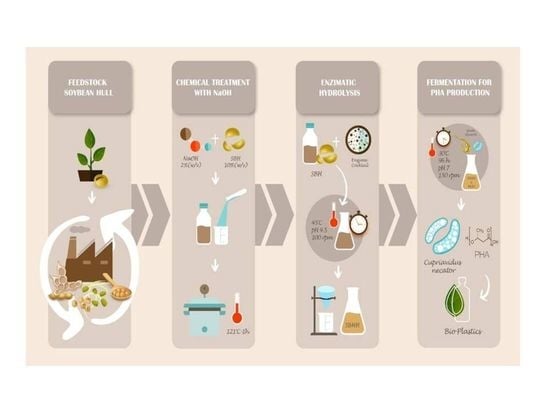
Production of Polyhydroxyalkanoates through Soybean Hull and Waste Glycerol Valorization: Subsequent Alkaline Pretreatment and Enzymatic Hydrolysis
Abstract
:1. Introduction
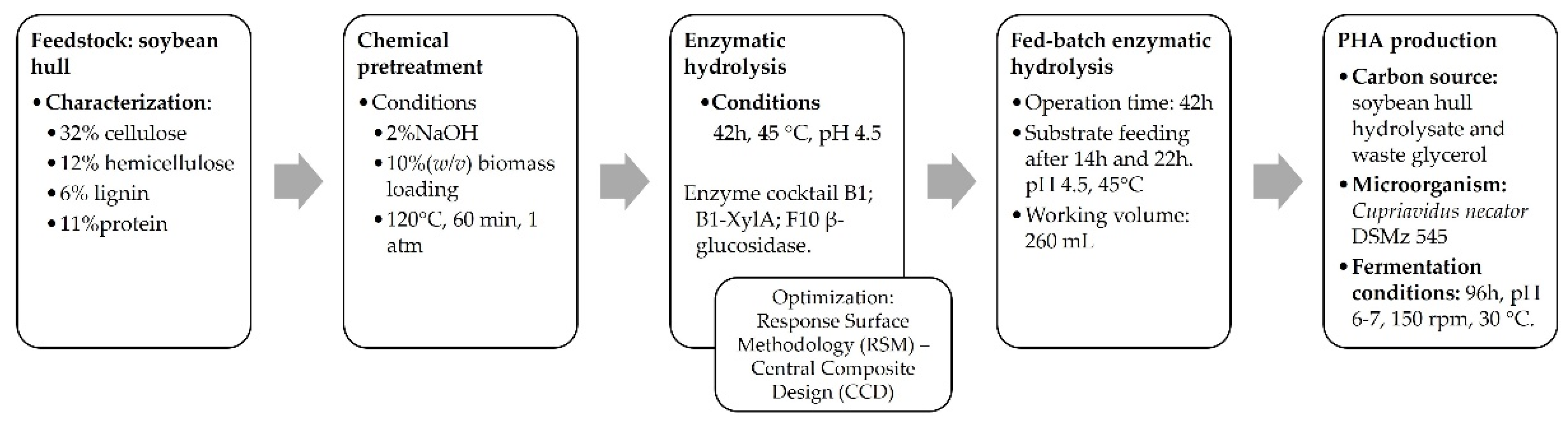
2. Material and Methods
2.1. Characterization of Soybean Hull
2.2. Pretreatment Conditions of Soybean Hulls
2.3. Optimization of Enzymatic Hydrolysis
2.3.1. First Step of SBH Enzymatic Hydrolysis Optimization
2.3.2. Second Step of SBH Fed-Batch Enzymatic Hydrolysis Optimization
2.4. PHA Production
2.5. Analytical Procedures
3. Results and Discussion
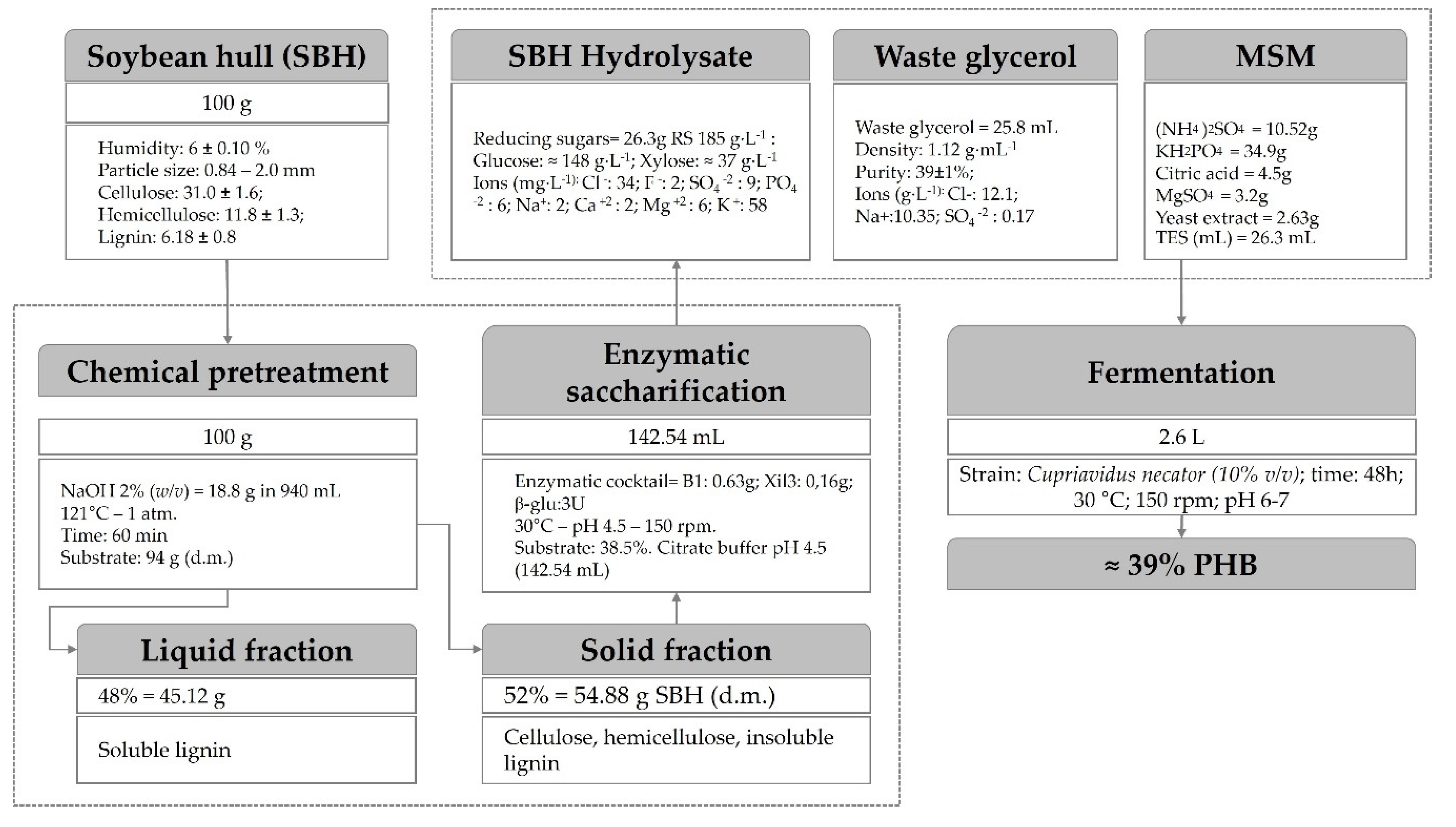
3.1. Characterization of Soybean Hull and Waste Glycerol
3.2. Alkaline Pretreatment of Soybean Hull
3.3. Optimization of Enzymatic Hydrolysis of Soybean Hull
3.3.1. Batch Enzymatic Hydrolysis Process
3.3.2. Fed-Batch Enzymatic Hydrolysis Process
3.4. PHA Production from Alternative Substrates
3.4.1. Inoculum Development and Preparation
3.4.2. PHB Production
3.4.3. PHB Production Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, T.; Zhao, D.; Khodadadi, M.; Len, C. Lignocellulosic biomass for bioethanol: Recent advances, technology trends, and barriers to industrial development. Curr. Opin. Green Sustain. Chem. 2020, 24, 56–60. [Google Scholar] [CrossRef]
- Nahak, B.; Preetam, S.; Sharma, D.; Shukla, S.; Syväjärvi, M.; Toncu, D.-C.; Tiwari, A. Advancements in net-zero pertinency of lignocellulosic biomass for climate neutral energy production. Renew. Sustain. Energy Rev. 2022, 161, 112393. [Google Scholar] [CrossRef]
- Niju, S.; Swathika, M.; Balajii, M. Pretreatment of lignocellulosic sugarcane leaves and tops for bioethanol production. In Lignocellulosic Biomass to Liquid Biofuels; Academic Press: Cambridge, MA, USA, 2020; pp. 301–324. [Google Scholar] [CrossRef]
- Embrapa. Soja Em Números (Safra 2020/21); Empresa Brasileira de Pesquisa Agropecuária Embrapa Soja Ministério Da Agricultura, Pecuária e Abastecimento; Embrapa: Brasília, Brazil, 2022. [Google Scholar]
- Amaro Bittencourt, G.; Porto de Souza Vandenberghe, L.; Valladares-Diestra, K.; Wedderhoff Herrmann, L.; Fátima Murawski de Mello, A.; Sarmiento Vásquez, Z.; Grace Karp, S.; Ricardo Soccol, C. Soybean Hulls as Carbohydrate Feedstock for Medium to High-Value Biomolecule Production in Biorefineries: A Review. Bioresour. Technol. 2021, 339, 125594. [Google Scholar] [PubMed]
- Abdul Raman, A.A.; Tan, H.W.; Buthiyappan, A. Two-Step Purification of Glycerol as a Value Added by Product from the Biodiesel Production Process. Front. Chem. 2019, 7, 774. [Google Scholar] [CrossRef]
- ANP Anuário Estatístico; Agência Nacional Do Petróleo, Gás Natural e Biocombustíveis: Rio de Janeiro, Brazil, 2021.
- Ju, J.-H.; Wang, D.; Heo, S.-Y.; Kim, M.-S.; Seo, J.-W.; Kim, Y.-M.; Kim, D.-H.; Kang, S.-A.; Kim, C.-H.; Oh, B.-R. Enhancement of 1,3-propanediol production from industrial by-product by Lactobacillus reuteri CH53. Microb. Cell Factories 2020, 19, 6. [Google Scholar] [CrossRef]
- Goyal, S.; Hernández, N.B.; Cochran, E.W. An update on the future prospects of glycerol polymers. Polym. Int. 2021, 70, 911–917. [Google Scholar] [CrossRef]
- UNEP Beat Plastic Pollution. Visual Feature | Beat Plastic Pollution (unep.org). Available online: https://www.unep.org/interactives/beat-plastic-pollution/ (accessed on 21 August 2021).
- PlasticsEurope. An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Bruxelles, Belgium, 2021. [Google Scholar]
- Environmental Protection Agency Facts and Figures about Materials, Waste and Recycling. Plastics: Material-Specific Data. Plastics: Material-Specific Data | US EPA. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 21 August 2021).
- Gahlawat, G. Polyhydroxyalkanoates Biopolymers—Production Strategies; Navard, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Li, M.; Wilkins, M. Fed-batch cultivation and adding supplements to increase yields of polyhydroxybutyrate production by Cupriavidus necator from corn stover alkaline pretreatment liquor. Bioresour. Technol. 2020, 299, 122676. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Karp, S.G.; Osipov, D.O.; Semenova, M.V.; Rozhkova, A.M.; Zorov, I.N.; Sinitsyna, O.A.; Soccol, C.R.; Sinitsyn, A.P. Effect of Novel Penicillium verruculosum Enzyme Preparations on the Saccharification of Acid- and Alkali-Pretreated Agro-Industrial Residues. Agronomy 2020, 10, 1348. [Google Scholar] [CrossRef]
- Sarmiento-Vásquez, Z.; Vandenberghe, L.; Rodrigues, C.; Tanobe, V.O.A.; Marín, O.; de Melo Pereira, G.V.; Ghislain Rogez, H.L.; Góes-Neto, A.; Soccol, C.R. Cocoa pod husk valorization: Alkaline-enzymatic pre-treatment for propionic acid production. Cellulose 2021, 28, 4009–4024. [Google Scholar] [CrossRef]
- Bittencourt, G.A.; Vandenberghe, L.P.D.S.; Valladares-Diestra, K.K.; Soccol, C.R. Soybean hull valorization for sugar production through the optimization of citric acid pretreatment and enzymatic hydrolysis. Ind. Crop. Prod. 2022, 186, 115178. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Qing, Q.; Guo, Q.; Zhou, L.; Gao, X.; Lu, X.; Zhang, Y. Comparison of alkaline and acid pretreatments for enzymatic hydrolysis of soybean hull and soybean straw to produce fermentable sugars. Ind. Crop. Prod. 2017, 109, 391–397. [Google Scholar] [CrossRef]
- Karp, S.G.; Rozhkova, A.M.; Semenova, M.V.; Osipov, D.O.; de Pauli, S.T.Z.; Sinitsyna, O.A.; Zorov, I.N.; de Souza Vandenberghe, L.P.; Soccol, C.R.; Sinitsyn, A.P. Designing enzyme cocktails from Penicillium and Aspergillus species for the enhanced saccharification of agro-industrial wastes. Bioresour. Technol. 2021, 330, 124888. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Alavi, S.; Vadlani, P.; Amanor-Boadu, V. Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars. Bioresour. Technol. 2011, 102, 7583–7590. [Google Scholar] [CrossRef]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Andrew Lin, K.Y.; Tsang, D.C.W.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Mozumder, M.S.I.; de Wever, H.; Volcke, E.I.P.; Garcia-Gonzalez, L. A robust fed-batch feeding strategy in-dependent of the carbon source for optimal polyhydroxybutyrate production. Process Biochem. 2014, 49, 365–373. [Google Scholar] [CrossRef]
- García, I.L.; López, J.A.; Dorado, M.P.; Kopsahelis, N.; Alexandri, M.; Papanikolaou, S.; Villar, M.A.; Koutinas, A.A. Evaluation of by-products from the biodiesel industry as fermentation feedstock for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Cupriavidus necator. Bioresour. Technol. 2013, 130, 16–22. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; de Almeida, M.C.M.D.; Grandfils, C.; da Fonseca, M.M.R. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicyclic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Evangeline, S.; Sridharan, T.B. Biosynthesis and statistical optimization of polyhydroxyalkanoate (PHA) produced by Bacillus cereus VIT-SSR1 and fabrication of biopolymer films for sustained drug release. Int. J. Biol. Macromol. 2019, 135, 945–958. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N. Production of polyhydroxybutyrate from wheat bran hydrolysate using Ralstonia eutropha through microbial fermentation. J. Biotechnol. 2016, 237, 13–17. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, B.; Mu, X. Review of Alkali-Based Pretreatment To Enhance Enzymatic Saccharification for Lignocellulosic Biomass Conversion. Ind. Eng. Chem. Res. 2016, 55, 8691–8705. [Google Scholar] [CrossRef]
- Ganesan, R.; Manigandan, S.; Shanmugam, S.; Chandramohan, V.P.; Sindhu, R.; Kim, S.-H.; Brindhadevi, K.; Pugazhendhi, A. A detailed scrutinize on panorama of catalysts in biodiesel synthesis. Sci. Total Environ. 2021, 777, 145683. [Google Scholar] [CrossRef]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.; Mohamed, T.A.; Zheng, G.; Qu, F.; Wang, F.; Zhao, Y.; Song, C. Lignocellulose biomass bioconversion during composting: Mechanism of action of lignocellulase, pretreatment methods and future perspectives. Chemosphere 2022, 286, 131635. [Google Scholar] [CrossRef]
- Saha, B.C.; Cotta, M.A. Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzym. Microb. Technol. 2007, 41, 528–532. [Google Scholar] [CrossRef]
- Camiscia, P.; Giordano, E.D.; Brassesco, M.E.; Fuciños, P.; Pastrana, L.; Cerqueira, M.; Picó, G.A.; Valetti, N.W. Comparison of soybean hull pre-treatments to obtain cellulose and chemical derivatives: Physical chemistry characterization. Carbohydr. Polym. 2018, 198, 601–610. [Google Scholar] [CrossRef]
- Bastos, J.A.; Remor, P.V.; Alino, J.H.L.; Frare, L.M.; Lofhagen, J.C.P.; Edwiges, T. Hydrolysate recycling improves economic feasibility of alkaline pretreatment for bioenergy production. J. Environ. Chem. Eng. 2021, 9, 105935. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, T.; Geng, W.; Yang, L. Comparison of sodium carbonate pretreatment for enzymatic hydrolysis of wheat straw stem and leaf to produce fermentable sugars. Bioresour. Technol. 2013, 137, 294–301. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Cortivo, P.R.D.; Hickert, L.R.; Rosa, C.A.; Ayub, M.A.Z. Conversion of fermentable sugars from hydrolysates of soybean and oat hulls into ethanol and xylitol by Spathaspora hagerdaliae UFMG-CM-Y303. Ind. Crop. Prod. 2020, 146, 112218. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-Escoto, H. Enzymatic hydrolysis of biomass at high-solids loadings through fed-batch operation. Biomass-Bioenergy 2018, 119, 191–197. [Google Scholar] [CrossRef]
- Mukasekuru, M.R.; Kaneza, P.; Sun, H.; Sun, F.F.; He, J.; Zheng, P. Fed-batch high-solids enzymatic saccharification of lignocellulosic substrates with a combination of additives and accessory enzymes. Ind. Crop. Prod. 2020, 146, 112156. [Google Scholar] [CrossRef]
- Gonzalez-Rios, J.A.; Valle-Pérez, A.U.; Amaya-Delgado, L.; Sanchez, A. A quick fed-batch saccharification strategy of wheat straw at high solid loadings improving lignocellulosic ethanol productivity. Biomass-Convers. Biorefinery 2021, 1–13. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; de Almeida, M.C.M.D.; da Fonseca, M.M.R.; de Carvalho, C.C.C.R. Adaptation of Cupriavidus necator to conditions favoring polyhydroxyalkanoate production. J. Biotechnol. 2012, 164, 309–317. [Google Scholar] [CrossRef]
- Sirohi, R.; Pandey, J.P.; Gaur, V.K.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef]
- Oh, Y.H.; Lee, S.H.; Jang, Y.-A.; Choi, J.W.; Hong, K.S.; Yu, J.H.; Shin, J.; Song, B.K.; Mastan, S.G.; David, Y.; et al. Development of rice bran treatment process and its use for the synthesis of polyhydroxyalkanoates from rice bran hydrolysate solution. Bioresour. Technol. 2015, 181, 283–290. [Google Scholar] [CrossRef]
- Silva, L.F.; Taciro, M.K.; Ramos, M.E.M.; Carter, J.M.; Pradella, J.G.C.; Gomez, J.G.C. Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 2004, 31, 245–254. [Google Scholar] [CrossRef]
- Ahn, J.; Jho, E.H.; Nam, K. Effect of C/N ratio on polyhydroxyalkanoates (PHA) accumulation by Cupriavidus necator and its implication on the use of rice straw hydrolysates. Environ. Eng. Res. 2015, 20, 246–253. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.-K. Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.Y.; Hussin, M.H.; Baidurah, S. Biosynthesis of poly(3-hydroxybutyrate) (PHB) by Cupriavidus necator from various pretreated molasses as carbon source. Biocatal. Agric. Biotechnol. 2019, 17, 51–59. [Google Scholar] [CrossRef]
- Gahlawat, G.; Soni, S.K. Valorization of waste glycerol for the production of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus necator and extraction in a sustainable manner. Bioresour. Technol. 2017, 243, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Mothes, G.; Schnorpfeil, C.; Ackermann, J.-U. Production of PHB from Crude Glycerol. Eng. Life Sci. 2007, 7, 475–479. [Google Scholar] [CrossRef]
- Wang, X.; Xia, K.; Yang, X.; Tang, C. Growth strategy of microbes on mixed carbon sources. Nat. Commun. 2019, 10, 1279. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Koller, M.; Dias, M.M.-D.S.; Calafell-Monfort, M.; Braunegg, G.; Marqués-Calvo, M.S. Influence of glycerol on poly(3-hydroxybutyrate) production by Cupriavidus necator and Burkholderia sacchari. Biochem. Eng. J. 2015, 94, 50–57. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Otari, S.V.; Jeon, J.-M.; Gurav, R.; Choi, Y.-K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Banu, J.R.; Yoon, J.-J.; et al. Biowaste-to-bioplastic (polyhydroxyalkanoates): Conversion technologies, strategies, challenges, and perspective. Bioresour. Technol. 2021, 326, 124733. [Google Scholar] [CrossRef]
- Poomipuk, N.; Reungsang, A.; Plangklang, P. Poly-β-hydroxyalkanoates production from cassava starch hydrolysate by Cupriavidus sp. KKU38. Int. J. Biol. Macromol. 2014, 65, 51–64. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, B.H.; Kim, B.S. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Ralstonia eutropha. Biochem. Eng. J. 2005, 23, 169–174. [Google Scholar] [CrossRef]



| Independent Variable | –1.682 | –1 | 0 | +1 | +1.682 |
|---|---|---|---|---|---|
| Substrate loading (% w/v) | 15.0 | 24.5 | 38.5 | 52.5 | 62.0 |
| Saccharification time (h) | 12.0 | 24.2 | 42.0 | 59.8 | 72.0 |
| Enzyme concentrations (g of enzyme/g SBH) | |||||
| B1 | 3.28 | 6.62 | 11.52 | 16.42 | 19.76 |
| β-glucosidase F10 | 16.40 | 33.10 | 57.59 | 82.08 | 98.78 |
| XylA | 0.82 | 1.66 | 2.88 | 4.10 | 4.49 |
| Ions | Concentration (mg·L−1) | ||
|---|---|---|---|
| Aqueous Extract SBH | SBH Hydrolysate | Waste Glycerol | |
| Soluble anions | |||
| Cl− | 34 | 135 | 12,150 |
| F− | 2 | 278 | nd |
| SO4−2 | 9 | 84 | 170 |
| PO4−3 | 6 | nd | nd |
| Br | nd | nd | nd |
| NOx− | nd | nd | nd |
| Soluble cations | |||
| Na+ | 2 | 630 | 10,350 |
| Ca+2 | 6 | 110 | nd |
| Mg+2 | 6 | 60 | nd |
| K+ | 58 | nd | nd |
| NH4+ | nd | nd | nd |
| Standard Run | Enzyme Cocktail | Substrate (d.m.) (% (w/v)) | Time (h) | Reducing Sugars (g∙L−1) | Yield (g Glucose/g SBH) | ||
|---|---|---|---|---|---|---|---|
| B1 (mg of Protein/g of Substrate) | β-Glucosidase (U/g of Substrate) | Xylanase A (mg of Protein/g of Substrate) | |||||
| 1 | 6.62 | 33.10 | 1.66 | 24.5 | 24.2 | 60.7 | 0.15 |
| 2 | 6.62 | 33.10 | 1.66 | 24.5 | 59.8 | 64.9 | 0.16 |
| 3 | 6.62 | 33.10 | 1.66 | 52.5 | 24.2 | 61.9 | 0.07 |
| 4 | 6.62 | 33.10 | 1.66 | 52.5 | 59.8 | 99.3 | 0.12 |
| 5 | 16.42 | 82.08 | 4.10 | 24.5 | 24.2 | 91.9 | 0.23 |
| 6 | 16.42 | 82.08 | 4.10 | 24.5 | 59.8 | 63.9 | 0.16 |
| 7 | 16.42 | 82.08 | 4.10 | 52.5 | 24.2 | 89.9 | 0.11 |
| 8 | 16.42 | 82.08 | 4.10 | 52.5 | 59.8 | 84.2 | 0.10 |
| 9 | 3.28 | 16.40 | 0.82 | 38.5 | 42.0 | 76.0 | 0.12 |
| 10 | 19.76 | 98.78 | 4.94 | 38.5 | 42.0 | 72.7 | 0.12 |
| 11 | 11.52 | 57.59 | 2.88 | 15.0 | 42.0 | 42.3 | 0.17 |
| 12 | 11.52 | 57.59 | 2.88 | 62.0 | 42.0 | 115.9 | 0.12 |
| 13 | 11.52 | 57.59 | 2.88 | 38.5 | 12.0 | 71.8 | 0.12 |
| 14 | 11.52 | 57.59 | 2.88 | 38.5 | 72.0 | 78.6 | 0.13 |
| 15 (C) | 11.52 | 57.59 | 2.88 | 38.5 | 42.0 | 86.6 | 0.14 |
| 16 (C) | 11.52 | 57.59 | 2.88 | 38.5 | 42.0 | 82.7 | 0.13 |
| 17 (C) | 11.52 | 57.59 | 2.88 | 38.5 | 42.0 | 79.4 | 0.13 |
| 18 (C) | 11.52 | 57.59 | 2.88 | 38.5 | 42.0 | 79.2 | 0.13 |
| Carbon Source | Inoculum | PHB Production | PHB | ||
|---|---|---|---|---|---|
| Biomass (g·L−1) | Carbon Source Consumption (%) | Biomass (g·L−1) | Carbon Source Consumption (%) | ||
| SBHH + WG | 6.7 ± 0.4 | 48 | 8.7 ± 0.1 | 50 | 39.0 ± 0.8 |
| SBHH | 9.0 ± 0.4 | 91 | 7.8 ± 0.2 | 77 | 31.8 ± 0.2 |
| WG | 4.5 ± 0.2 | 17 | 6.9 ± 0.2 | 44 | 25.4 ± 1.6 |
| LGG | 10.3 ± 0.2 | 81 | 9.2 ± 0.2 | 90 | 39.4 ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmiento-Vásquez, Z.; Vandenberghe, L.P.d.S.; Karp, S.G.; Soccol, C.R. Production of Polyhydroxyalkanoates through Soybean Hull and Waste Glycerol Valorization: Subsequent Alkaline Pretreatment and Enzymatic Hydrolysis. Fermentation 2022, 8, 433. https://doi.org/10.3390/fermentation8090433
Sarmiento-Vásquez Z, Vandenberghe LPdS, Karp SG, Soccol CR. Production of Polyhydroxyalkanoates through Soybean Hull and Waste Glycerol Valorization: Subsequent Alkaline Pretreatment and Enzymatic Hydrolysis. Fermentation. 2022; 8(9):433. https://doi.org/10.3390/fermentation8090433
Chicago/Turabian StyleSarmiento-Vásquez, Zulma, Luciana Porto de Souza Vandenberghe, Susan Grace Karp, and Carlos Ricardo Soccol. 2022. "Production of Polyhydroxyalkanoates through Soybean Hull and Waste Glycerol Valorization: Subsequent Alkaline Pretreatment and Enzymatic Hydrolysis" Fermentation 8, no. 9: 433. https://doi.org/10.3390/fermentation8090433
APA StyleSarmiento-Vásquez, Z., Vandenberghe, L. P. d. S., Karp, S. G., & Soccol, C. R. (2022). Production of Polyhydroxyalkanoates through Soybean Hull and Waste Glycerol Valorization: Subsequent Alkaline Pretreatment and Enzymatic Hydrolysis. Fermentation, 8(9), 433. https://doi.org/10.3390/fermentation8090433