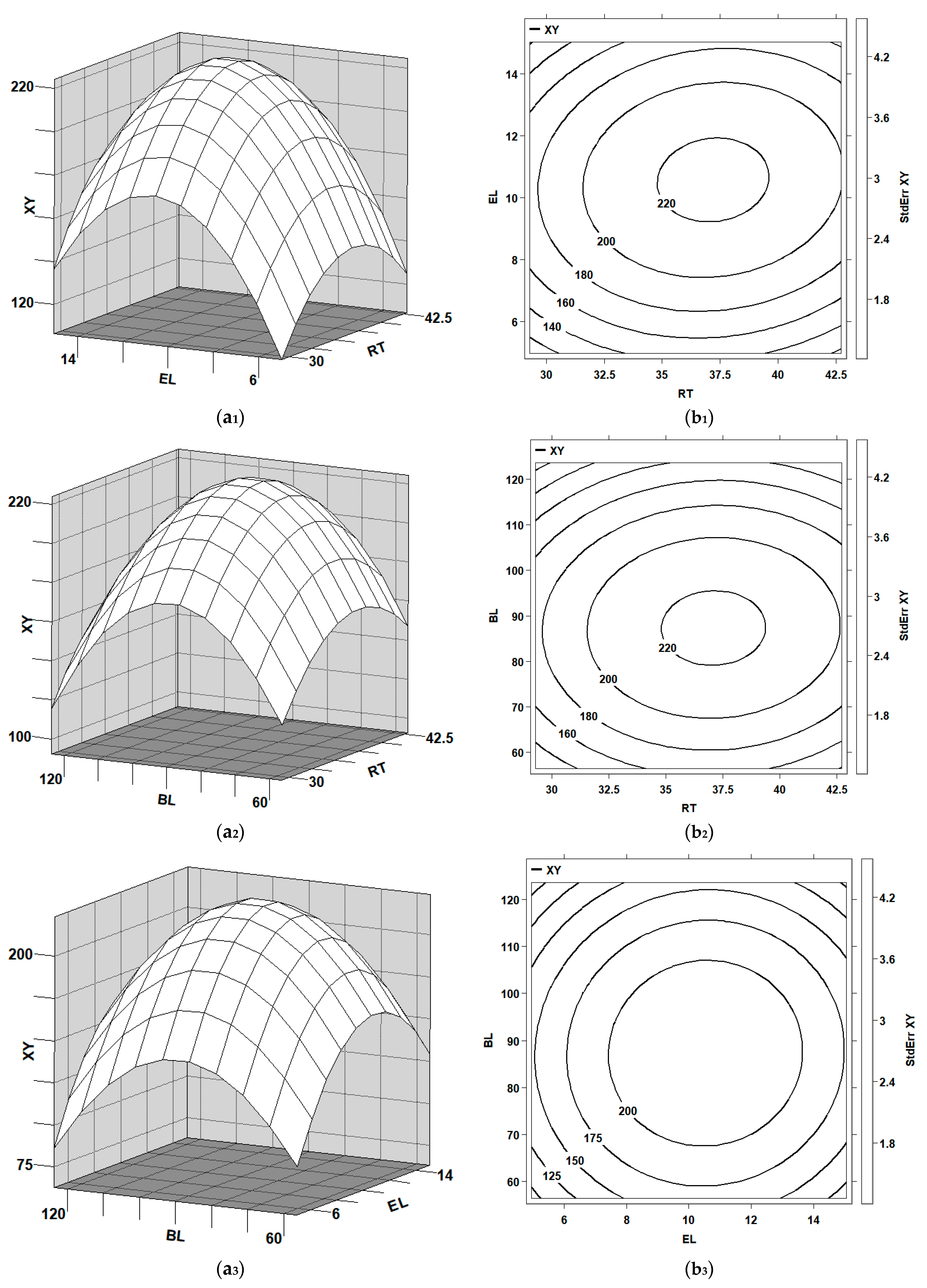
Three-dimensional response surface plots and the corresponding contour plots to demonstrate the combined effects of each variable’s pair on glucose and xylose yields are shown in
Figure 3 and
Figure 4, respectively. As shown in
Figure 3(a
1,b
1) and
Figure 4(a
1,b
1), the optimal region of the reaction time was 35.0 h–37.5 h and that of the enzyme loading was 10.0 FPU/g
ds–12.0 FPU/g
ds while the biomass loading was fixed at its middle level (90 g/L). As shown in
Figure 3(a
2,b
2) and
Figure 4(a
2,b
2), the optimal region of the reaction time was 35.0 h–37.5 h and that of the biomass loading was 80 g/L–90 g/L while the enzyme loading was fixed at its middle level (10.0 FPU/g
ds). As shown in
Figure 3(a
3,b
3) and
Figure 4(a
3,b
3), the optimal region of enzyme loading was 10.0 FPU/g
ds–12.0 FPU/g
ds and that of biomass loading was 80 g/L–90 g/L while reaction time was fixed at its middle level (36.0 h).
According to canonical analysis, the maximal glucose yield (551.1 mg/gds) could be obtained while the pretreated WS was hydrolyzed at 50 °C and pH 4.8 for 36.9 h (X1 = 0.22817) with 10.9 FPU/gds of enzyme loading (X2 = 0.29383) and 88 g/L of biomass loading (X3 = −0.08301). The maximal xylose (224.9 mg/gds) could be obtained while the pretreated WS was hydrolyzed at 50 °C and pH 4.8 for 37.2 h (X1 = 0.29130) by adopting 10.6 FPU/gds of enzyme loading (X2 = 0.19146) and 87 g/L of biomass loading (X3 = −0.12648) with a supplement of Tween-80 (0.3% w/v).
After an adjustment, three confirmatory experiments for enzymatic hydrolysis were carried out at 50 °C and pH 4.8 for 37.0 h with the use of 10.8 FPU/gds of enzyme loading and 88 g/L of biomass loading. The average yields of glucose (552.7 mg/gds) and xylose (223.6 mg/gds) could be obtained, which were close to the predicted values. Compared with the yields of glucose (234.2 mg/gds) and xylose (92.5 mg/gds) before optimization, the optimization resulted in a 1.36-fold increase in the glucose yield and a 1.42-fold increase in the xylose yield. After the calculation, cellulose and hemicellulose conversion were 94.0% and 83.5%, respectively.
Comparisons of sugar yields, along with enzymatic hydrolysis conditions, between different reports are shown in
Table 6. It is obvious that the glucose yield (562.9 mg/g
ds) described by Yu et al. (2020) [
24] was higher than that (552.7 mg/g
ds) in this work. Compared with the other six reports, the glucose yield (552.7 mg/g
ds) in this work was more competitive. As shown in
Table 6, the xylose yield (223.6 mg/g
ds) in this work was higher than those in the other five reports. Though the higher glucose yield (562.9 mg/g
ds) and shorter reaction time (12 h) described by Yu et al. (2020) [
24] were more competitive than those in this work, the adoption of commercial cellulases and lower biomass loading (30 g/L) could increase the enzyme cost and requirement of reaction vessels. Compared with the biomass loading and reaction time in the other six reports, the higher biomass loading (88 g/L) and shorter reaction time (37.0 h) in this work could reduce the enzymatic hydrolysis cost and enhance hydrolysis efficiency. Direct comparisons of enzyme loading in different reports were not available, as the enzyme assay conditions differed from each other. Even so, the enzyme loading (10.8 FPU/g
ds) in this work was lower than 66.3 FPU/g
ds [
24], 38.2 FPU/g
ds [
27,
28], 40.4 FPU/g
ds [
36], 39.7 FPU/g
ds [
37] and 53.9 FPU/g
ds [
38]. The adoption of lower enzyme loading could reduce the enzyme input cost. Though 10.0 FPU/g
ds adopted by Patel et al. (2017) [
39] was lower than that (10.8 FPU/g
ds) in this work, the adoption of commercial cellulases in that work could enhance the enzyme input cost. In addition, the cellulose and hemicellulose conversions (94.0%, 83.5%) in this work were the most competitive among the reports in
Table 6. Higher cellulose conversion and hemicellulose conversion were related to higher enzymatic digestibility of holocellulose in the pretreated WS. Therefore, the optimized enzymatic hydrolysis conditions could enhance the sugar yields, along with higher cellulose and hemicellulose conversion, by using a lower enzyme loading, higher biomass loading and shorter reaction time.