Analysis of Metabolic Differences in the Water Extract of Shenheling Fermented by Lactobacillus fermentum Based on Nontargeted Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Fermentation of SHL
2.3. Metabolite Extraction
2.4. LC–MS/MS Conditions
2.5. Statistical Analysis
3. Results
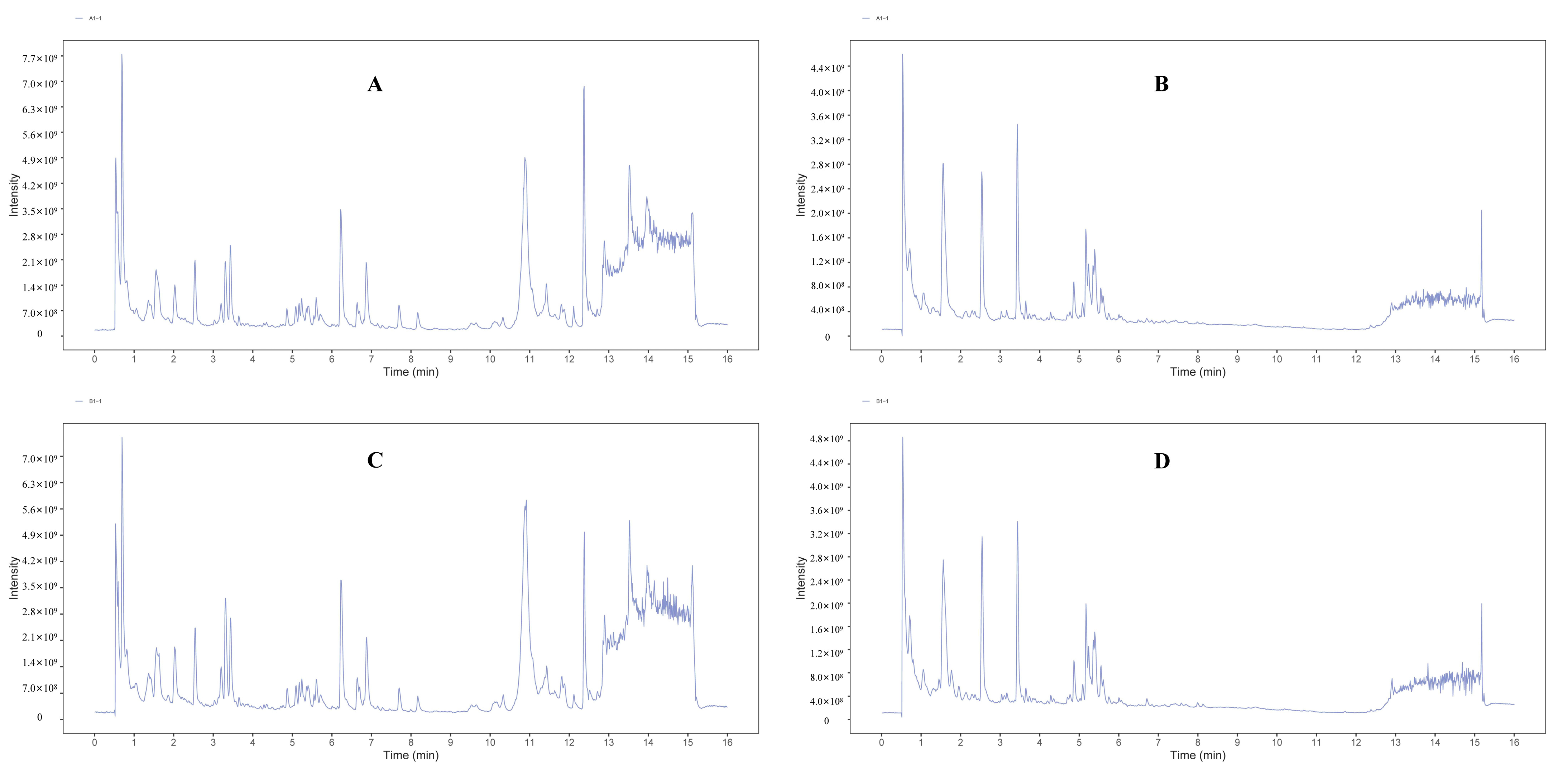
3.1. UHPLC-QE-MS Metabolic Profile Analysis
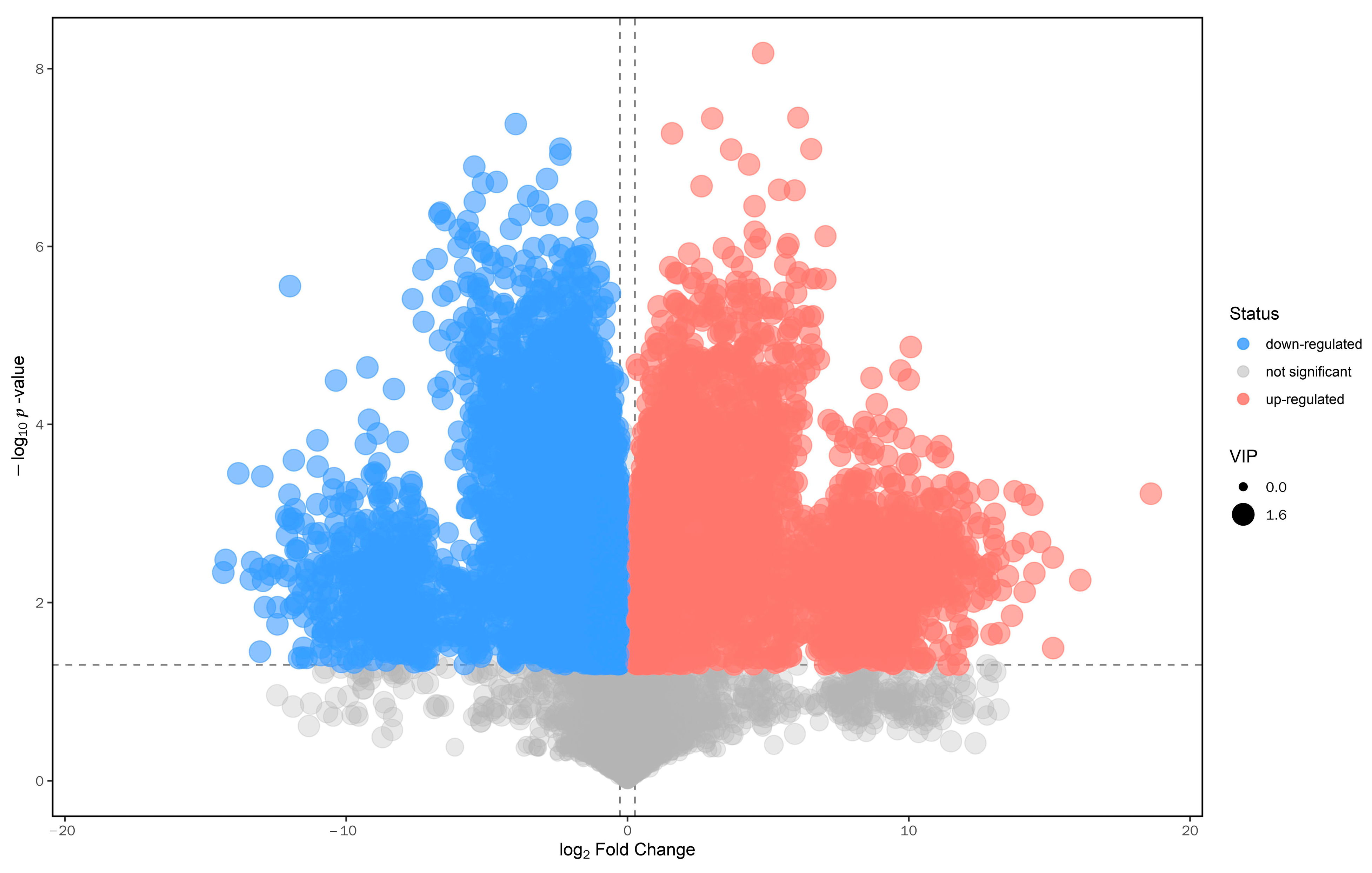
3.2. Multivariate Statistical Analysis of Metabolites before and after Fermentation of SHLE
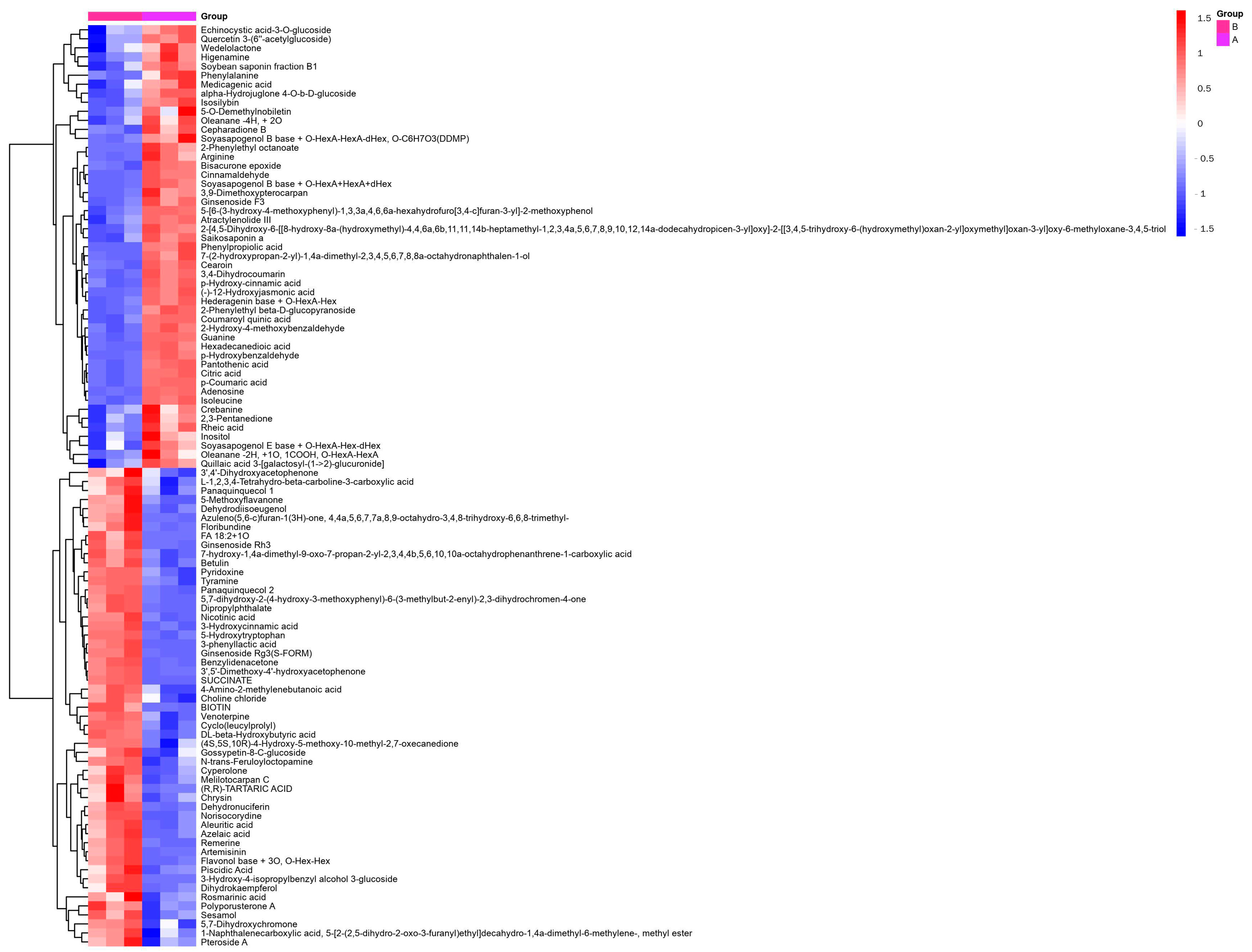
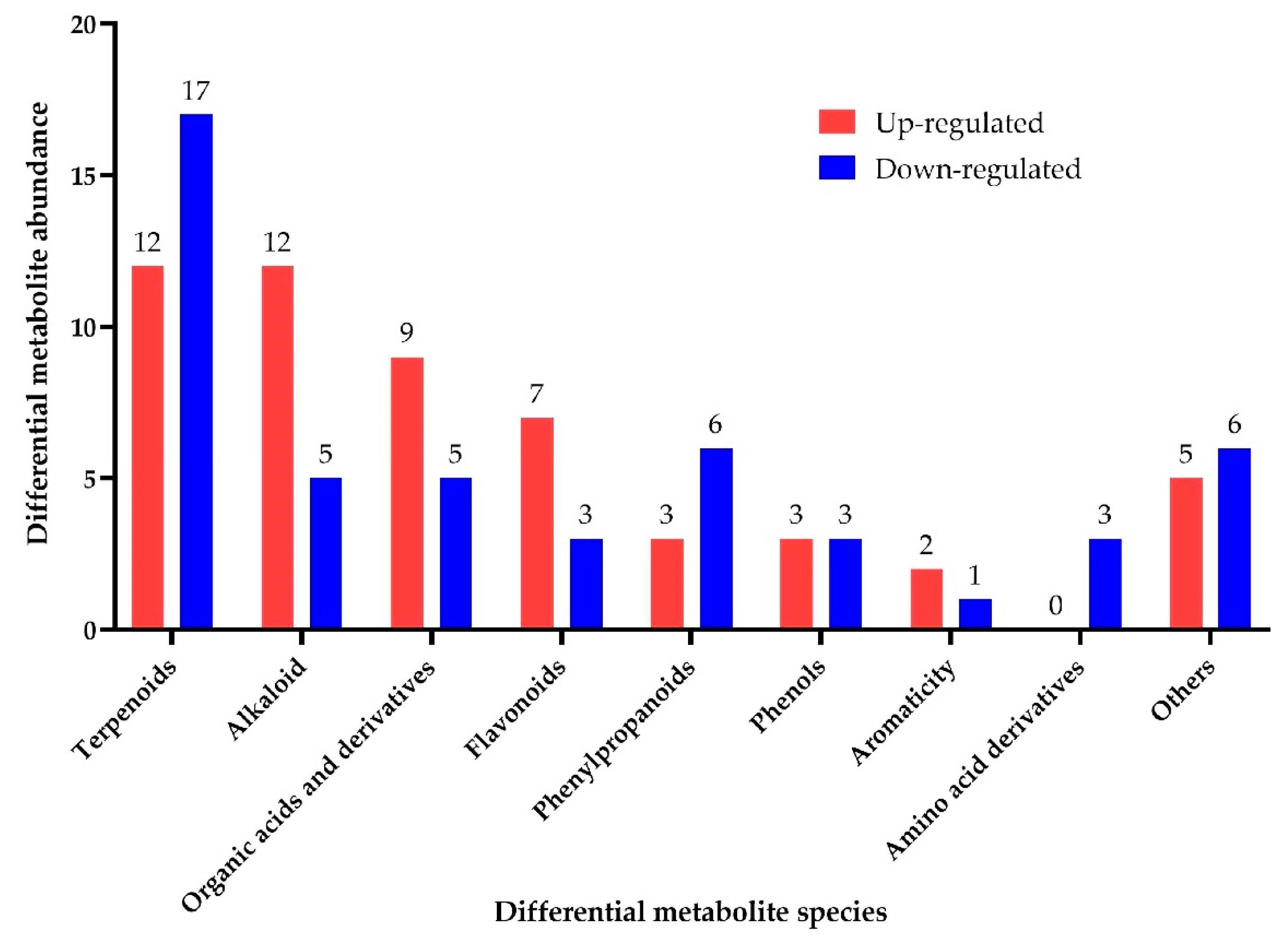
3.3. Analysis of Differential Metabolites before and after Fermentation of SHLE
3.3.1. Differential Terpenoids before and after Fermentation of SHLE
3.3.2. Differential Alkaloid Compounds before and after Fermentation of SHLE
3.3.3. Differential Organic Acids and Derivatives before and after Fermentation of SHLE
3.3.4. Differential Flavonoids before and after Fermentation of SHLE
3.4. Metabolic Pathway Analysis of Differential Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, X.-T.; Zhang, W.; Zhang, Y.; Chen, D.; Wang, W.; Ma, W.; Qu, H.; Qian, J.-Y.; Gu, R. In Vitro Anti-Obesity Effect of Shenheling Extract (SHLE) Fermented with Lactobacillus fermentum grx08. Foods 2022, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang, Y.; Zhang, H. Anticancer effects of ginsenoside Rg3 (Review). Int. J. Mol. Med. 2017, 39, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tan, J.; Long, X.; Yi, R.; Zhao, X.; Park, K.-Y. Anti-obesity effect of fermented lemon peel on high-fat diet-induced obese mice by modulating the inflammatory response. J. Food. Biochem. 2022, 46, e14200. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, M.; Woo, M.; Lee, K.H.; An, B.K.; Yokozawa, T.; Song, Y.O. Comparison of the Effects of Nonfermented and Fermented Panax ginseng Root Against Hypertriglycemia in High-Fat Diet-Fed Mice. J. Med. Food 2018, 21, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.N.; Kim, S.H.; Dey, D.K.; Park, S.M.; Nasif, O.; Bajpai, V.K.; Kang, S.C.; Lee, J.; Park, J.G. 5-O-Demethylnobiletin Alleviates CCl(4)-Induced Acute Liver Injury by Equilibrating ROS-Mediated Apoptosis and Autophagy Induction. Int. J. Mol. Sci. 2021, 22, 1083. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Monteiro, A.F.M.; de Oliveira Viana, J.; Mendonca Junior, F.J.B.; Ishiki, H.M.; Tchouboun, E.N.; Santos, R.; Scotti, M.T. Multi-Target Drugs Against Metabolic Disorders. Endocr. Metab. Immune. Disord. Drug. Targets. 2019, 19, 402–418. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Zhou, L.; Zhou, X.; Xie, B.; Zhang, W.; Sun, J. Prevention and treatment of COVID-19 using Traditional Chinese Medicine: A review. Phytomedicine 2021, 85, 153308. [Google Scholar] [CrossRef]
- Hwang, J.E.; Kim, K.T.; Paik, H.D. Improved Antioxidant, Anti-inflammatory, and Anti-adipogenic Properties of Hydroponic Ginseng Fermented by Leuconostoc mesenteroides KCCM 12010P. Molecules 2019, 24, 3359. [Google Scholar] [CrossRef]
- Song, M.-W.; Park, J.-Y.; Lee, H.-S.; Kim, K.-T.; Paik, H.-D. Co-Fermentation by Lactobacillus brevis B7 Improves the Antioxidant and Immunomodulatory Activities of Hydroponic Ginseng-Fortified Yogurt. Antioxidants 2021, 10, 1447. [Google Scholar] [CrossRef]
- Kim, C.; Ji, J.; Ho Baek, S.; Lee, J.H.; Ha, I.J.; Lim, S.S.; Yoon, H.J.; Je Nam, Y.; Ahn, K.S. Fermented dried Citrus unshiu peel extracts exert anti-inflammatory activities in LPS-induced RAW264.7 macrophages and improve skin moisturizing efficacy in immortalized human HaCaT keratinocytes. Pharm. Biol. 2019, 57, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Charchoghlyan, H.; Lee, J.S.; Kim, M. Bioactive compounds and antioxidant activities of the Korean lotus leaf (Nelumbo nucifera) condiment: Volatile and nonvolatile metabolite profiling during fermentation. Int. J. Food. Sci. Technol. 2015, 50, 1988–1995. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.; Park, J.H.; Lee, J.S.; Kim, M. Development of novel Meju starter culture using plant extracts with reduced Bacillus cereus counts and enhanced functional properties. Sci. Rep. 2017, 7, 11409. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Lactic acid fermentation: A novel approach to eliminate unpleasant aroma in pea protein isolates. Lwt 2021, 150, 111927. [Google Scholar] [CrossRef]
- Gao, P.; Xu, G. Mass-spectrometry-based microbial metabolomics: Recent developments and applications. Anal. Bioanal. Chem. 2015, 407, 669–680. [Google Scholar] [CrossRef]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef]
- Godzien, J.; Ciborowski, M.; Martínez-Alcázar, M.P.; Paulina Samczuk; Adam Kretowski; Barbas, C. Rapid and reliable identification of phospholipids for untargeted metabolomics with LC-ESI-QTOF-MS/MS. J. Proteome. Res. 2015, 14, 3204–3216. [Google Scholar] [CrossRef]
- Dunn, W.B.; Wilson, I.D.; Nicholls, A.W.; Broadhurst, D. The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. Bioanalysis 2012, 4, 2249–2264. [Google Scholar] [CrossRef]
- Huang, B.M.; Zha, Q.L.; Chen, T.B.; Xiao, S.Y.; Xie, Y.; Luo, P.; Wang, Y.P.; Liu, L.; Zhou, H. Discovery of markers for discriminating the age of cultivated ginseng by using UHPLC-QTOF/MS coupled with OPLS-DA. Phytomedicine 2018, 45, 8–17. [Google Scholar] [CrossRef]
- Ai, J.; Wu, Q.; Battino, M.; Bai, W.; Tian, L. Using untargeted metabolomics to profile the changes in roselle (Hibiscus sabdariffa L.) anthocyanins during wine fermentation. Food. Chem. 2021, 364, 130425. [Google Scholar] [CrossRef]
- Kim, R.; Son, S.-R.; Lee, N.-K.; Kim, J.-Y.; An, G.; Choi, J.-H.; Jang, D.S. Cytotoxic Properties of C17 Polyacetylenes from the Fresh Roots of Panax ginseng on Human Epithelial Ovarian Cancer Cells. Molecules 2022, 27, 7027. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghanayem, A.A. Phytochemical analysis of Cymbopogon flexuosus (lemongrass) oil, its antifungal activity, and role in inhibiting biofilm formation in Candida albicans MTCC854. J. King. Saud. Univ. Sci. 2022, 34. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C(17) and C(18) Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Zhao, Q.; Yang, B.; Cong, D.; Zhou, Y.; Lei, X.; Zhang, X. Ginsenoside Rh3 Inhibits Proliferation and Induces Apoptosis of Colorectal Cancer Cells. Pharmacology 2020, 105, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Park, J.S.; Lee, E.J.; Lee, S.Y.; Kim, D.H.; Kang, J.L.; Kim, H.S. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: Critical role of 5′-adenosine monophosphate-activated protein kinase signaling pathway. J. Agric. Food. Chem. 2015, 63, 3472–3480. [Google Scholar] [CrossRef]
- Lee, H.; Kong, G.; Tran, Q.; Kim, C.; Park, J.; Park, J. Relationship Between Ginsenoside Rg3 and Metabolic Syndrome. Front. Pharm. 2020, 11, 130. [Google Scholar] [CrossRef]
- Liu, X.; Mi, X.; Wang, Z.; Zhang, M.; Hou, J.; Jiang, S.; Wang, Y.; Chen, C.; Li, W. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell. Death Dis. 2020, 11, 454. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Son, B.M.; Damodharan, K.; Suh, J.-W.; Yang, S.H. Fermentative transformation of ginsenoside Rb1 from Panax ginseng C. A. Meyer to Rg3 and Rh2 by Lactobacillus paracasei subsp. tolerans MJM60396. Biotechnol. Bioprocess. Eng. 2016, 21, 587–594. [Google Scholar] [CrossRef]
- Tang, J.J.; Li, J.G.; Qi, W.; Qiu, W.W.; Li, P.S.; Li, B.L.; Song, B.L. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell. Metab. 2011, 13, 44–56. [Google Scholar] [CrossRef]
- Li, K.K.; Xu, F.; Gong, X.J. Isolation, Purification and Quantification of Ginsenoside F(5) and F(3) Isomeric Compounds from Crude Extracts of Flower Buds of Panax ginseng. Molecules 2016, 21, 315. [Google Scholar] [CrossRef]
- Andersen, G.; Marcinek, P.; Sulzinger, N.; Schieberle, P.; Krautwurst, D. Food sources and biomolecular targets of tyramine. Nutr. Rev. 2019, 77, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, Z.; Li, Q. Nicotinic acid supplementation contributes to the amelioration of Alzheimer's disease in mouse models. Ann. Transl. Med. 2022, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef]
- Zhang, N.; Lian, Z.; Peng, X.; Li, Z.; Zhu, H. Applications of Higenamine in pharmacology and medicine. J. Ethnopharmacol. 2017, 196, 242–252. [Google Scholar] [CrossRef]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Gerez, C.L.; Carbajo, M.S.; Rollan, G.; Torres Leal, G.; Font de Valdez, G. Inhibition of citrus fungal pathogens by using lactic acid bacteria. J. Food. Sci. 2010, 75, M354–M359. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.; Salgado, J.M.; Cortés, S.; Domínguez, J.M. Antimicrobial activity of d-3-phenyllactic acid produced by fed-batch process against Salmonella enterica. Food. Control 2012, 25, 274–284. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, K.T.; Kwon, J.; Lee, H.J.; Lee, D.; Mar, W. Neuroprotection against 6-OHDA-induced oxidative stress and apoptosis in SH-SY5Y cells by 5,7-Dihydroxychromone: Activation of the Nrf2/ARE pathway. Life Sci. 2015, 130, 25–30. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, C.; Li, X.; Liang, L.; Zhang, M.; Chen, B.; Liu, X.; Yang, D. Protective Effect of Dihydrokaempferol on Acetaminophen-Induced Liver Injury by Activating the SIRT1 Pathway. Am. J. Chin. Med. 2021, 49, 705–718. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Xu, N.; Zhang, X.C.; Zhu, Y.Y.; Liu, S.W.; Chang, Y.N. Chrysin Improves Glucose and Lipid Metabolism Disorders by Regulating the AMPK/PI3K/AKT Signaling Pathway in Insulin-Resistant HepG2 Cells and HFD/STZ-Induced C57BL/6J Mice. J. Agric. Food. Chem. 2021, 69, 5618–5627. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham Ul, H.; IqraYasmin, U.H.; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Liu, X.; Hu, M.; Ye, C.; Liao, L.; Ding, C.; Sun, L.; Liang, J.; Chen, Y. Isosilybin regulates lipogenesis and fatty acid oxidation via the AMPK/SREBP-1c/PPARalpha pathway. Chem. Biol. Interact. 2022, 368, 110250. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Liu, M.; Guo, C.; Lian, X.; Shen, Y.; Liu, Y.; Qian, Y.; Zhang, L.; Wang, W.; Chen, D.; et al. Analysis of Metabolic Differences in the Water Extract of Shenheling Fermented by Lactobacillus fermentum Based on Nontargeted Metabolomics. Fermentation 2023, 9, 44. https://doi.org/10.3390/fermentation9010044
Yan X, Liu M, Guo C, Lian X, Shen Y, Liu Y, Qian Y, Zhang L, Wang W, Chen D, et al. Analysis of Metabolic Differences in the Water Extract of Shenheling Fermented by Lactobacillus fermentum Based on Nontargeted Metabolomics. Fermentation. 2023; 9(1):44. https://doi.org/10.3390/fermentation9010044
Chicago/Turabian StyleYan, Xiantao, Min Liu, Congcong Guo, Xinyue Lian, Yun Shen, Yang Liu, Yi Qian, Longfei Zhang, Wenqiong Wang, Dawei Chen, and et al. 2023. "Analysis of Metabolic Differences in the Water Extract of Shenheling Fermented by Lactobacillus fermentum Based on Nontargeted Metabolomics" Fermentation 9, no. 1: 44. https://doi.org/10.3390/fermentation9010044
APA StyleYan, X., Liu, M., Guo, C., Lian, X., Shen, Y., Liu, Y., Qian, Y., Zhang, L., Wang, W., Chen, D., Qian, J., & Gu, R. (2023). Analysis of Metabolic Differences in the Water Extract of Shenheling Fermented by Lactobacillus fermentum Based on Nontargeted Metabolomics. Fermentation, 9(1), 44. https://doi.org/10.3390/fermentation9010044





